A Method for Ferulic Acid Production from Rice Bran Oil Soapstock Using a Homogenous System
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Equipments
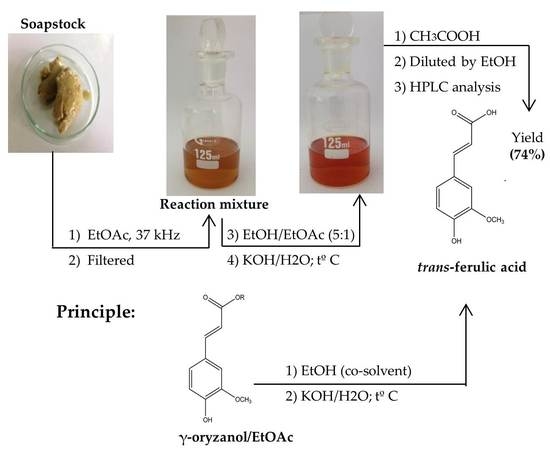
2.2. Extraction of the γ-oryzanol from Soapstock
2.3. Homogeneous Hydrolysis of γ-oryzanol
2.3.1. Selection of Solvents
2.3.2. Hydrolysis Process by Heating Method
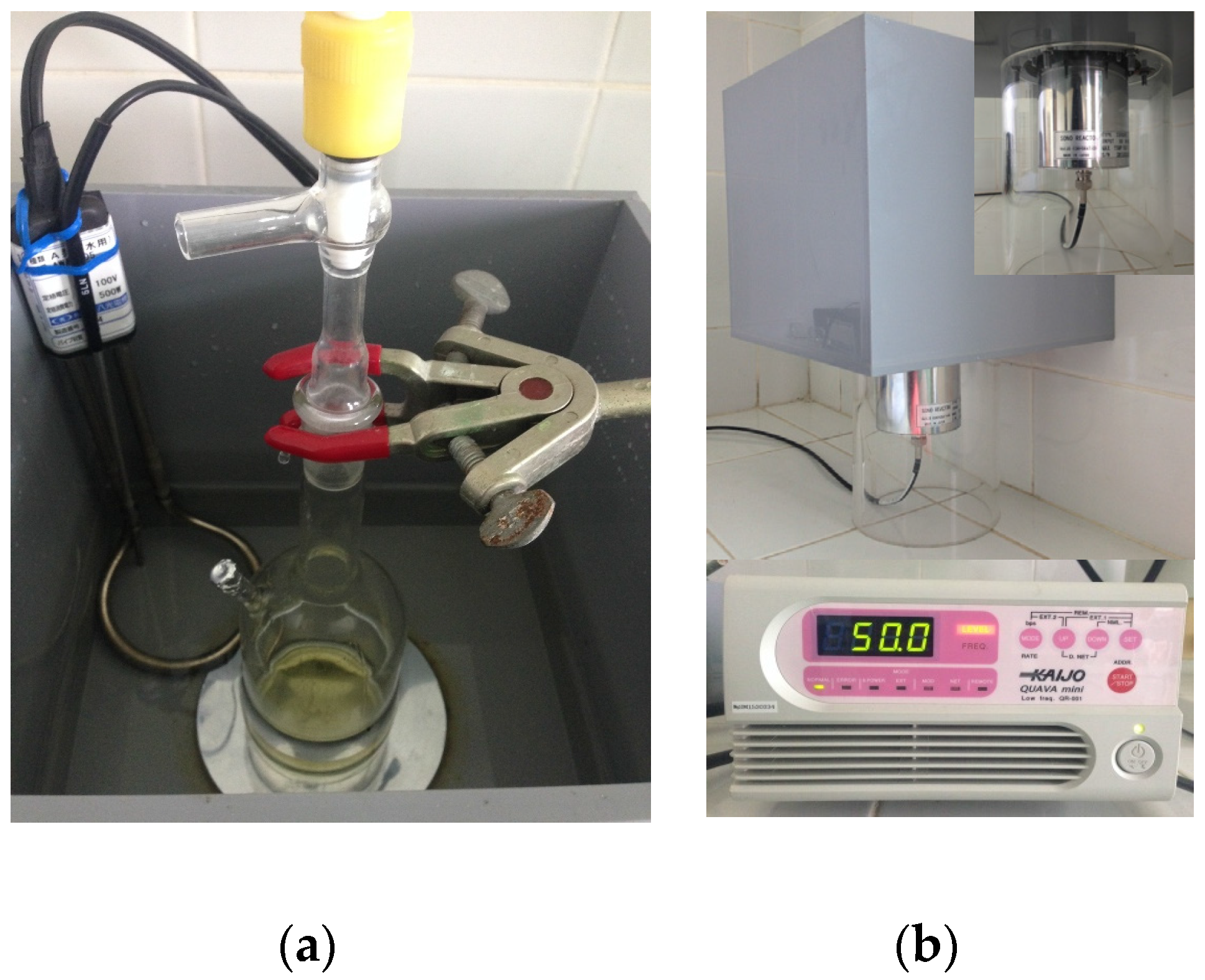
2.3.3. Ultrasound-Assisted Hydrolysis
2.4. Homogenous Hydrolysis of Ory-Extracts and Their Storage
2.5. Quantitative Analysis Method of γ-oryzanol, FA, and Ethyl Ferulate
3. Results and Discussion
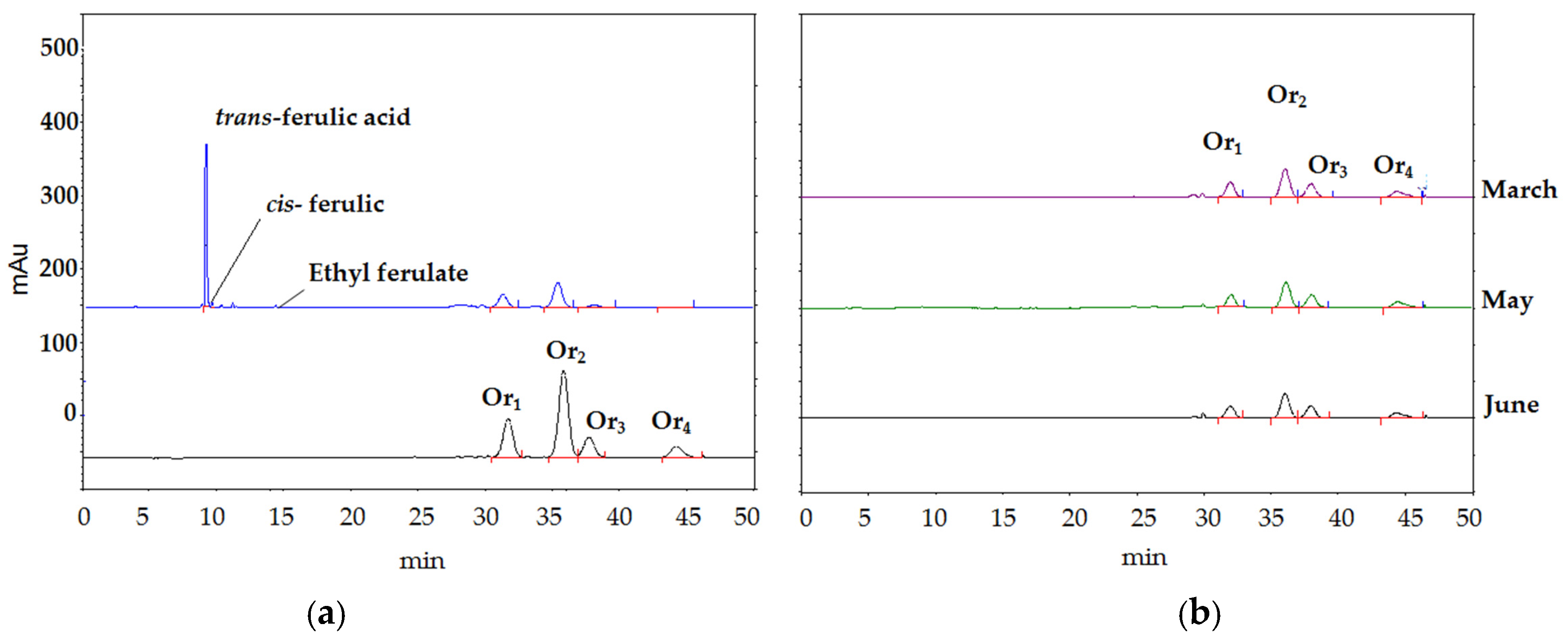
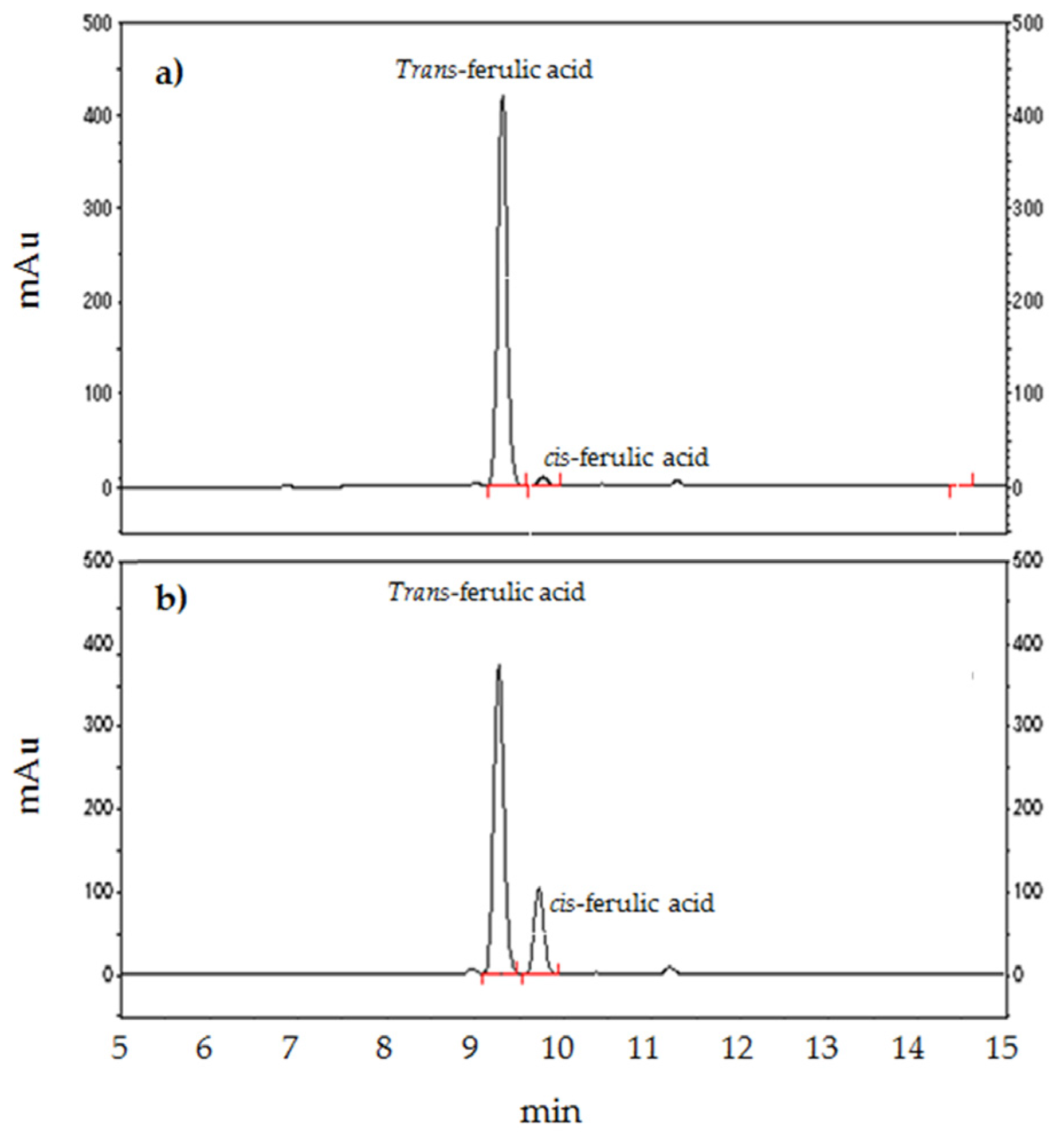
3.1. HPLC Chromatography
3.2. Hydrolysis of γ-oryzanol with Homogeneous Systems
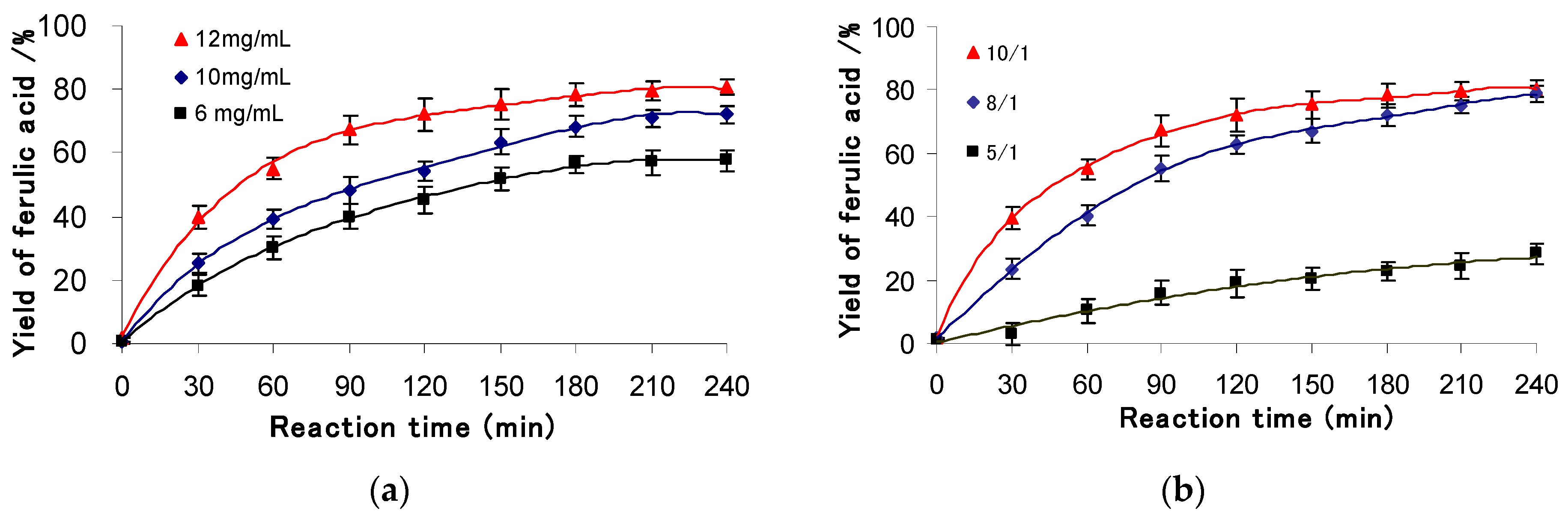
3.2.1. Effect of Initial Concentration of γ-oryzanol and KO Ratio
3.2.2. Effect of the Cosolvent Ratio
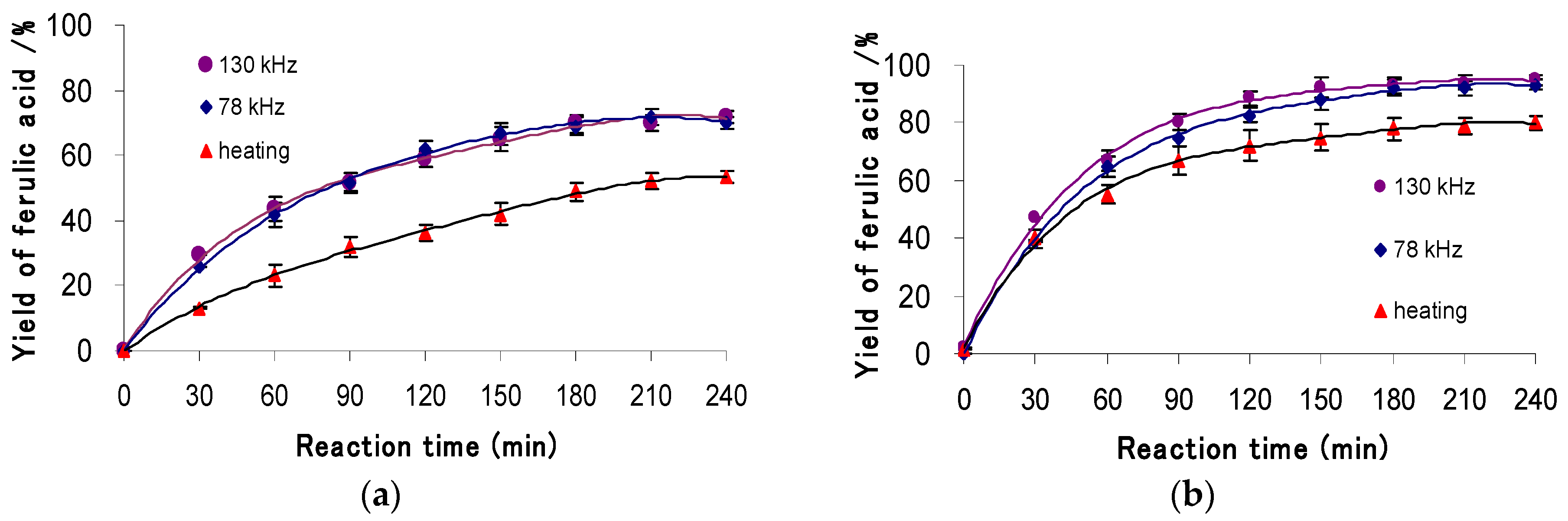
3.2.3. Effect of Temperature and Ultrasound Irradiation
3.3. Hydrolysis of Ory-Extract from RBO Soapstock
3.4. The Cis-Trans Isomerization of FA during Storage
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Graf, E. Antioxidant potential of ferulic acid. Free Radic. Biol. Med. 1992, 13, 435–448. [Google Scholar] [CrossRef]
- Saija, A.; Tomaino, A.; Trombetta, D.; De Pasquale, A.; Uccella, N.; Barbuzzi, T.; Paolino, D.; Bonina, F. In vitro and in vivo evaluation of caffeic and ferulic acids as topical photoprotective agents. Int. J. Pharm. 2000, 199, 39–47. [Google Scholar] [CrossRef]
- Itagaki, S.; Kurokawa, T.; Nakata, C.; Saito, Y.; Oikawa, S.; Kobayashi, M.; Hirano, T.; Iseki, K. In vitro and in vivo antioxidant properties of ferulic acid: A comparative study with other natural oxidation inhibitors. Food Chem. 2009, 114, 466–471. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Ferulic and p-coumaric acids extraction by alkaline hydrolysis of brewer's spent grain. Ind. Crops Prod. 2007, 25, 231–237. [Google Scholar] [CrossRef]
- Prasad, N.R.; Ramachandran, S.; Pugalendi, K.V.; Menon, V.P. Ferulic acid inhibits UV-B–induced oxidative stress in human lymphocytes. Nutr. Res. 2007, 27, 559–564. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Zhang, W.; Cao, S.W. Extraction of ferulic acid and caffeic acid with ionic liquids. Chin. J. Anal. Chem. 2007, 35, 1726–1730. [Google Scholar]
- Srinivasan, M.; Sudheer, A.R.; Pillai, K.R.; Kumar, P.R.; Sudhakaran, P.R.; Menon, V.P. Influence of ferulic acid on γ-radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes. Toxicol. 2006, 228, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Woranuch, S.; Yoksan, R. Preparation, characterization and antioxidant property of water-soluble ferulic acid grafted chitosan. Carbohydr. Polym. 2013, 96, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Moghadasian, M.H. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chem. 2008, 109, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Buranov, A.U.; Mazza, G. Extraction and purification of ferulic acid from flax shives, wheat and corn bran by alkaline hydrolysis and pressurised solvents. Food Chem. 2009, 115, 1542–1548. [Google Scholar] [CrossRef]
- Salleh, N.H.M.; Daud, M.Z.M.; Arbain, D.; Ahmad, M.S.; Ismail, K.S.K. Optimization of alkaline hydrolysis of paddy straw for ferulic acid extraction. Ind. Crops Prod. 2011, 34, 1635–1640. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Shen, P.; Wang, C.; Shen, Y. Microwave-assisted extraction and high-speed counter-current chromatography purification of ferulic acid from radix angelicae sinensis. Sep. Purif. Technol. 2006, 52, 18–21. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, W. Ultrasonic extraction of ferulic acid from ligusticum chuanxiong. J. Chin. Inst. Chem. Eng. 2008, 39, 653–656. [Google Scholar] [CrossRef]
- Lerma-García, M.J.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F.; Mendonça, C.R.B.; Ramis-Ramos, G. Composition, industrial processing and applications of rice bran γ-oryzanol. Food Chem. 2009, 115, 389–404. [Google Scholar] [CrossRef]
- Krishna, A.G.G.; Khatoon, S.; Shiela, P.M.; Sarmandal, C.V.; Indira, T.N.; Mishra, A. Effect of refining of crude rice bran oil on the retention of oryzanol in the refined oil. J. the Am. Oil Chem. Soc. 2001, 78, 127–131. [Google Scholar] [CrossRef]
- Taniguchi, H.; Nomura, E.; Tsuno, T.; Minami, S.; Kato, K.; Hayashi, C. Method of manufacturing ferulic acid. Patent US5288902A, 22 February 1994. [Google Scholar]
- Tuulmets, A.; Salmar, S. Effect of ultrasound on ester hydrolysis in aqueous ethanol. Ultrason. Sonochem. 2001, 8, 209–212. [Google Scholar] [CrossRef]
- Tuulmets, A.; Raik, P. Ultrasonic acceleration of ester hydrolyses. Ultrason. Sonochem. 1999, 6, 85–87. [Google Scholar] [CrossRef]
- Tuulmets, A. Ultrasound and polar homogeneous reactions. Ultrason. Sonochem. 1997, 4, 189–193. [Google Scholar] [CrossRef]
- Tuulmets, A.; Cravotto, G.; Salmar, S.; Jarv, J. Sonochemistry of homogeneous ionic reactions. Mini-Rev. Org. Chem. 2010, 7, 204–211. [Google Scholar] [CrossRef]
- Zhao, C.J.; Zhang, Y.K.; Li, C.Y.; He, X.; Yang, L.; Fu, Y.J.; Zhang, J.J.; Zhao, W.Y.; Zu, Y.G. Development of an ionic liquid-based ultrasonic/microwave-assisted simultaneous distillation and extraction method for separation of camptothecin, 10-hydroxycamptothecin, vincoside-lactam, and essential oils from the fruits of camptotheca acuminata decne. Appl. Sci. 2016, 6, 293. [Google Scholar] [CrossRef]
- Liao, J.Q.; Qu, B.D.; Zheng, N. Extraction of glycyrrhizic acid from glycyrrhiza uralensis using ultrasound and its process extraction model. Appl. Sci. 2016, 6, 319. [Google Scholar] [CrossRef]
- Varma, R.S. Greener and sustainable chemistry. Appl. Sci. 2014, 4, 493–497. [Google Scholar] [CrossRef]
- Introduction to Wilmar Agro Vietnam. Available online: http://www.wilmar-agro.com.vn/wilmar-agro.com.vn/introduction/introduction.html (accessed on 30 June 2017).
- Maeda, Y.; Thanh, L.T.; Imamura, K.; Izutani, K.; Okitsu, K.; Boi, L.V.; Ngoc Lan, P.; Tuan, N.C.; Yoo, Y.E.; Takenaka, N. New technology for the production of biodiesel fuel. Green Chem. 2011, 13, 1124–1128. [Google Scholar] [CrossRef]
- Nanzai, B.; Okitsu, K.; Takenaka, N.; Bandow, H.; Tajima, N.; Maeda, Y. Effect of reaction vessel diameter on sonochemical efficiency and cavitation dynamics. Ultrason. Sonochem. 2009, 16, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Sakunpak, A.; Suksaeree, J.; Pathompaka, P.; Charoonratanaa, T.; Sermkaew, N. Antioxidant individual γ-oryzanol screening in cold pressed rice bran oil of different thai rice varieties by hplc-dpph method. Int. J. Pharm. Pharm. Sci. 2014, 6, 592–597. [Google Scholar]
- Seetharamaiah, G.S.; Prabhakar, J.V. Oryzanol content of indian rice bran oil and its extraction ftpm soap stock. J. Food Sci. Technol. 1986, 23, 270–273. [Google Scholar]
- Santos, H.M.; Lodeiro, C.; Capelo-Martínez, J.-L. The power of ultrasound. In Ultrasound Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; pp. 1–16. [Google Scholar]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Yates, B.; Abbot, J.; Chen, C.L. Oxidation of lignin model compounds containing an α-carbonyl group and a ring-conjugated double-bond by hydrogen peroxide-uv photolysis. Holzforsch. 1996, 50, 226–232. [Google Scholar] [CrossRef]
- Biliaderis, C.G.; Izydorczyk, M.S. (Eds.) Functional Food Carbohydrates; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2006; p. 261. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truong, H.T.; Do Van, M.; Duc Huynh, L.; Thi Nguyen, L.; Do Tuan, A.; Le Xuan Thanh, T.; Duong Phuoc, H.; Takenaka, N.; Imamura, K.; Maeda, Y. A Method for Ferulic Acid Production from Rice Bran Oil Soapstock Using a Homogenous System. Appl. Sci. 2017, 7, 796. https://doi.org/10.3390/app7080796
Truong HT, Do Van M, Duc Huynh L, Thi Nguyen L, Do Tuan A, Le Xuan Thanh T, Duong Phuoc H, Takenaka N, Imamura K, Maeda Y. A Method for Ferulic Acid Production from Rice Bran Oil Soapstock Using a Homogenous System. Applied Sciences. 2017; 7(8):796. https://doi.org/10.3390/app7080796
Chicago/Turabian StyleTruong, Hoa Thi, Manh Do Van, Long Duc Huynh, Linh Thi Nguyen, Anh Do Tuan, Thao Le Xuan Thanh, Hung Duong Phuoc, Norimichi Takenaka, Kiyoshi Imamura, and Yasuaki Maeda. 2017. "A Method for Ferulic Acid Production from Rice Bran Oil Soapstock Using a Homogenous System" Applied Sciences 7, no. 8: 796. https://doi.org/10.3390/app7080796