Effect of Organic Stabilizers on Silver Nanoparticles Fabricated by Femtosecond Pulsed Laser Ablation
Abstract
:1. Introduction
2. Materials and Methods
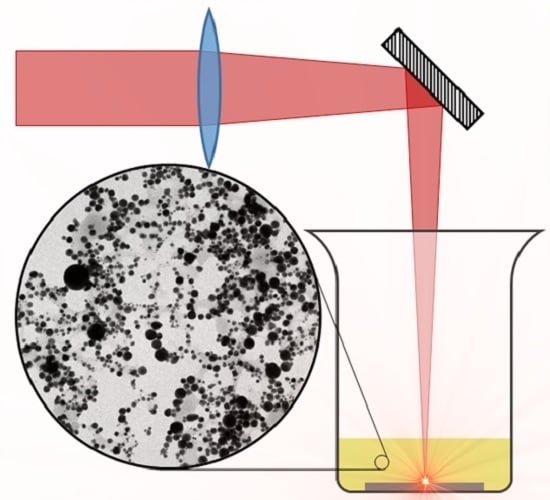
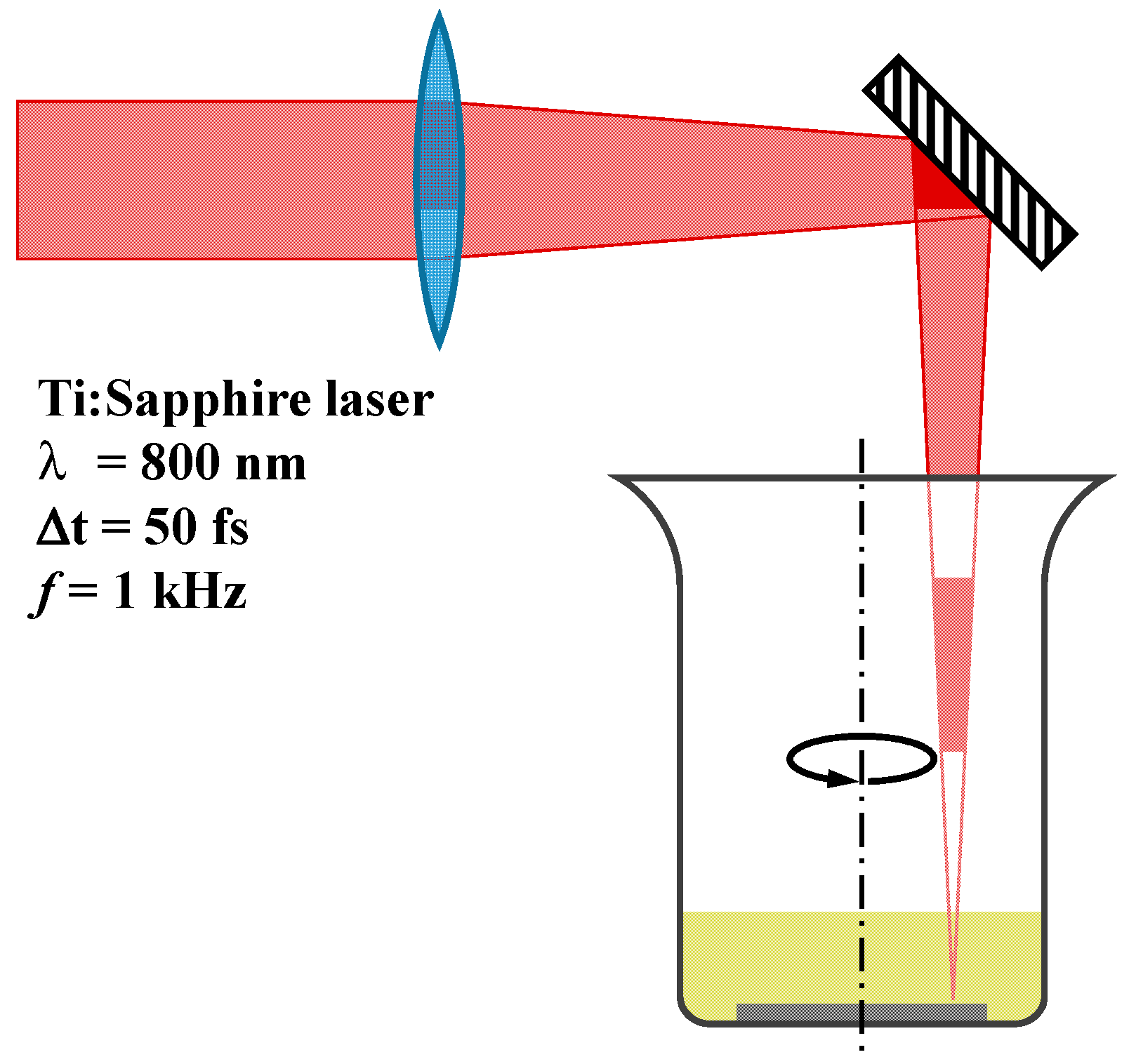
2.1. Experimental
2.2. Optical Fits
3. Results and Discussion
3.1. Morphology and Nanoparticle Size Distribution
3.2. Optical Absorption Spectra
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pulsed Laser Deposition of Thin Films: Applications-Led Growth of Functional Materials; Eason, R. (Ed.) John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; ISBN 978-0-470-05212-9. [Google Scholar]
- Amoruso, S.; Bruzzese, R.; Vitiello, M.; Nedialkov, N.N.; Atanasov, P.A. Experimental and theoretical investigations of femtosecond laser ablation of aluminum in vacuum. J. Appl. Phys. 2005, 98, 044907. [Google Scholar] [CrossRef]
- Teghil, R.; D’Alessio, L.; De Bonis, A.; Galasso, A.; Villani, P.; Santagata, A. Femtosecond pulsed laser ablation and deposition of titanium carbide. Thin. Solid Films 2006, 515, 1411–1418. [Google Scholar] [CrossRef]
- Sanz, M.; de Nalda, R.; Marco, J.F.; Izquierdo, J.G.; Bañares, L.; Castillejo, M. Femtosecond pulsed laser deposition of nanostructured CdS films. J. Phys. Chem. C 2010, 114, 4864–4868. [Google Scholar] [CrossRef]
- Sanz, M.; Walczak, M.; de Nalda, R.; Oujja, M.; Marco, J.F.; Rodriguez, J.; Izquierdo, J.G.; Bañares, L.; Castillejo, M. Femtosecond pulsed laser deposition of nanostructured TiO2 films. Appl. Surf. Sci. 2009, 255, 5206–5210. [Google Scholar] [CrossRef]
- Gámez, F.; Plaza-Reyes, A.; Hurtado, P.; Guillén, E.; Anta, J.A.; Martínez-Haya, B.; Pérez, S.; Sanz, M.; Castillejo, M.; Izquierdo, J.G.; et al. Nanoparticle TiO2 films prepared by pulsed laser deposition: Laser desorption and cationization of model adsorbates. J. Phys. Chem. C 2010, 114, 17409–17415. [Google Scholar] [CrossRef]
- Krishnan, R.; David, C.; Ajikumar, P.K.; Nithya, R.; Tripura Sundari, S.; Dash, S.; Panigrahi, B.K.; Kamruddin, M.; Tyagi, A.K.; Jayaram, V.; et al. Reactive pulsed laser deposition of titanium nitride thin films: Effect of reactive gas pressure on the structure, composition, and properties. J. Mater. 2013, 2013, e128986. [Google Scholar] [CrossRef]
- Deno, H.; Kamemoto, T.; Nemoto, S.; Koshio, A.; Kokai, F. Formation of TiN–Ir particle films using pulsed-laser deposition and their electrolytic properties in producing hypochlorous acid. Appl. Surf. Sci. 2008, 254, 2776–2782. [Google Scholar] [CrossRef]
- Craciun, D.; Stefan, N.; Socol, G.; Dorcioman, G.; McCumiskey, E.; Hanna, M.; Taylor, C.R.; Bourne, G.; Lambers, E.; Siebein, K.; et al. Very hard TiN thin films grown by pulsed laser deposition. Appl. Surf. Sci. 2012, 260, 2–6. [Google Scholar] [CrossRef]
- Narayan, R.J. Pulsed laser deposition of functionally gradient diamondlike carbon–metal nanocomposites. Diam. Relat. Mater. 2005, 14, 1319–1330. [Google Scholar] [CrossRef]
- Peña-Rodríguez, O.; González-Izquierdo, J.; Rivera, A.; Balabanian, G.; Olivares, J.; Perlado, J.M.; Bañares, L. Embedded silver nanoparticle multilayers fabricated by femtosecond pulsed laser deposition. Opt. Mater. Express. 2014, 4, 1943–1952. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. What controls the composition and the structure of nanomaterials generated by laser ablation in liquid solution? Phys. Chem. Chem. Phys. 2013, 15, 3027–3046. [Google Scholar] [CrossRef] [PubMed]
- Simakin, A.V.; Voronov, V.V.; Shafeev, G.A.; Brayner, R.; Bozon-Verduraz, F. Nanodisks of Au and Ag produced by laser ablation in liquid environment. Chem. Phys. Lett. 2001, 348, 182–186. [Google Scholar] [CrossRef]
- Dolgaev, S.I.; Simakin, A.V.; Voronov, V.V.; Shafeev, G.A.; Bozon-Verduraz, F. Nanoparticles produced by laser ablation of solids in liquid environment. Appl. Surf. Sci. 2002, 186, 546–551. [Google Scholar] [CrossRef]
- Simakin, A.V.; Voronov, V.V.; Kirichenko, N.A.; Shafeev, G.A. Nanoparticles produced by laser ablation of solids in liquid environment. Appl. Phys. A 2004, 79, 1127–1132. [Google Scholar] [CrossRef]
- Tsuji, T.; Kakita, T.; Tsuji, M. Preparation of nano-size particles of silver with femtosecond laser ablation in water. Appl. Surf. Sci. 2003, 206, 314–320. [Google Scholar] [CrossRef]
- Tsuji, T.; Thang, D.-H.; Okazaki, Y.; Nakanishi, M.; Tsuboi, Y.; Tsuji, M. Preparation of silver nanoparticles by laser ablation in polyvinylpyrrolidone solutions. Appl. Surf. Sci. 2008, 254, 5224–5230. [Google Scholar] [CrossRef]
- Tsuji, T.; Yahata, T.; Yasutomo, M.; Igawa, K.; Tsuji, M.; Ishikawa, Y.; Koshizaki, N. Preparation and investigation of the formation mechanism of submicron-sized spherical particles of gold using laser ablation and laser irradiation in liquids. Phys. Chem. Chem. Phys. 2013, 15, 3099–3107. [Google Scholar] [CrossRef] [PubMed]
- Kabashin, A.V.; Meunier, M. Synthesis of colloidal nanoparticles during femtosecond laser ablation of gold in water. J. Appl. Phys. 2003, 94, 7941–7943. [Google Scholar] [CrossRef]
- Sylvestre, J.-P.; Poulin, S.; Kabashin, A.V.; Sacher, E.; Meunier, M.; Luong, J.H.T. Surface chemistry of gold nanoparticles produced by laser ablation in aqueous media. J. Phys. Chem. B 2004, 108, 16864–16869. [Google Scholar] [CrossRef]
- Eliezer, S.; Eliaz, N.; Grossman, E.; Fisher, D.; Gouzman, I.; Henis, Z.; Pecker, S.; Horovitz, Y.; Fraenkel, M.; Maman, S.; et al. Synthesis of nanoparticles with femtosecond laser pulses. Phys. Rev. B 2004, 69, 144119. [Google Scholar] [CrossRef]
- Barcikowski, S.; Menéndez-Manjón, A.; Chichkov, B.; Brikas, M.; Račiukaitis, G. Generation of nanoparticle colloids by picosecond and femtosecond laser ablations in liquid flow. Appl. Phys. Lett. 2007, 91, 083113. [Google Scholar] [CrossRef]
- Poondi, D.; Singh, J. Synthesis of metastable silver-nickel alloys by a novel laser-liquid-solid interaction technique. J. Mater. Sci. 2000, 35, 2467–2476. [Google Scholar] [CrossRef]
- Poondi, D.; Dobbins, T.; Singh, J. A novel laser-liquid-solid interaction technique for synthesis of silver, nickel and immiscible silver-nickel alloys from liquid precursors. J. Mater. Sci. 2000, 35, 6237–6243. [Google Scholar] [CrossRef]
- Izgaliev, A.T.; Simakin, A.V.; Shafeev, G.A. Formation of the alloy of Au and Ag nanoparticles upon laser irradiation of the mixture of their colloidal solutions. Quantum Electron. 2004, 34, 47–50. [Google Scholar] [CrossRef]
- Liu, Q.X.; Wang, C.X.; Zhang, W.; Wang, G.W. Immiscible silver–nickel alloying nanorods growth upon pulsed-laser induced liquid/solid interfacial reaction. Chem. Phys. Lett. 2003, 382, 1–5. [Google Scholar] [CrossRef]
- Liang, C.; Shimizu, Y.; Sasaki, T.; Koshizaki, N. Synthesis of ultrafine SnO2−x nanocrystals by pulsed laser-induced reactive quenching in liquid medium. J. Phys. Chem. B 2003, 107, 9220–9225. [Google Scholar] [CrossRef]
- Chen, J.; Dong, Q.; Yang, J.; Guo, Z.; Song, Z.; Lian, J. The irradiation effect of a Nd–YAG pulsed laser on the CeO2 target in the liquid. Mater. Lett. 2004, 58, 337–341. [Google Scholar] [CrossRef]
- Sasaki, T.; Liang, C.; Nichols, W.T.; Shimizu, Y.; Koshizaki, N. Fabrication of oxide base nanostructures using pulsed laser ablation in aqueous solutions. Appl. Phys. A 2004, 79, 1489–1492. [Google Scholar] [CrossRef]
- Yang, G.W.; Wang, J.B. Carbon nitride nanocrystals having cubic structure using pulsed laser induced liquid–solid interfacial reaction. Appl. Phys. A 2000, 71, 343–344. [Google Scholar] [CrossRef]
- Wang, J.B.; Zhang, C.Y.; Zhong, X.L.; Yang, G.W. Cubic and hexagonal structures of diamond nanocrystals formed upon pulsed laser induced liquid–solid interfacial reaction. Chem. Phys. Lett. 2002, 361, 86–90. [Google Scholar] [CrossRef]
- Wang, J.B.; Yang, G.W. Phase transformation between diamond and graphite in preparation of diamonds by pulsed-laser induced liquid-solid interface reaction. J. Phys. Condens. Matter. 1999, 11, 7089. [Google Scholar] [CrossRef]
- Anikin, K.V.; Melnik, N.N.; Simakin, A.V.; Shafeev, G.A.; Voronov, V.V.; Vitukhnovsky, A.G. Formation of ZnSe and CdS quantum dots via laser ablation in liquids. Chem. Phys. Lett. 2002, 366, 357–360. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Rivas, L.; Sanchez-Cortes, S.; García-Ramos, J.V.; Morcillo, G. Growth of silver colloidal particles obtained by citrate reduction to increase the raman enhancement factor. Langmuir 2001, 17, 574–577. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Shape-controlled synthesis of gold and silver nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- Bastús, N.G.; Merkoçi, F.; Piella, J.; Puntes, V. Synthesis of highly monodisperse citrate-stabilized silver nanoparticles of up to 200 nm: Kinetic control and catalytic properties. Chem. Mater. 2014, 26, 2836–2846. [Google Scholar] [CrossRef]
- Moskovits, M.; Vlčková, B. Adsorbate-induced silver nanoparticle aggregation kinetics. J. Phys. Chem. B 2005, 109, 14755–14758. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Lee, C.-H.; Joo, S.-W.; Lee, K. Kinetics of gold nanoparticle aggregation: Experiments and modeling. J. Colloid Interface Sci. 2008, 318, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, S.; Praharaj, S.; Basu, S.; Ghosh, S.K.; Jana, S.; Pande, S.; Vo-Dinh, T.; Jiang, H.; Pal, T. Self-assembly of silver nanoparticles: Synthesis, stabilization, optical properties, and application in surface-enhanced raman scattering. J. Phys. Chem. B 2006, 110, 13436–13444. [Google Scholar] [CrossRef] [PubMed]
- Fumitaka, M.; Kohno, J.; Takeda, Y.; Kondow, T.; Sawabe, H. Formation and size control of silver nanoparticles by laser ablation in aqueous solution. J. Phys. Chem. B 2000, 104, 9111–9117. [Google Scholar] [CrossRef]
- Fumitaka, M.; Kohno, J.; Takeda, Y.; Kondow, T.; Sawabe, H. Formation of gold nanoparticles by laser ablation in aqueous solution of surfactant. J. Phys. Chem. B 2001, 105, 5114–5120. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Yeh, C.-S. Laser ablation method: Use of surfactants to form the dispersed Ag nanoparticles. Colloids Surf. Physicochem. Eng. Asp. 2002, 197, 133–139. [Google Scholar] [CrossRef]
- Sylvestre, J.-P.; Kabashin, A.V.; Sacher, E.; Meunier, M.; Luong, J.H.T. Stabilization and size control of gold nanoparticles during laser ablation in aqueous cyclodextrins. J. Am. Chem. Soc. 2004, 126, 7176–7177. [Google Scholar] [CrossRef] [PubMed]
- Peña, O.; Rodríguez-Fernández, L.; Rodríguez-Iglesias, V.; Kellermann, G.; Crespo-Sosa, A.; Cheang-Wong, J.C.; Silva-Pereyra, H.G.; Arenas-Alatorre, J.; Oliver, A. Determination of the size distribution of metallic nanoparticles by optical extinction spectroscopy. Appl. Opt. 2009, 48, 566. [Google Scholar] [CrossRef] [PubMed]
- Peña, O.; Pal, U. Scattering of electromagnetic radiation by a multilayered sphere. Comput. Phys. Commun. 2009, 180, 2348–2354. [Google Scholar] [CrossRef]
- Yang, W. Improved recursive algorithm for light scattering by a multilayered sphere. Appl. Opt. 2003, 42, 1710. [Google Scholar] [CrossRef] [PubMed]
- Abramowitz, M. Handbook of Mathematical Functions; Dover Publications Inc.: Long Island, NY, USA, 1965; ISBN 0-486-61272-4. [Google Scholar]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; Wiley-Interscience: Weinheim, Germany, 1998; ISBN 0-471-29340-7. [Google Scholar]
- Peña-Rodríguez, O.; González Pérez, P.P.; Pal, U. MieLab: A software tool to perform calculations on the scattering of electromagnetic waves by multilayered spheres. Int. J. Spectrosc. 2011, 2011, 1–10. [Google Scholar] [CrossRef]
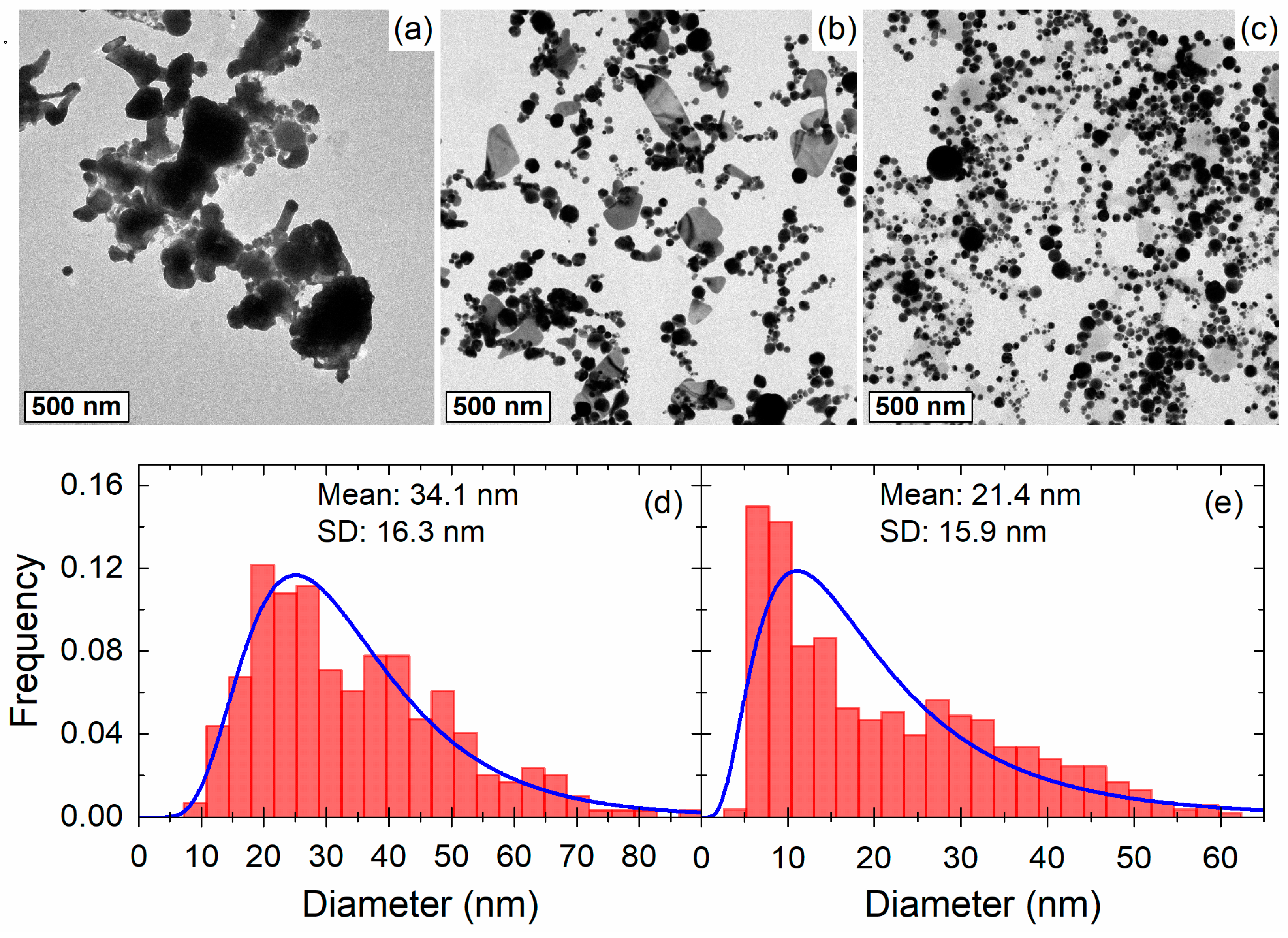
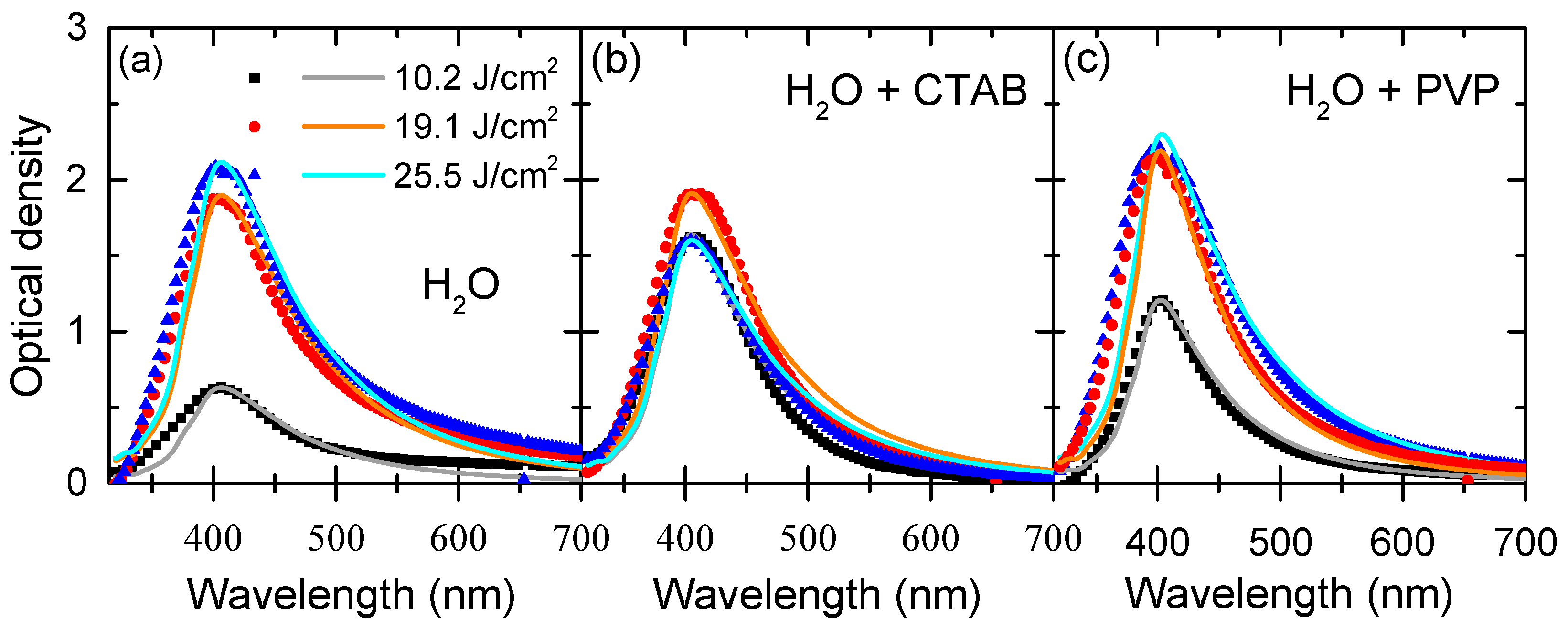
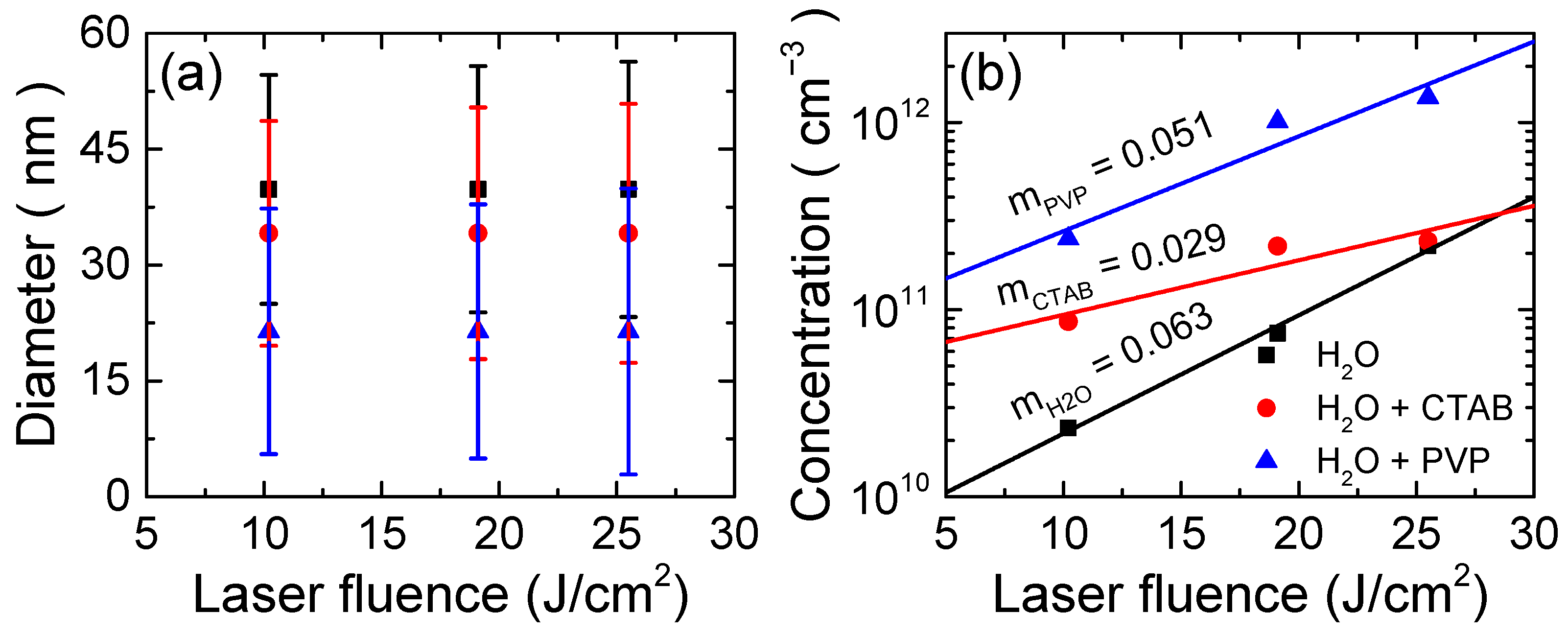
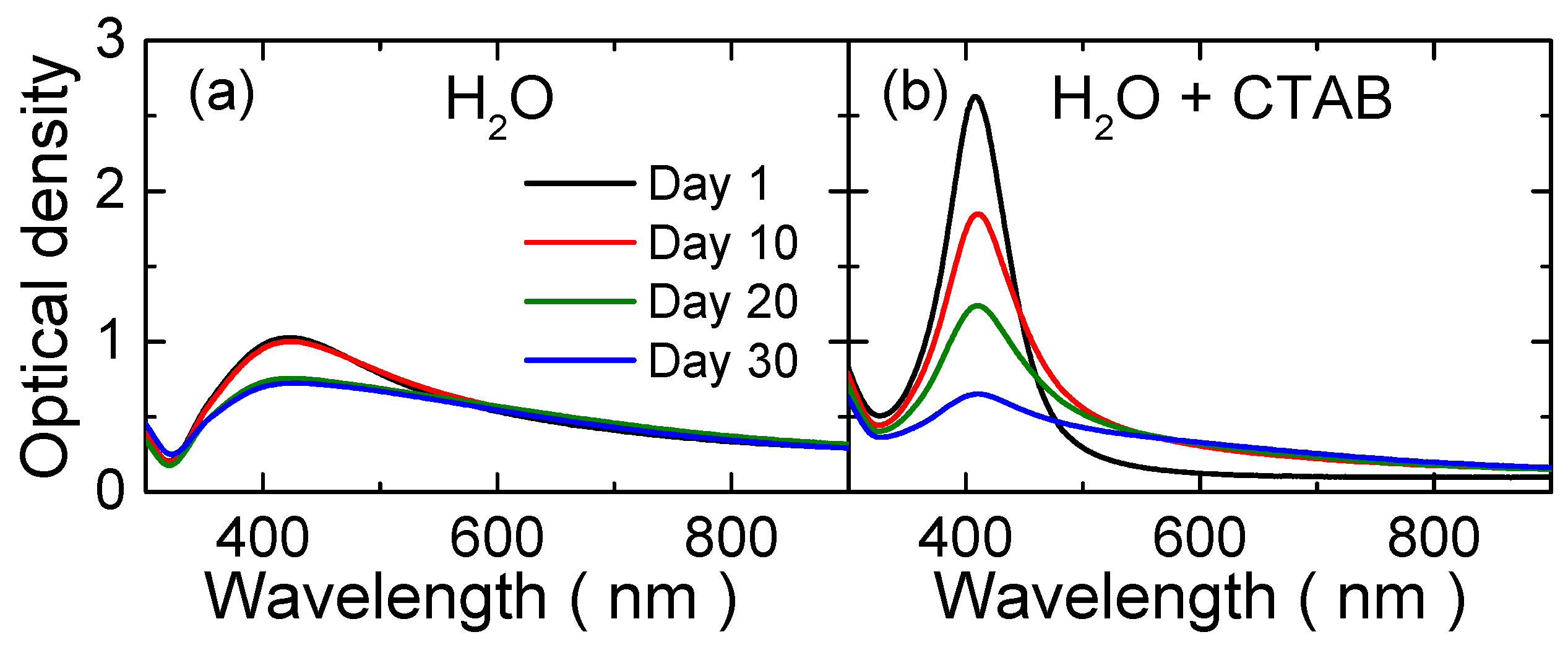
| Medium | Fluence (J/cm2) | Diameter (nm) | Standard Deviation (nm) | Concentration (Particles/cm3) |
|---|---|---|---|---|
| H2O | 10.2 | 40 ± 5 | 14.8 | 2.3 × 1010 |
| 19.1 | 40 ± 5 | 15.9 | 7.5 × 1010 | |
| 25.5 | 40 ± 5 | 16.5 | 2.2 × 1011 | |
| H2O + CTAB | 10.2 | 34 ± 3 | 14.5 | 8.6 × 1010 |
| 19.1 | 34 ± 3 | 16.3 | 2.2 × 1011 | |
| 25.5 | 34 ± 3 | 16.8 | 2.3 × 1011 | |
| H2O + PVP | 10.2 | 21 ± 2 | 15.9 | 2.4 × 1011 |
| 19.1 | 21 ± 2 | 16.5 | 1.0 × 1012 | |
| 25.5 | 21 ± 2 | 18.5 | 1.4 × 1012 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Núñez, P.; González-Izquierdo, J.; González-Rubio, G.; Guerrero-Martínez, A.; Rivera, A.; Perlado, J.M.; Bañares, L.; Peña-Rodríguez, O. Effect of Organic Stabilizers on Silver Nanoparticles Fabricated by Femtosecond Pulsed Laser Ablation. Appl. Sci. 2017, 7, 793. https://doi.org/10.3390/app7080793
Díaz-Núñez P, González-Izquierdo J, González-Rubio G, Guerrero-Martínez A, Rivera A, Perlado JM, Bañares L, Peña-Rodríguez O. Effect of Organic Stabilizers on Silver Nanoparticles Fabricated by Femtosecond Pulsed Laser Ablation. Applied Sciences. 2017; 7(8):793. https://doi.org/10.3390/app7080793
Chicago/Turabian StyleDíaz-Núñez, Pablo, Jesús González-Izquierdo, Guillermo González-Rubio, Andrés Guerrero-Martínez, Antonio Rivera, José Manuel Perlado, Luis Bañares, and Ovidio Peña-Rodríguez. 2017. "Effect of Organic Stabilizers on Silver Nanoparticles Fabricated by Femtosecond Pulsed Laser Ablation" Applied Sciences 7, no. 8: 793. https://doi.org/10.3390/app7080793