Comparison of Microencapsulated Phase Change Materials Prepared at Laboratory Containing the Same Core and Different Shell Material
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Solé, A.; Martorell, I.; Cabeza, L.F. State of the art on gas—Solid thermochemical energy storage systems and reactors for building applications. Renew. Sustain. Energy Rev. 2015, 47, 386–398. [Google Scholar] [CrossRef]
- Vandegaer, J.E. Microencapsulation: Processes and Applications; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Konuklu, Y.; Ostry, M.; Paksoy, H.O.; Charvat, P. Review on Using Microencapsulated Phase Change Materials (PCM) in Building Applications. Energy Build. 2015, 106, 134–155. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Martínez, M.; Cabeza, L.F.; Fernández, A.I. Types, methods, techniques, and applications for Microencapsulated Phase Change Materials (MPCM): A review. Renew. Sustain. Energy Rev. 2016, 53, 1059–1075. [Google Scholar] [CrossRef]
- Sari, A.; Alkan, C.; Biçer, A.; Altuntaş, A.; Bilgin, C. Micro/nanoencapsulated n-nonadecane with poly(methyl methacrylate) shell for thermal energy storage. Energy Convers. Manag. 2014, 86, 614–621. [Google Scholar] [CrossRef]
- Sari, A.; Alkan, C.; Bilgin, C. Micro/nano encapsulation of some paraffin eutectic mixtures with poly(methyl methacrylate) shell: Preparation, characterization and latent heat thermal energy storage properties. Appl. Energy 2014, 136, 217–227. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, H.; Xia, T.D.; Zhang, T.; Feng, H.X. Fabrication and performances of microencapsulated paraffin composites with polymethylmethacrylate shell based on ultraviolet irradiation-initiated. Mater. Chem. Phys. 2012, 135, 181–187. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Konuklu, Y.; Fernández, A.I. Preparation and exhaustive characterization of paraffin or palmitic acid microcapsules as novel phase change material. Sol. Energy 2015, 112, 300–309. [Google Scholar] [CrossRef]
- Sánchez-Silva, L.; Rodríguez, J.F.; Romero, A.; Borreguero, A.M.; Carmona, M.; Sánchez, P. Microencapsulation of PCMs with a styrene-methyl methacrylate copolymer shell by suspension-like polymerisation. Chem. Eng. J. 2010, 157, 216–222. [Google Scholar] [CrossRef]
- Sari, A.; Alkan, C.; Altintaş, A. Preparation, characterization and latent heat thermal energy storage properties of micro-nanoencapsulated fatty acids by polystyrene shell. Appl. Therm. Eng. 2014, 73, 1158–1166. [Google Scholar] [CrossRef]
- Sari, A.; Alkan, C.; Kahraman Döǧüşcü, D.; Biçer, A. Micro/nano-encapsulated n-heptadecane with polystyrene shell for latent heat thermal energy storage. Sol. Energy Mater. Sol. Cells 2014, 126, 42–50. [Google Scholar] [CrossRef]
- Sánchez, L.; Sánchez, P.; de Lucas, A.; Carmona, M.; Rodríguez, J.F. Microencapsulation of PCMs with a polystyrene shell. Colloid Polym. Sci. 2007, 285, 1377–1385. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Z.H.; Zeng, X.R. Preparation, characterization, and thermal properties of microPCMs containing n-dodecanol by using different types of styrene-maleic anhydride as emulsifier. Colloid Polym. Sci. 2009, 287, 549–560. [Google Scholar] [CrossRef]
- Pisello, A.L.; D’ Alessandro, A.; Fabiani, C.; Fiorelli, A.P.; Ubertini, F.; Cabeza, L.F.; Materazzi, A.L.; Cotana, F. Multifunctional Analysis of Innovative PCM-filled Concretes. Energy Procedia 2017, 111, 81–90. [Google Scholar] [CrossRef]
- Bajāre, D.; Kazjonovs, J.; Korjakins, A.; Merijs-Meri, R. Development of Cement and Lime Based Plaster with Microencapsulated Phase Change Materials (PCM). In Proceedings of the 12th International Conference on Energy Storage, Lleida, Spain, 16–18 May 2012; pp. 1–7. [Google Scholar]
- Rozanna, D.; Salmiah, A.; Chuah, T.G.; Medyan, R.; Thomas Choong, S.Y.; Sa’Ari, M. A study on thermal characteristics of phase change material (PCM) in gypsum board for building application. J. Oil Palm Res. 2005, 17, 41–46. [Google Scholar]
- Benita, S. Microencapsulation: Methods and Industrial Applications; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Wang, H.; Wang, J.P.; Wang, X.; Li, W.; Zhang, X. Preparation and Properties of Microencapsulated Phase Change Materials Containing Two-Phase Core Materials. Ind. Eng. Chem. Res. 2013, 52, 14706–14712. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, S.; Li, J.; Tang, G. Preparation and thermal reliabilities of microencapsulated phase change materials with binary cores and acrylate-based polymer shells. Thermochim. Acta 2014, 588, 38–46. [Google Scholar] [CrossRef]
- Sari, A.; Alkan, C.; Özcan, A.N. Synthesis and characterization of micro/nano capsules of PMMA/capric-stearic acid eutectic mixture for low temperature-thermal energy storage in buildings. Energy Build. 2015, 90, 106–113. [Google Scholar] [CrossRef]
- Fernández Lladó, J. Análisis Del Comportamiento Al Fuego De Materiales De Construcción Con Adición De Pcmr; Universitat Politècnica de Catalunya (UPC): Barcelona, Spain, 2012. [Google Scholar]
- Borreguero, A.M.; Garrido, I.; Valverde, J.L.; Rodríguez, J.F.; Carmona, M. Thermal Characterization of Gypsum Composites by Using Differential Scanning Calorimetry. Introduction Thermal Energy Storage Materials. In Proceedings of the Energy and Environment Knowledge Week (E2KW) 2013, Toledo, Spain, 20–22 November 2013. [Google Scholar]
- Eddhahak-Ouni, A.; Drissi, S.; Colin, J.; Neji, J.; Care, S. Experimental and multi-scale analysis of the thermal properties of Portland cement concretes embedded with microencapsulated Phase Change Materials (PCMs). Appl. Therm. Eng. 2014, 64, 32–39. [Google Scholar] [CrossRef]
- Sari-Bey, S.; Fois, M.; Krupa, I.; Ibos, L.; Benyoucef, B.; Candau, Y. Thermal characterization of polymer matrix composites containing microencapsulated paraffin in solid or liquid state. Energy Convers. Manag. 2014, 78, 796–804. [Google Scholar] [CrossRef]
- Franquet, E.; Gibout, S.; Tittelein, P.; Zalewski, L.; Dumas, J.P. Experimental and theoretical analysis of a cement mortar containing microencapsulated PCM. Appl. Therm. Eng. 2014, 73, 30–38. [Google Scholar] [CrossRef]
- Delgado, M.; Lázaro, A.; Mazo, J.; Zalba, B. Review on phase change material emulsions and microencapsulated phase change material slurries: Materials, heat transfer studies and applications. Renew. Sustain. Energy Rev. 2012, 16, 253–273. [Google Scholar] [CrossRef]
- Joulin, A.; Zalewski, L.; Lassue, S.; Naji, H. Experimental investigation of thermal characteristics of a mortar with or without a micro-encapsulated phase change material. Appl. Therm. Eng. 2014, 66, 171–180. [Google Scholar] [CrossRef]
- Serrano, S.; Barreneche, C.; Rincón, L.; Boer, D.; Cabeza, L.F. Optimization of three new compositions of stabilized rammed earth incorporating PCM: Thermal properties characterization and LCA. Constr. Build. Mater. 2013, 47, 872–878. [Google Scholar] [CrossRef]
- Serrano, S.; Barreneche, C.; Rincón, L.; Boer, D.; Cabeza, L.F. Stabilized rammed earth incorporating PCM: Optimization and improvement of thermal properties and Life Cycle Assessment. Energy Procedia 2012, 30, 461–470. [Google Scholar] [CrossRef]
- Menoufi, K.; Castell, A.; Navarro, L.; Pérez, G.; Boer, D.; Cabeza, L.F. Evaluation of the environmental impact of experimental cubicles using Life Cycle Assessment: A highlight on the manufacturing phase. Appl. Energy 2012, 92, 534–544. [Google Scholar] [CrossRef]
- Konuklu, Y. Microencapsulation of phase change material with poly(ethylacrylate) shell for thermal energy storage. Int. J. Energy Res. 2014, 38, 2019–2029. [Google Scholar] [CrossRef]
- Konuklu, Y.; Paksoy, H.O.; Unal, M.; Konuklu, S. Microencapsulation of a fatty acid with Poly(melamine-urea-formaldehyde). Energy Convers. Manag. 2014, 80, 382–390. [Google Scholar] [CrossRef]
- Konuklu, Y.; Unal, M.; Paksoy, H.O. Microencapsulation of caprylic acid with different wall materials as phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2014, 120, 536–542. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Barreneche, C.; Martínez, M.; Šumiga, B.; Fernández, A.I.; Cabeza, L.F. Mechanical response evaluation of microcapsules from different slurries. Renew. Energy 2016, 85, 732–739. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Oncins, G.; Barreneche, C.; Martínez, M.; Fernández, A.I.; Cabeza, L.F. Physico-chemical and mechanical properties of microencapsulated phase change material. Appl. Energy 2013, 109, 441–448. [Google Scholar] [CrossRef]
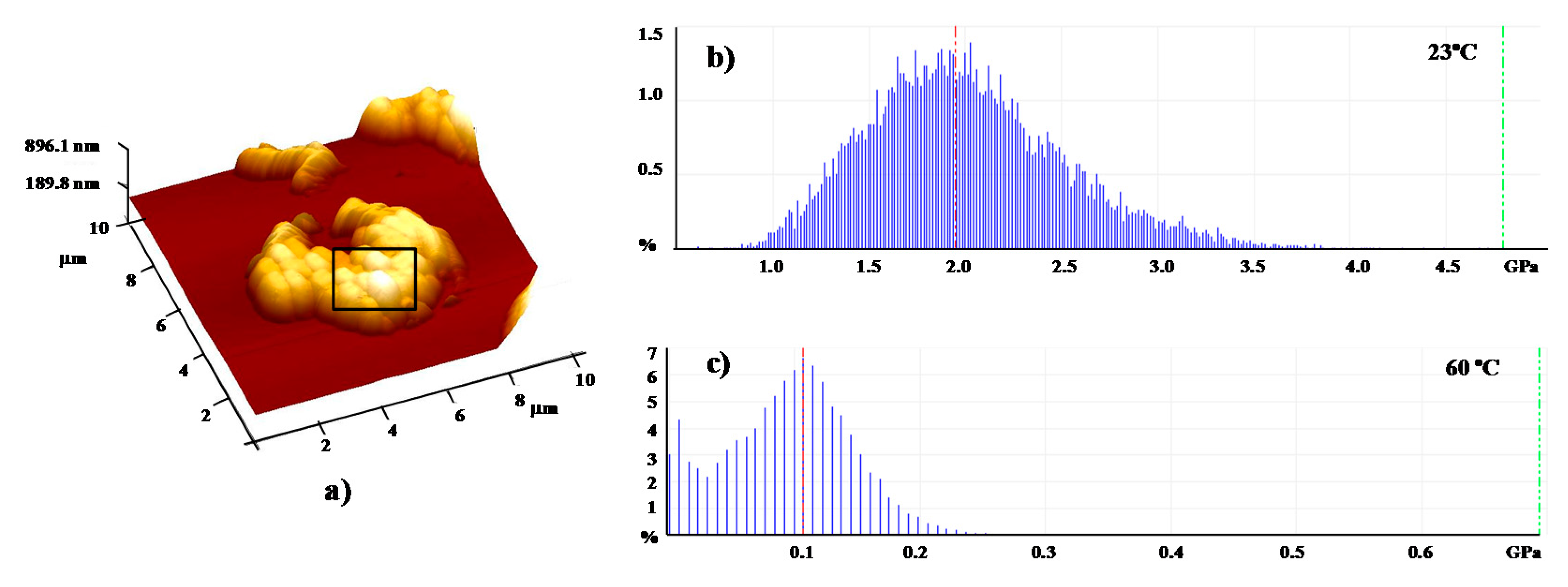
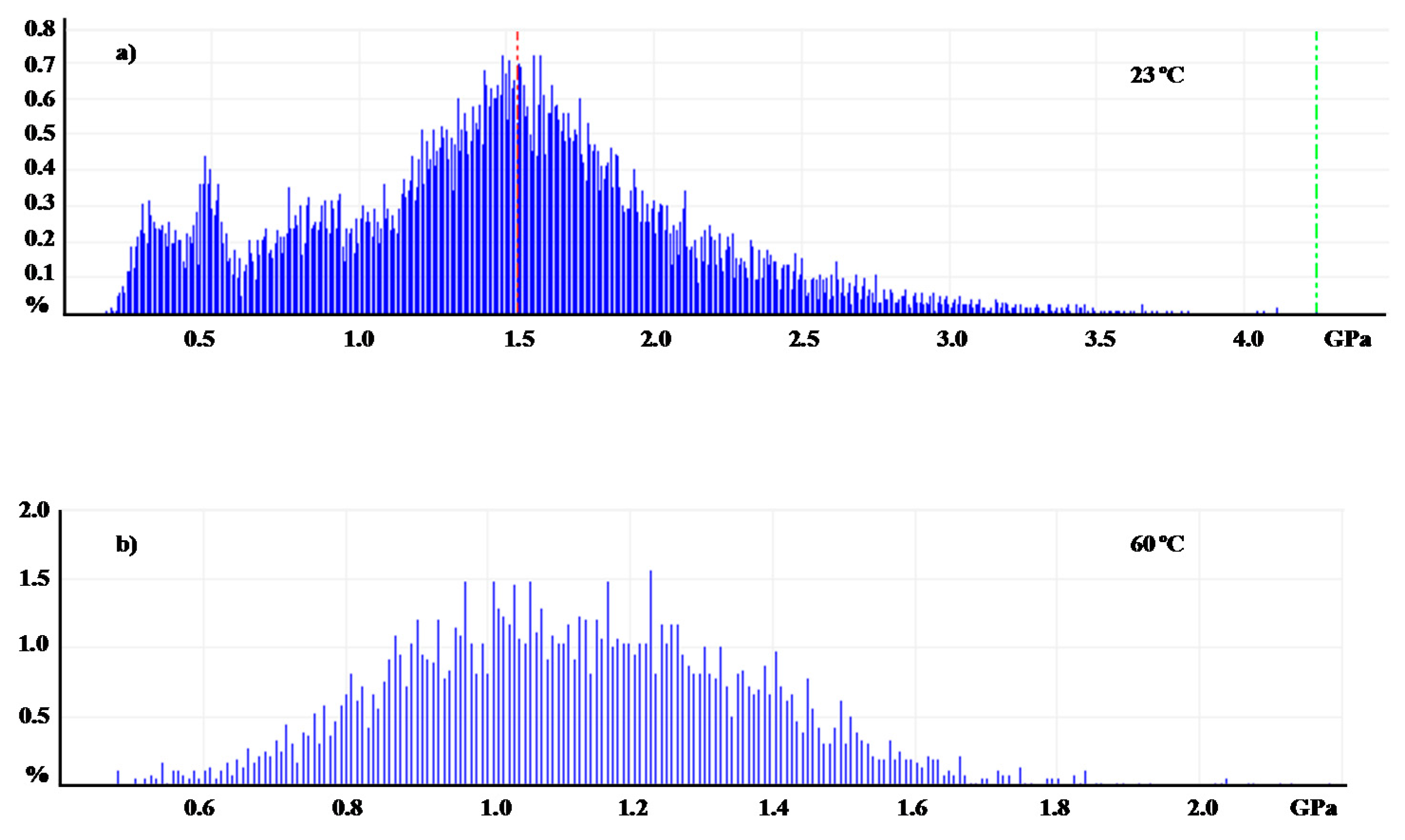
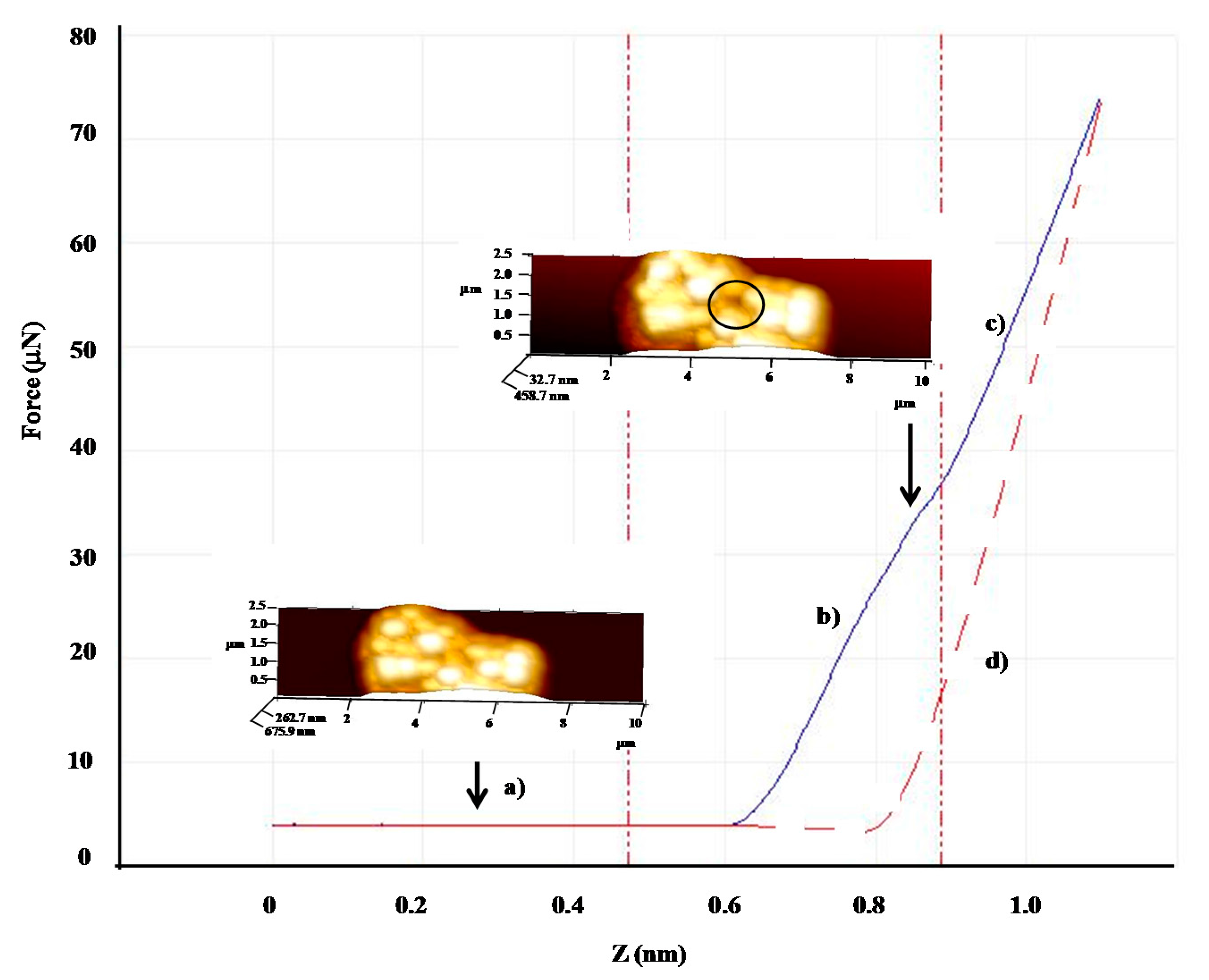
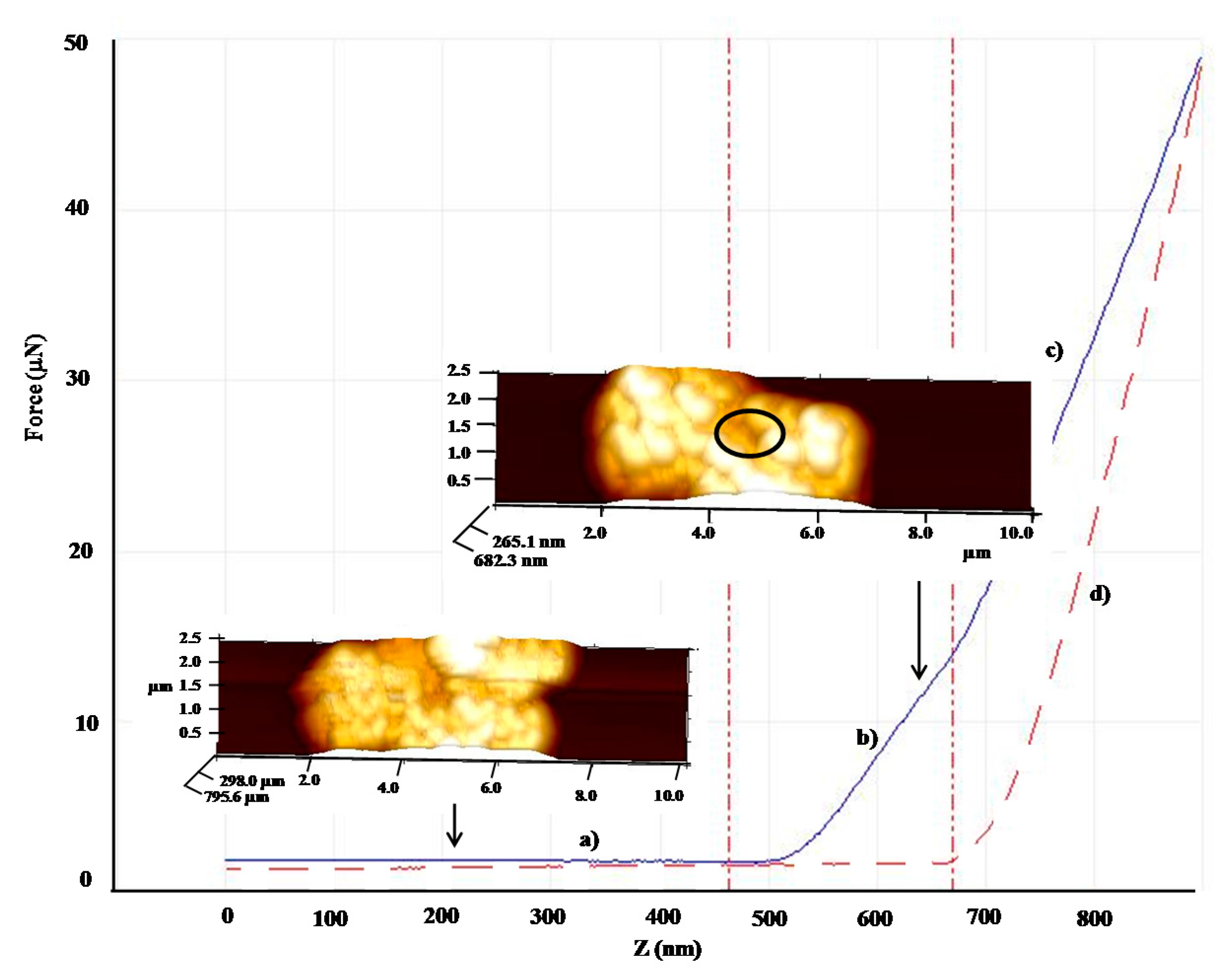
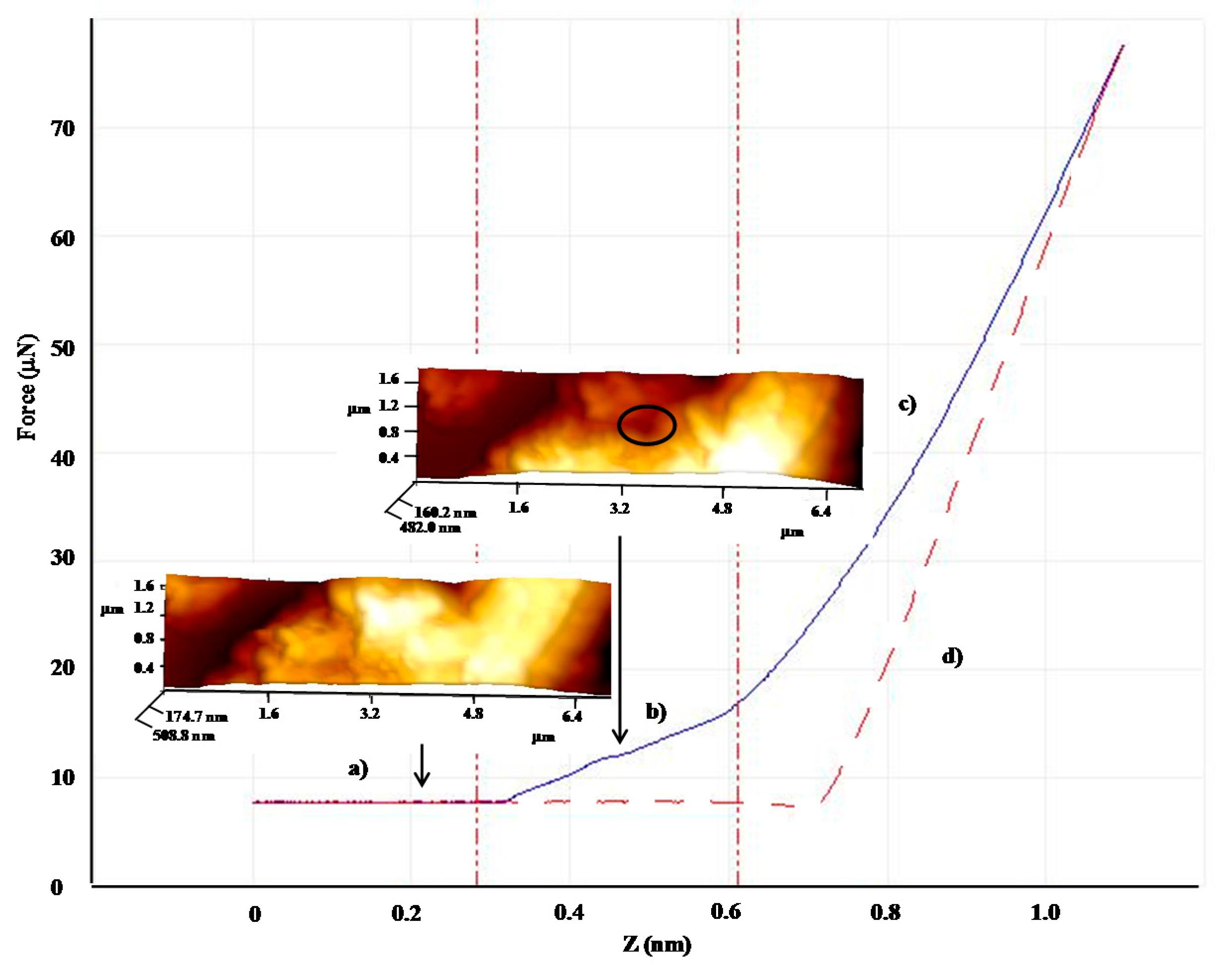
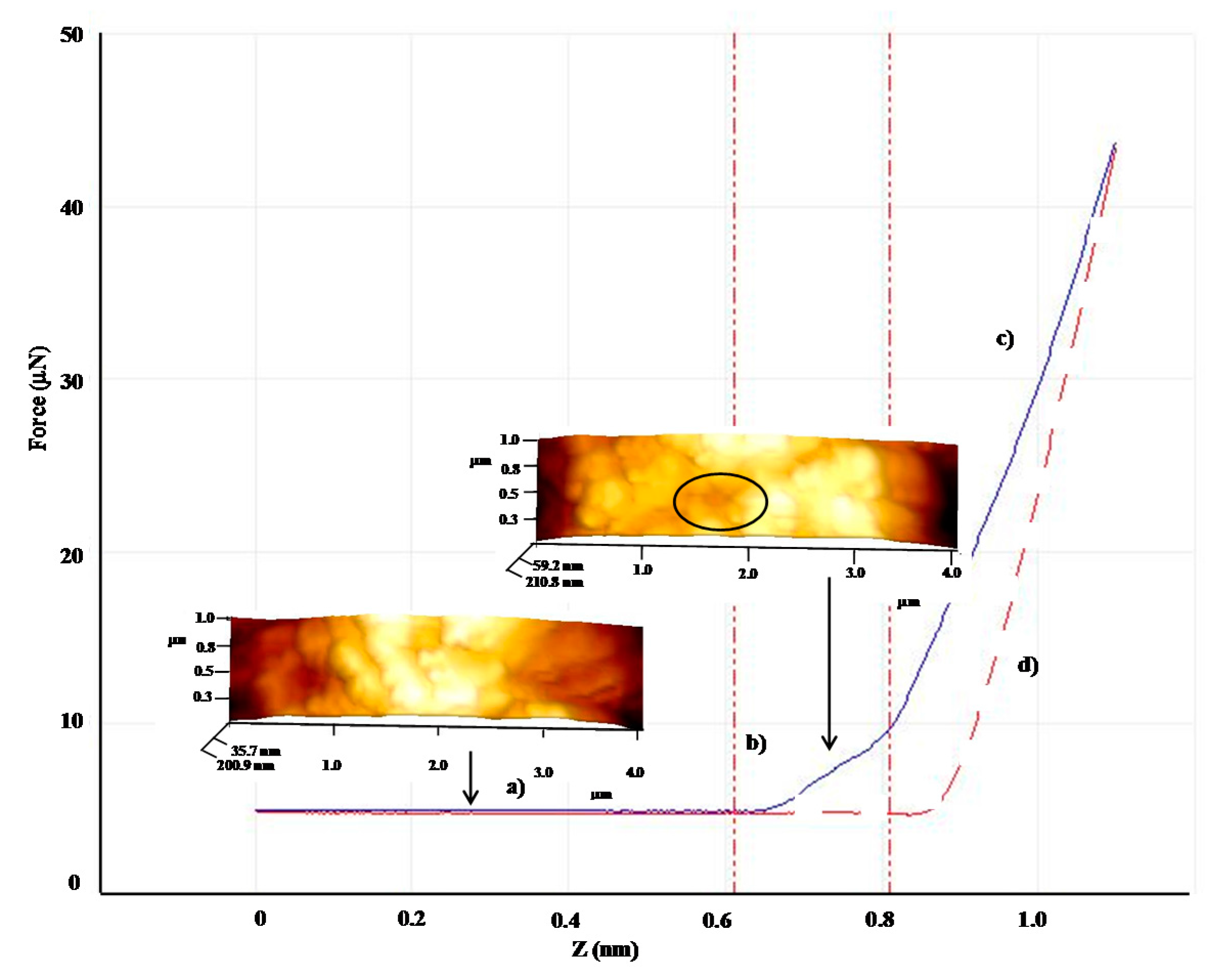
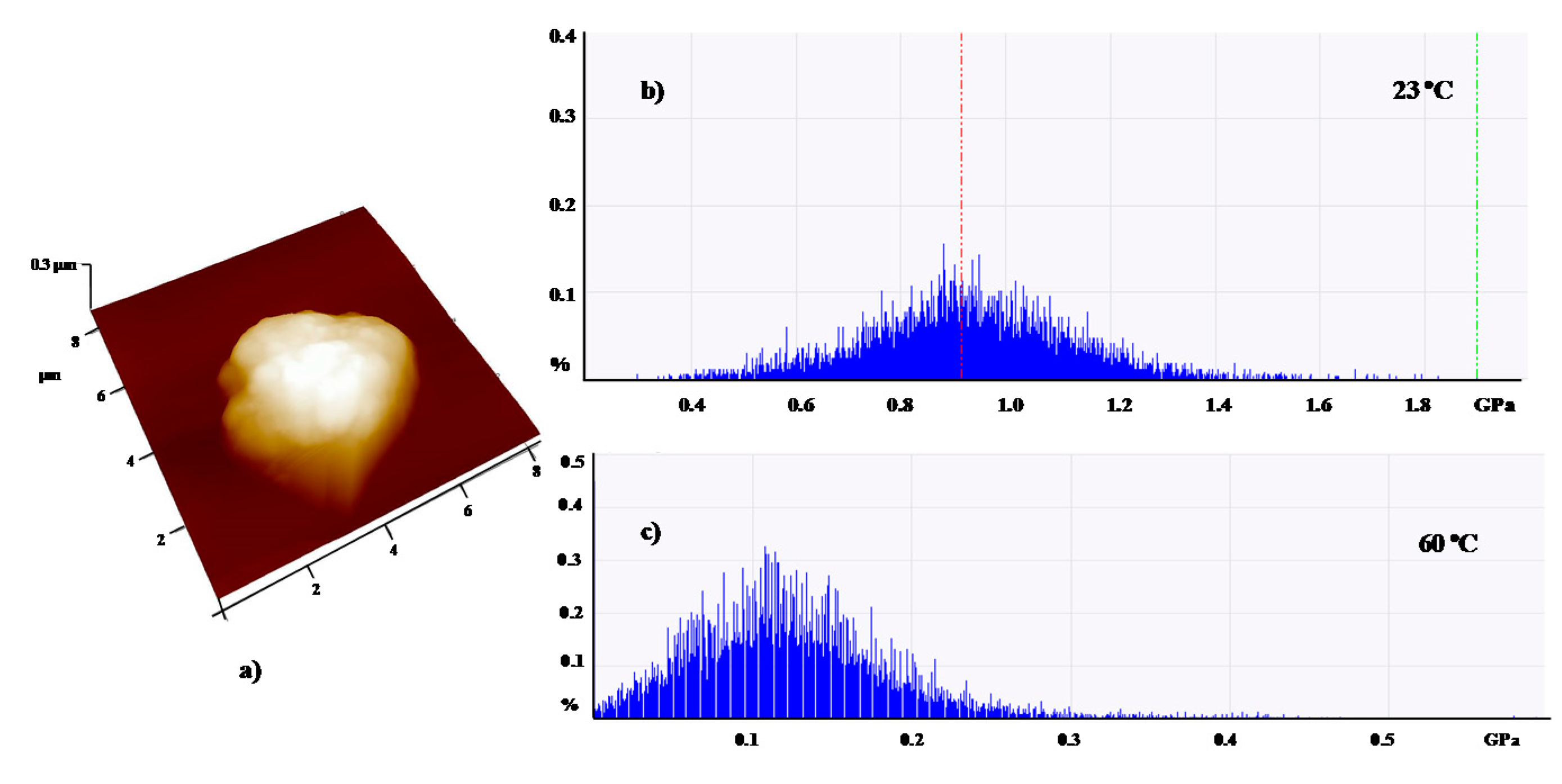
| Shell:PCM Encapsulation Mass Ratio | PMMA:Tetracosane 1:3 | PS:Tetracosane 1:3 | PS:Tetracosane 1:1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature and % Reduction | 23 °C | 60 °C | Reduction (%) | 23 °C | 60 °C | Reduction (%) | 23 °C | 60 °C | Reduction (%) |
| E (GPa) | 2.7 | 0.1 | 96 | 1.5 | 1.1 | 27 | 0.9 | 0.1 | 87 |
| F (μN) | 31.2 | 11.4 | 64 | 13.2 | 5.3 | 60 | - | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giro-Paloma, J.; Alkan, C.; Chimenos, J.M.; Fernández, A.I. Comparison of Microencapsulated Phase Change Materials Prepared at Laboratory Containing the Same Core and Different Shell Material. Appl. Sci. 2017, 7, 723. https://doi.org/10.3390/app7070723
Giro-Paloma J, Alkan C, Chimenos JM, Fernández AI. Comparison of Microencapsulated Phase Change Materials Prepared at Laboratory Containing the Same Core and Different Shell Material. Applied Sciences. 2017; 7(7):723. https://doi.org/10.3390/app7070723
Chicago/Turabian StyleGiro-Paloma, Jessica, Cemil Alkan, Josep Maria Chimenos, and Ana Inés Fernández. 2017. "Comparison of Microencapsulated Phase Change Materials Prepared at Laboratory Containing the Same Core and Different Shell Material" Applied Sciences 7, no. 7: 723. https://doi.org/10.3390/app7070723






