An Innovative Dual-Column System for Heavy Metallic Ion Sorption by Natural Zeolite
Abstract
:1. Introduction
- The evaluation of predominantly single- or dual-component HMI system combinations;
- The implementation of primarily slender column aspect ratios (i.e., bed depth/particle diameter, column height/diameter), causing a challenge to eventual scale-up design;
- The use of inconsistent and/or vague sorbent compaction techniques, and;
- The application of simple, idealized flow patterns (i.e., set single and continuous flow rate).
2. Experimental Design
2.1. Packed Fixed-Bed Column Design Considerations
2.2. Natural Zeolite Mineral
2.3. Heavy Metallic Ion Solution
2.4. Analytical Procedure and Quality Control
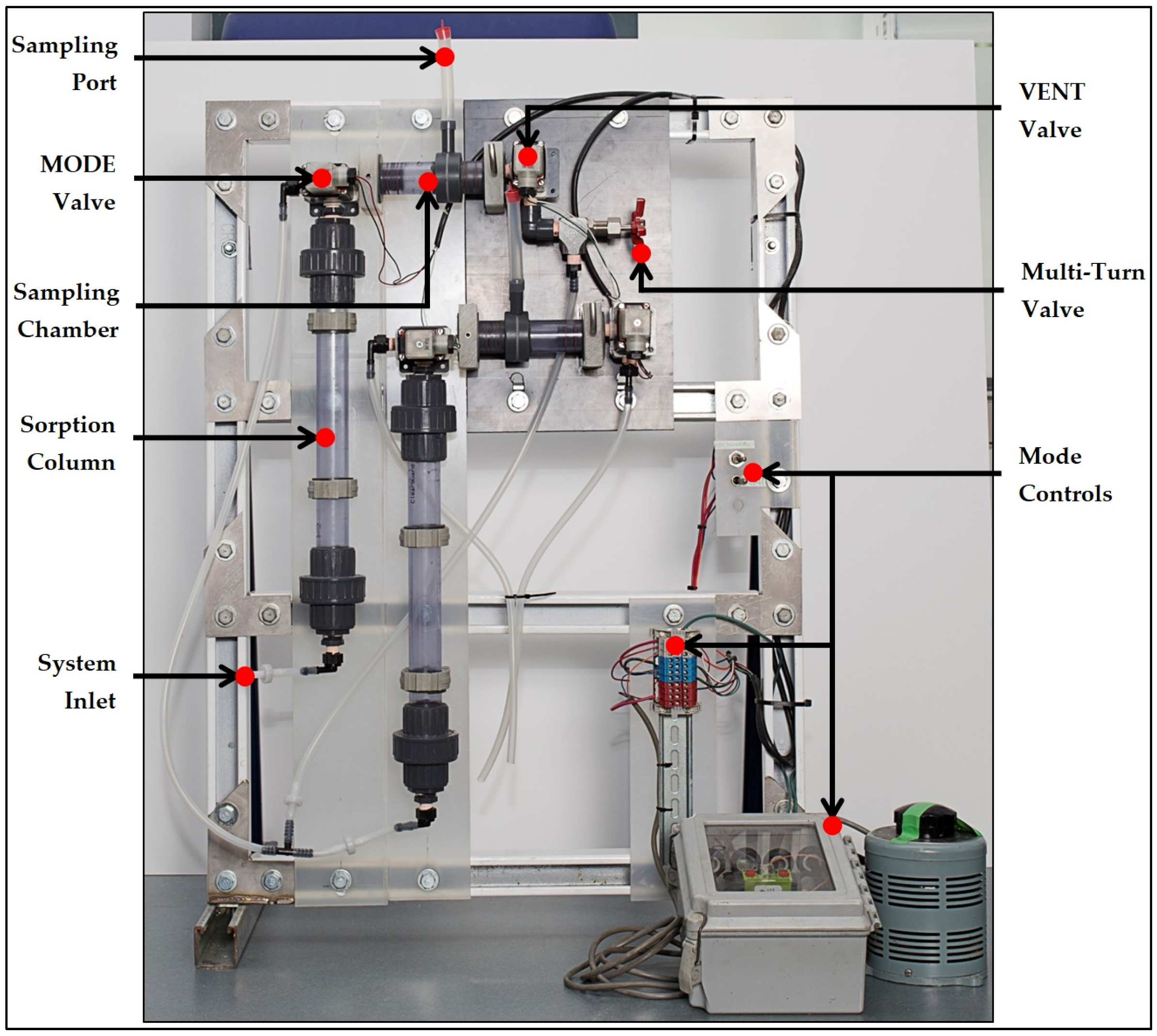
2.5. Sorption System Design
- Zeolite Compaction Technique
- ○
- Regulated Layers of Dry Mass
- ○
- Systematic Tampered Compaction
- Column Dimensions
- ○
- Modular Design
- ○
- Internal Diameter (1 in)
- ○
- Sorption Column Height (1 ft)
- Flow Configuration
- ○
- Upflow Distribution
- ○
- Dual-Column Series Connection
- ○
- Methodical Flow Rate Variability
- Pump Type
- ○
- Diaphragm Metering
- Sampling Method
- ○
- Automated Mode Controls
- ○
- Customized Sampling Chambers
- ○
- Modes’ Interchange in Five (5)-minute Intervals
- Analysis Period
- ○
- Three (3)-hour Contact Period
- HMI Multi-Component Influent Stock
- Metering Pump
- Silicon Tubing and Polyvinyl chloride (PVC) Connections
- Check Valves
- Automatable Solenoid Valves (symbol S)
- Packed Fixed-Bed Sorption Columns
- Custom Sampling Chambers
- Sampling Ports
- Effluent Collection Basin
2.5.1. Column Dimensions
2.5.2. Flow Rate and Configuration
2.5.3. Sampling Method
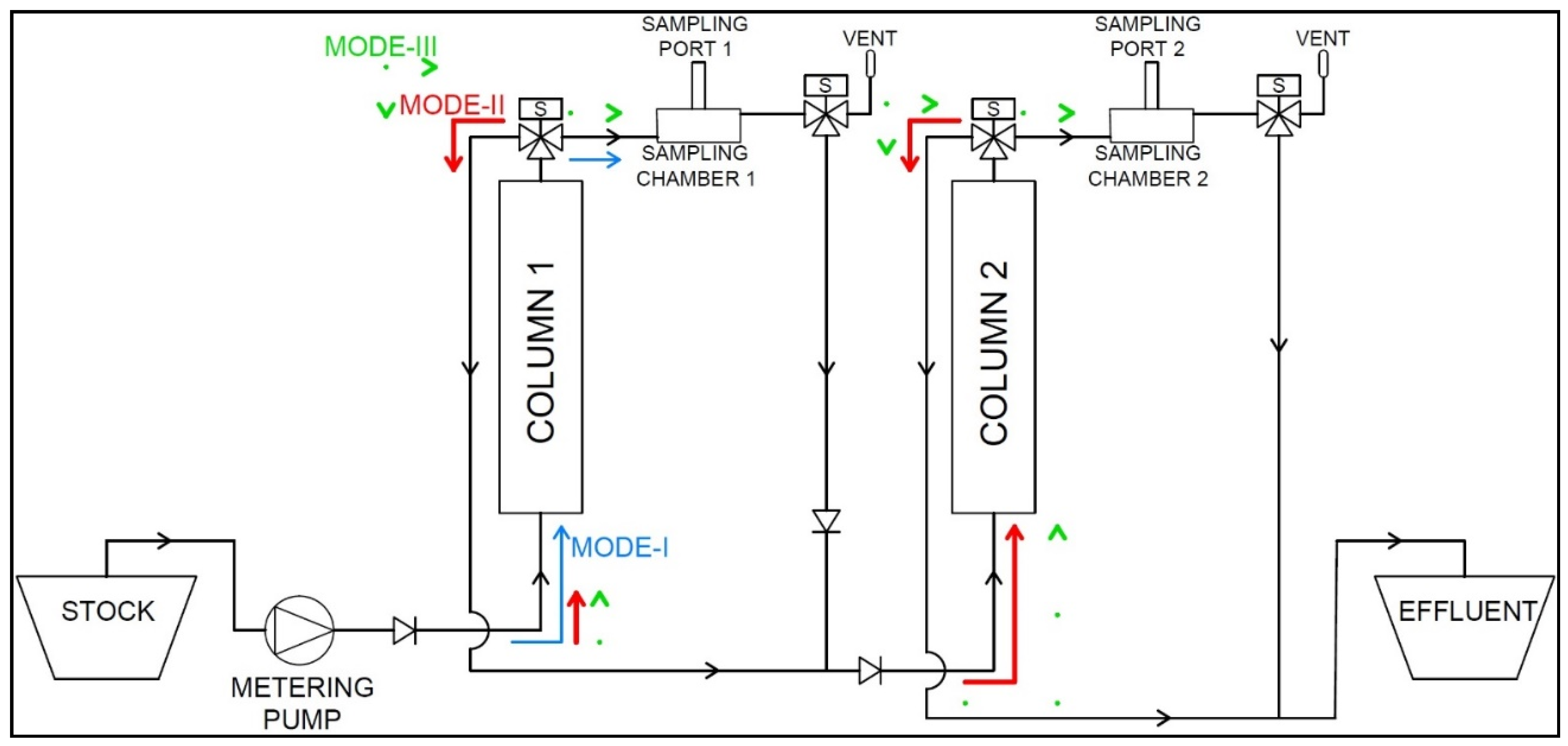
2.5.4. System Modes of Operation
- Mode-I
- ○
- Sorption System Activation
- ○
- Fill Sorption Column 1 and Sample Chamber 1
- Mode-II
- ○
- Flow Circulation through Sorption Columns 1 (C1) and 2 (C2)
- ○
- Detour of flow to Sampling Chambers 1 (SC1) and 2 (SC2)
- ○
- VENT Valve Activation for Sample Collection
- Mode-III
- ○
- Flow Rate Division
- ○
- Concurrently ‘Pulse’ Fill Sampling Chambers 1 and 2
3. Results and Discussion
3.1. Preliminary Batch Mode Results
3.2. Automated Column Sorption System
3.2.1. Sampling Sequence and Flow Rate
3.2.2. Acidity Levels
3.2.3. Hydraulic Conductivity Considerations
3.2.4. Heavy Metallic Ion Concentration Analysis
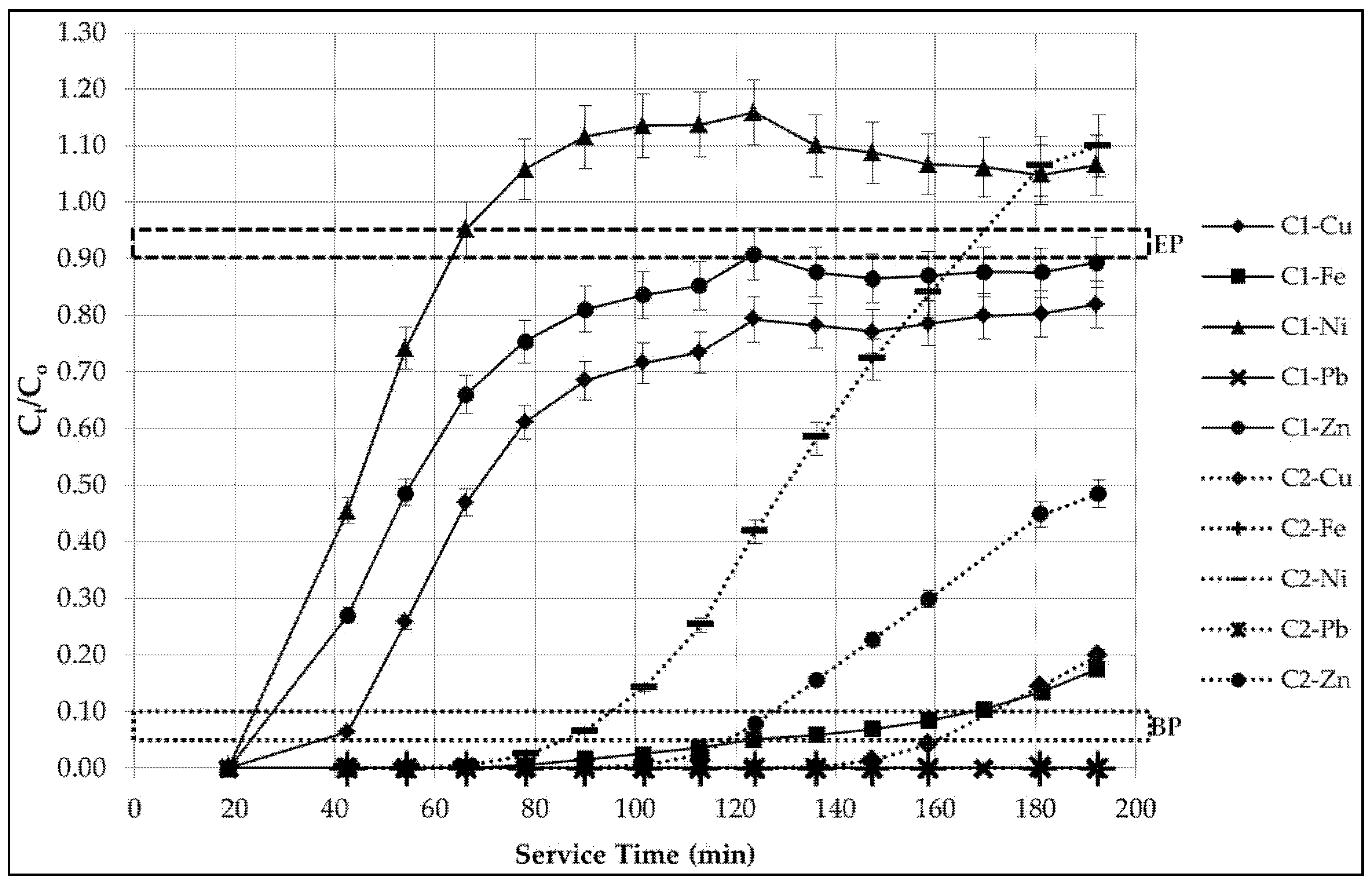
3.2.5. Breakthrough Curve, Capacity and Usage Rate Analysis
- The zeolite holds the greatest preference towards to Pb2+ ion, based on its complete removal throughout the analysis period;
- The zeolite demonstrates the least preference towards the Ni2+ ion;
- A more sudden breakpoint occurring after just 25 min and 90 min of service time in columns C1 and C2, respectively;
- An approximate exhaustion point after just 65 min and 165 min of service time in columns C1 and C2, respectively;
- The Fe3+ ion is removed entirely and sustained throughout the analysis period in C2, and;
- The removal of both the Cu2+ and Zn2+ ions begin to plateau at 120 min of service time in C1, acting in parallel and do not reach the lower threshold of the exhaustion point in both columns throughout the analysis period.
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vaca-Mier, M.; Lopez-Callejas, R.; Gehr, R.; Jimenez-Cisneros, B.E.; Alvarez, P.J.J. Heavy metal removal with mexican clinoptilolite: multi-component ionic exchange. Wat. Res. 2001, 35, 373–378. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options—A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Mohan, D.; Chander, S. Removal and recovery of metal ions from acid mine drainage using lignite—A low cost sorbent. J. Hazard. Mater. 2006, B137, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Motsi, T.; Rowson, N.A.; Simmons, M.J.H. Kinetic studies of the removal of heavy metals from acid mine drainage by natural zeolite. Int. J. Miner. Process. 2011, 101, 42–49. [Google Scholar] [CrossRef]
- Baraket, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 276–282. [Google Scholar] [CrossRef]
- Vukojevic Medvidovic, N.; Peric, J.; Trgo, M.; Nuic, I.; Ugrina, M. Design of fixed bed column for lead removal on natural zeolite based on batch Studies. Chem. Biochem. Eng. Q. 2013, 27, 21–28. [Google Scholar]
- Nuic, I.; Trgo, M.; Vukojevic Medvidovic, N. The application of the packed bed reactor theory to Pb and Zn uptake from the binary solution onto the fixed bed of natural zeolite. Chem. Eng. J. 2016, 295, 347–357. [Google Scholar] [CrossRef]
- Han, R.; Zou, W.; Li, H.; Li, Y.; Shi, J. Copper(II) and lead(II) removal from aqueous solution in fixed-bed columns by manganese oxide coated zeolite. J. Hazard. Mater. 2006, 137, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, M.A.; Inglezakis, V.J.; Moustakas, K.G.; Malamis, S.P.; Loizidou, M.D. Removal of Cu(II) in fixed bed and batch reactors using natural zeolite and exfoliated vermiculite as adsorbents. Desalination 2007, 215, 133–142. [Google Scholar] [CrossRef]
- Stylianou, M.A.; Hadjiconstantinou, M.P.; Inglezakis, V.J.; Moustakas, K.G.; Loizidou, M.D. Use of natural clinoptilolite for the removal of lead, copper and zinc in fixed bed column. J. Hazard. Mater. 2007, 143, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Nuic, M.; Trgo, J.; Peric, N.; Vukojevic Medvidovic, N. Analysis of breakthrough curves of Pb and Zn sorption from binary solutions on natural clinoptilolite. Micropor. Mesopor. Mater. 2013, 167, 55–61. [Google Scholar] [CrossRef]
- Peric, J.; Trgo, M.; Vukojevic Medvidovic, N. Removal of zinc, copper and lead by natural zeolite—A comparison of adsorption isotherms. Water Res. 2004, 38, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Sun, X.; Wang, L.; Sun, X. Evaluation of zeolites synthesized from fly ash as potential adsorbents for wastewater containing heavy metals. J. Environ. Sci. 2009, 21, 127–136. [Google Scholar] [CrossRef]
- Anari-Anaraki, M.; Nezamzadeh-Ejhieh, A. Modification of an Iranian clinoptilolite nano-particles by hexadecyltrimethyl ammonium cationic surfactant and dithizone for removal of Pb(II) from aqueous solution. J. Colloid Interf. Sci. 2015, 440, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Borandegi, M.; Nezamzadeh-Ejhieh, A. Enhanced removal efficiency of clinoptilolite nano-particles toward Co(II) from aqueous solution by modification with glutamic acid. Colloids Surf. A Physicochem. Eng. Asp. 2015, 479, 35–45. [Google Scholar] [CrossRef]
- Curkovic, L.; Cerjan-Stefanovic, S.; Filipan, T. Metal ion exchange by natural and modified zeolites. Water. Res. 1997, 31, 1379–1382. [Google Scholar] [CrossRef]
- Helfferich, F. Equilibria; Kinetics; Ion-Exchange in Columns. In Ion Exchange; Series in Advanced Chemistry; McGraw-Hill Book Company: New York, NY, USA, 1962; pp. 95–322, 421–506. [Google Scholar]
- Inglezakis, V.J.; Poulopoulos, S.G. Chapter 4—Adsorption and Ion-Exchange (Kinetics). In Adsorption, Ion Exchange and Catalysis—Design of Operations and Environmental Applications, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2006; pp. 262–266. ISBN 13 978-0-444-52783-7. [Google Scholar]
- Trgo, M.; Peric, J.; Vukojevic Medvidovic, N. A comparative study of ion exchange kinetics in zinc/lead—Modified zeolite-clinoptilolite systems. J. Hazard. Mater. 2006, 136, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Tsitsishvili, G.V. Perspectives of Natural Zeolite Applications. Occurrence. In Properties and Utilization of Natural Zeolites—2nd International Conference 1985; Akademiai Kiado: Budapest, Hungary, 1988; pp. 367–393. [Google Scholar]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Shirzadi, A. Enhancement of the photocatalytic activity of Ferrous Oxide by doping onto the nano-clinoptilolite particles towards photodegradation of tetracycline. Chemosphere 2014, 107, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Inglezakis, V.J.; Loizidou, M.D.; Grigoropoulou, H.P. Equilibrium and kinetic ion exchange studies of Pb2+, Cr3+, Fe3+ and Cu2+ on natural clinoptilolite. Water Res. 2002, 36, 2784–2792. [Google Scholar] [CrossRef]
- Nuic, I.; Trgo, M.; Peric, J.; Vukojevic Medvidovic, N. Uptake of Pb and Zn from a binary solution onto different fixed bed depths of natural zeolite—The BDST model approach. Clay Miner. 2015, 50, 91–101. [Google Scholar] [CrossRef]
- Ersoy, B.; Celik, M.S. Electrokinetic properties of clinoptilolite with mono- and multivalent electrolytes. Micropor. Mesopor. Mater. 2002, 55, 305–312. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Grigoropoulou, H. Effects of operating conditions on the removal of heavy metals by zeolite in fixed bed reactors. J. Hazard. Mater. 2004, 112, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Inglezakis, V.J. Ion exchange and adsorption fixed bed operations for wastewater treatment—Part I: Modelling fundamentals and hydraulics analysis. J. Eng. Stud. Res. 2010, 16, 29–41. [Google Scholar]
- Erdol Aydin, N.; Nasun Saygili, G. Column experiments to remove copper from wastewaters using natural zeolite. Int. J. Environ. Waste Manag. 2009, 3, 319–326. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Papadeas, C.D.; Loizidou, M.D.; Grigoropoulou, H.P. Effects of pretreatment on physical and ion exchange properties of natural clinoptilolite. Environ. Technol. 2001, 22, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Inglezakis, V.J. Ion exchange and adsorption fixed bed operations for wastewater treatment—Part II: scale-up and approximate design methods. J. Eng. Stud. Res. 2010, 16, 42–50. [Google Scholar]
- Ciosek, A.L.; Luk, G.K. Lead Removal from mine tailings with multiple metallic ions. Int. J. Water Wastewater Treat. 2017, 3, 1–9. [Google Scholar] [CrossRef]
- Ciosek, A.L.; Luk, G.K. Kinetic modelling of the removal of multiple heavy metallic ions in mine waste by natural zeolite sorption. Water 2017, 9, 482. [Google Scholar] [CrossRef]
- Vukojevic Medvidovic, N.; Peric, J.; Trgo, M. Column performance in lead removal from aqueous solutions by fixed bed of natural zeolite–clinoptilolite. Sep. Purif. Technol. 2006, 49, 237–244. [Google Scholar] [CrossRef]
- Reed, B.E.; Jamil, M.; Thomas, B. Effect of pH, empty bed contact time and hydraulic loading rate on lead removal by granular activated carbon columns. Water Environ. Res. 1996, 68, 877–882. [Google Scholar] [CrossRef]
- Peric, J.; Trgo, M.; Vukojevic Medvidovic, N.; Nuic, I. The Effect of Zeolite Fixed Bed Depth on Lead Removal from Aqueous Solutions. Sep. Sci. Technol. 2009, 44, 3113–3127. [Google Scholar] [CrossRef]
- Bear River Zeolite Co. Inc. Zeolite—Specifications and MSDS. Available online: http://www.bearriverzeolite.com (accessed on 1 September 2012 and 1 April 2017).
- Inglezakis, V.J.; Hadjiandreou, K.J.; Loizidou, M.D.; Grigoropoulou, H.P. Pretreatment of natural clinoptilolite in a laboratory-scale ion exchange packed bed. Water Res. 2001, 35, 2161–2166. [Google Scholar] [CrossRef]
- Mullin, J. Physical and thermal properties. In Crystallization, 4th ed.; Read Educational and Professional Publishing Ltd: Woburn, MA, USA, 2001; pp. 76–77, IBSN 0-7506-4833-3. [Google Scholar]
- Wilson, L.J. Canada-Wide Survey of Acid Mine Drainage Characteristics. Project Report 3.22.1—Job No. 50788. Mineral Sciences Laboratories Division Report MSL 94–32 (CR). Ontario Ministry of Northern Development and Mines. Mine Environment Neutral Drainage (MEND) Program. Canada, 1994. Available online: http://mend-nedem.org/wp-content/uploads/2013/01/3.22.1.pdf (accessed on 30 October 2014).
- Canadian Minister of Justice—Metal Mining Effluent Regulations. Consolidation SOR/2002-222. Justice Laws—Government of Canada. Available online: http://laws-lois.justice.gc.ca (accessed on 1 September 2014).
- Wingenfelder, U.; Hansen, C.; Furrer, G.; Schulin, R. Removal of Heavy Metals from Mine Waters from Natural Zeolites. Environ. Sci. Technol. 2005, 39, 4606–4613. [Google Scholar] [CrossRef] [PubMed]
- Inglezakis, V.J.; Loizidou, M.D.; Grigoropoulou, H.P. Ion exchange of Pb2+, Cu2+, Fe3+, and Cr3+ on natural clinoptilolite: Selectivity determination and influence of acidity on metal uptake. J. Colloid Interface Sci. 2003, 261, 49–54. [Google Scholar] [CrossRef]
- Minceva, M.; Fajgar, R.; Markovska, L.; Meshko, V. Comparative Study of Zn2+, Cd2+, and Pb2+ Removal From Water Solution Using Natural Clinoptilolitic Zeolite and Commercial Granulated Activated Carbon: Equilibrium of Adsorption. Sep. Sci. Technol. 2008, 43, 2117–2143. [Google Scholar] [CrossRef]
- Ouki, S.K.; Kavannagh, M. Treatment of metals-contaminated wastewaters by use of natural zeolites. Water. Sci. Tech. 1999, 39, 115–122. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Part 1000-Introduction, Part 3000-METALS. In Standard Methods for the Examination of Water and Wastewater, 22nd ed.; The American Public Health Association (APHA): Washington, DC, USA; The American Water Works Association (AWWA): Denver, CO, USA; The Water Environment Federation (WEF): Alexandria, VA, USA, 2012; pp. 1.1–68, 3.1–112, ISSN 978-087553-013-0. [Google Scholar]
- Perkin Elmer Inc. Atomic Spectroscopy—A Guide to Selecting the Appropriate Technique and System: World Leader in AA, ICP-OES, and ICP-MS; Perkin Elmer Inc.: Waltham, MA, USA, 2011. [Google Scholar]
- Perkin Elmer Inc. WinLab32 for ICP—Instrument Control Software, Version 5.0; Perkin Elmer Inc.: Waltham, MA, USA, 2010. [Google Scholar]
- ASTM D2434–68. Standard Test Method for Permeability of Granular Soils (Constant Head). ASTM International, West Conshohocken, PA. 2000. Available online: www.astm.org (accessed on 1 March 2016).
- Naja, G.; Volesky, B. Multi-metal biosorption in a fixed-bed flow-through column. Colloid. Surf. 2006, 281, 194–201. [Google Scholar] [CrossRef]
- Othman, M.Z.; Roddick, F.A.; Snow, R. Removal of Dissolved Organic Compounds in Fixed-Bed Columns: Evaluation of Low-Rank Coal Adsorbents. Water Res. 2001, 35, 2943–2949. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Buszewski, B.; Terzyk, A.P.; Namiesnik, J. Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+, and Cd2+) adsorption on clinoptilolite. J. Colloid Interface Sci. 2006, 304, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zinck, J. Review of Disposal, Reprocessing and Reuse Options for Acidic Drainage Treatment Sludge. Report 3.42.3. Mine Environment Neutral Drainage (MEND) Program. Mining Association of Canada. CANMET Mining and Mineral Sciences Laboratories. Canada, 2005. Available online: http://mend-nedem.org/wp-content/uploads/2013/01/3.42.3.pdf (accessed on 30 October 2014).
- Dinardo, O.; Kondos, P.D.; MacKinnon, D.J.; McCready, R.G.L.; Riveros, P.A.; Skaff, M. Study on Metals Recovery/Recycling from Acid Mine Drainage Phase IA: Literature Survey. Report 3.21.1a. Mine Environment Neutral Drainage (MEND) Program. CANMET, Energy, Mines and Resources Canada and WTC, Environment Canada. 1991. Available online: http://mend-nedem.org/wp-content/uploads/2013/01/3.21.1a.pdf (accessed on 30 October 2014).
| Chemical Composition | Mineral Component | 85%–95% Clinoptilolite (non-crystalline silica opaline balance) | ||
| Cation Exchange Capacity (CEC) | 180–220 meq/100 g (as ammonium, -N) (high) | |||
| Maximum Water Retention | >55 wt % (hydrophilic) | |||
| Overall Surface Area | 24.9 m2/g (large) | |||
| Bulk Density | approx. 55–60 lb/f3 | |||
| Hardness | Moh’s No. 4 (high) | |||
| pH | 7–8.64 | |||
| Colour | Pale Green | |||
| MSDS Composition Information | Chemical | wt % | CAS No. | |
| Clinoptilolite | 90–97 | 12173-10-3 | ||
| Water | 3–10 | 7732-18-5 | ||
| Analytical Rock Data | SiO2 | 67.4% | ||
| Al2O3 | 10.6% | |||
| MgO | 0.45% | |||
| K2O | 4.19% | |||
| MnO | <0.01% | |||
| CaO | 2.23% | |||
| TiO2 | 0.27% | |||
| Fe2O3 | 1.70% | |||
| Na2O | 0.59% | |||
| P2O5 | 0.10% | |||
| Loss-On-Ignition (LOI) 925 °C | 11.40% | |||
| Major Cation Range | Ca | 1.60%–2.0% | ||
| K | 2.93%-3.47% | |||
| Na | <0.5% | |||
| Sample ID | Analyte | Int (Corr) | RSD (Corr Int) | Conc (Calib) (mg/L) |
|---|---|---|---|---|
| M-X | Cu 327.393 | 188,070.71 | 0.27 | 34.26 |
| Fe 238.204 | 79,641.94 | 0.39 | 19.18 | |
| Ni 231.604 | 41,071.22 | 0.50 | 29.81 | |
| Pb 220.353 | 32,330.16 | 0.52 | 105.28 | |
| Zn 206.200 | 55,015.91 | 0.38 | 32.31 | |
| M-Y | Cu 327.393 | 186,885.03 | 0.74 | 34.04 |
| Fe 238.204 | 79,083.95 | 0.90 | 19.04 | |
| Ni 231.604 | 40,721.53 | 0.48 | 29.55 | |
| Pb 220.353 | 31,973.87 | 0.31 | 104.12 | |
| Zn 206.200 | 54,758.60 | 1.09 | 32.16 | |
| M-Z | Cu 327.393 | 202,742.71 | 0.91 | 36.93 |
| Fe 238.204 | 85,771.53 | 1.02 | 20.65 | |
| Ni 231.604 | 44,652.28 | 3.73 | 32.41 | |
| Pb 220.353 | 35,199.76 | 3.77 | 114.63 | |
| Zn 206.200 | 60,176.84 | 4.12 | 35.34 | |
| MM | Cu 327.393 | 192,776.82 | 0.63 | 35.11 |
| Fe 238.204 | 81,419.80 | 0.80 | 19.60 | |
| Ni 231.604 | 41,667.28 | 0.38 | 30.24 | |
| Pb 220.353 | 32,738.45 | 0.40 | 106.61 | |
| Zn 206.200 | 56,170.16 | 0.94 | 32.99 |
| MODE | Function | Flow Description | Time (min:s) |
|---|---|---|---|
| I | Fill C1 | Primed Inlet to C1 Base | 2:26 |
| C1 Base to C1 Top | 11:05 | ||
| C1 Top to SC1 Drip | 15:10 | ||
| Fill SC1 | |||
| II | Sample C1-A | 18:50 | |
| Fill C2 | C2 Inlet to C2 Base | 24:08 | |
| C2 Base to C2 Top | 32:35 | ||
| C2 Top to SC2 Drip | 36:14 | ||
| III | Fill SC1 and SC2 | ||
| II | Sample C1-B and C2-B | 42:50 | |
| III | Fill SC1 and SC2 | 48:04 | |
| II | Sample C1-1 and C2-1 | 54:27 |
| Sample | MODE | Start Time (min:s) | End Time (min:s) | |
|---|---|---|---|---|
| SC1 | SC2 | |||
| C1-A | I | 15:10 | 18:50 | - |
| II | 18:50 | |||
| Cx-B | III | 36:40 | 42:42 | 42:49 |
| II | 42:50 | |||
| Cx-1 | III | 48:04 | 54:10 | 54:27 |
| II | 54:27 | |||
| Cx-2 | III | 59:39 | 66:15 | 66:34 |
| II | 66:34 | |||
| Cx-3 | III | 71:37 | 77:55 | 78:19 |
| II | 78:20 | |||
| Cx-4 | III | 83:24 | 89:56 | 89:56 |
| II | 89:57 | |||
| Cx-5 | III | 95:05 | 101:32 | 101:45 |
| II | 101:46 | |||
| Cx-6 | III | 106:52 | 112:53 | 113:10 |
| II | 113:11 | |||
| TW1 | 115:45 | |||
| Cx-7 | III | 118:10 | 123:45 | 123:56 |
| II | 123:57 | |||
| Cx-8 | III | 129:00 | 136:07 | 136:19 |
| II | 136:20 | |||
| Cx-9 | III | 141:22 | 147:29 | 147:34 |
| II | 147:35 | |||
| Cx-10 | III | 152:40 | 158:35 | 158:45 |
| II | 158:46 | |||
| Cx-11 | III | 163:45 | 169:40 | 169:45 |
| II | 169:46 | |||
| Cx-12 | III | 174:50 | 181:06 | 181:04 |
| II | 181:10 | |||
| Cx-13 | III | 186:11 | 192:08 | 192:36 |
| II | PUMP OFF | |||
| TW2 | 195:00 | |||
| Sample | pH Level | |
|---|---|---|
| SC1 | SC2 | |
| C1-A | 6.34 | - |
| Cx-3 | 4.79 | 6.84 |
| Cx-6 | 3.99 | 6.72 |
| TW1 | 6.05 | |
| Cx-9 | 3.86 | 6.33 |
| Cx-13 | 3.60 | 5.76 |
| TW2 | 5.44 | |
| Sample | HMI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu2+ | Fe3+ | Ni2+ | Pb2+ | Zn2+ | |||||||
| meq/L | %R | meq/L | %R | meq/L | %R | meq/L | %R | meq/L | %R | ||
| C1 | C1-A | 0.000 | 100.00 | 0.000 | 99.98 | 0.007 | 99.67 BP | 0.0003 | 99.98 | 0.002 | 99.92 BP |
| C1-B | 0.129 | 93.55 BP | 0.000 | 99.99 | 0.911 | 54.47 | 0.0006 | 99.97 | 0.541 | 72.96 | |
| C1-1 | 0.517 | 74.17 | 0.000 | 99.99 | 1.483 | 25.85 | 0.0004 | 99.98 | 0.974 | 51.30 | |
| C1-2 | 0.938 | 53.09 | 0.001 | 99.93 | 1.906 | 4.71 | 0.0006 | 99.97 | 1.320 | 34.02 | |
| C1-3 | 1.221 | 38.93 | 0.011 | 99.46 | 2.116 | 0.00 | 0.0003 | 99.98 | 1.507 | 24.66 | |
| C1-4 | 1.369 | 31.54 | 0.030 | 98.49 | 2.231 | 0.00 | 0.0006 | 99.97 | 1.622 | 18.91 | |
| C1-5 | 1.431 | 28.47 | 0.052 | 97.42 | 2.269 | 0.00 | 0.0004 | 99.98 | 1.671 | 16.43 | |
| C1-6 | 1.468 | 26.60 | 0.072 | 96.40 | 2.273 | 0.00 | 0.0005 | 99.97 | 1.703 | 14.83 | |
| C1-7 | 1.584 | 20.78 | 0.102 | 94.90 BP | 2.316 | 0.00 | 0.0005 | 99.98 | 1.816 | 9.20 | |
| C1-8 | 1.563 | 21.86 | 0.118 | 94.08 | 2.199 | 0.00 | 0.0005 | 99.98 | 1.751 | 12.43 | |
| C1-9 | 1.543 | 22.84 | 0.138 | 93.11 | 2.174 | 0.00 | 0.0004 | 99.98 | 1.730 | 13.51 | |
| C1-10 | 1.571 | 21.44 | 0.167 | 91.64 | 2.134 | 0.00 | 0.0004 | 99.98 | 1.739 | 13.04 | |
| C1-11 | 1.598 | 20.12 | 0.209 | 89.55 | 2.123 | 0.00 | 0.0004 | 99.98 | 1.752 | 12.38 | |
| C1-12 | 1.604 | 19.79 | 0.268 | 86.59 | 2.096 | 0.00 | 0.0001 | 100.00 | 1.750 | 12.49 | |
| C1-13 | 1.638 | 18.09 | 0.349 | 82.54 | 2.130 | 0.00 | 0.0002 | 99.99 | 1.786 | 10.71 | |
| C2 | C2-B | 0.00 | 100.00 | 0.0004 | 99.98 | 0.002 | 99.90 | 0.0003 | 99.99 | 0.002 | 99.88 |
| C2-1 | 0.00 | 100.00 | 0.0003 | 99.98 | 0.002 | 99.88 | 0.0003 | 99.99 | 0.001 | 99.94 | |
| C2-2 | 0.00 | 100.00 | 0.0003 | 99.98 | 0.012 | 99.42 | 0.0004 | 99.98 | 0.001 | 99.95 | |
| C2-3 | 0.00 | 100.00 | 0.0002 | 99.99 | 0.046 | 97.68 | 0.0006 | 99.97 | 0.001 | 99.96 | |
| C2-4 | 0.00 | 100.00 | 0.0002 | 99.99 | 0.131 | 93.43 BP | 0.0002 | 99.99 | 0.001 | 99.93 | |
| C2-5 | 0.00 | 100.00 | 0.0002 | 99.99 | 0.285 | 85.75 | 0.0002 | 99.99 | 0.009 | 99.55 | |
| C2-6 | 0.00 | 100.00 | 0.0003 | 99.98 | 0.505 | 74.77 | 0.0003 | 99.99 | 0.049 | 97.56 | |
| C2-7 | 0.00 | 100.00 | 0.0002 | 99.99 | 0.835 | 58.26 | 0.0003 | 99.98 | 0.155 | 92.23 BP | |
| C2-8 | 0.004 | 99.80 | 0.0002 | 99.99 | 1.163 | 41.85 | 0.0003 | 99.99 | 0.312 | 84.39 | |
| C2-9 | 0.029 | 98.57 | 0.0002 | 99.99 | 1.444 | 27.82 | 0.0004 | 99.98 | 0.455 | 77.24 | |
| C2-10 | 0.085 | 95.77 BP | 0.0003 | 99.99 | 1.675 | 16.26 | 0.0004 | 99.98 | 0.597 | 70.17 | |
| C2-12 | 0.289 | 85.57 | 0.0002 | 99.99 | 2.126 | 0.00 | 0.0006 | 99.97 | 0.895 | 55.23 | |
| C2-13 | 0.399 | 80.07 | 0.0003 | 99.98 | 2.198 | 0.00 | 0.0004 | 99.98 | 0.969 | 51.53 | |
| TW1 | 0.0514 | 97.43 | 0.0004 | 99.98 | 0.3077 | 84.61 | 0.0004 | 99.98 | 0.1107 | 94.46 | |
| TW2 | 0.1659 | 91.71 | 0.0057 | 99.72 | 1.0207 | 48.97 | 0.0000 | 100.00 | 0.4088 | 79.56 | |
| HMI | Sorption Column 1 | Sorption Column 2 | ||||
|---|---|---|---|---|---|---|
| (L) | (meq/g) | (g/L) | (L) | (meq/g) | (g/L) | |
| Cu2+ | 0.3295 | 0.00433 | 461.67 | 0.7257 | 0.00954 | 209.59 |
| Fe3+ | 0.9926 | 0.01305 | 153.24 | - | - | - |
| Ni2+ | 0.1342 | 0.00176 | 1133.54 | 0.3547 | 0.00466 | 428.80 |
| Pb2+ | - | - | - | - | - | - |
| Zn2+ | 0.1342 | 0.00176 | 1133.54 | 0.5380 | 0.00707 | 282.71 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciosek, A.L.; Luk, G.K. An Innovative Dual-Column System for Heavy Metallic Ion Sorption by Natural Zeolite. Appl. Sci. 2017, 7, 795. https://doi.org/10.3390/app7080795
Ciosek AL, Luk GK. An Innovative Dual-Column System for Heavy Metallic Ion Sorption by Natural Zeolite. Applied Sciences. 2017; 7(8):795. https://doi.org/10.3390/app7080795
Chicago/Turabian StyleCiosek, Amanda L., and Grace K. Luk. 2017. "An Innovative Dual-Column System for Heavy Metallic Ion Sorption by Natural Zeolite" Applied Sciences 7, no. 8: 795. https://doi.org/10.3390/app7080795





