High Conductivity and Adhesion of Cu-Cr-Zr Alloy for TFT Gate Electrode
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
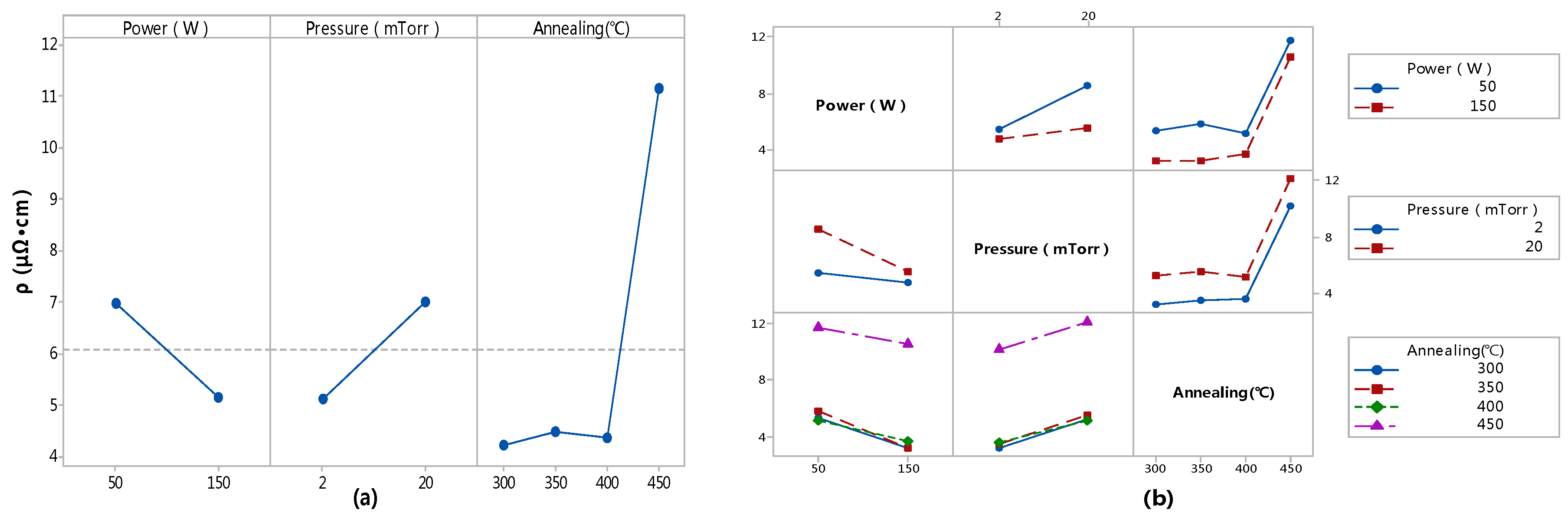
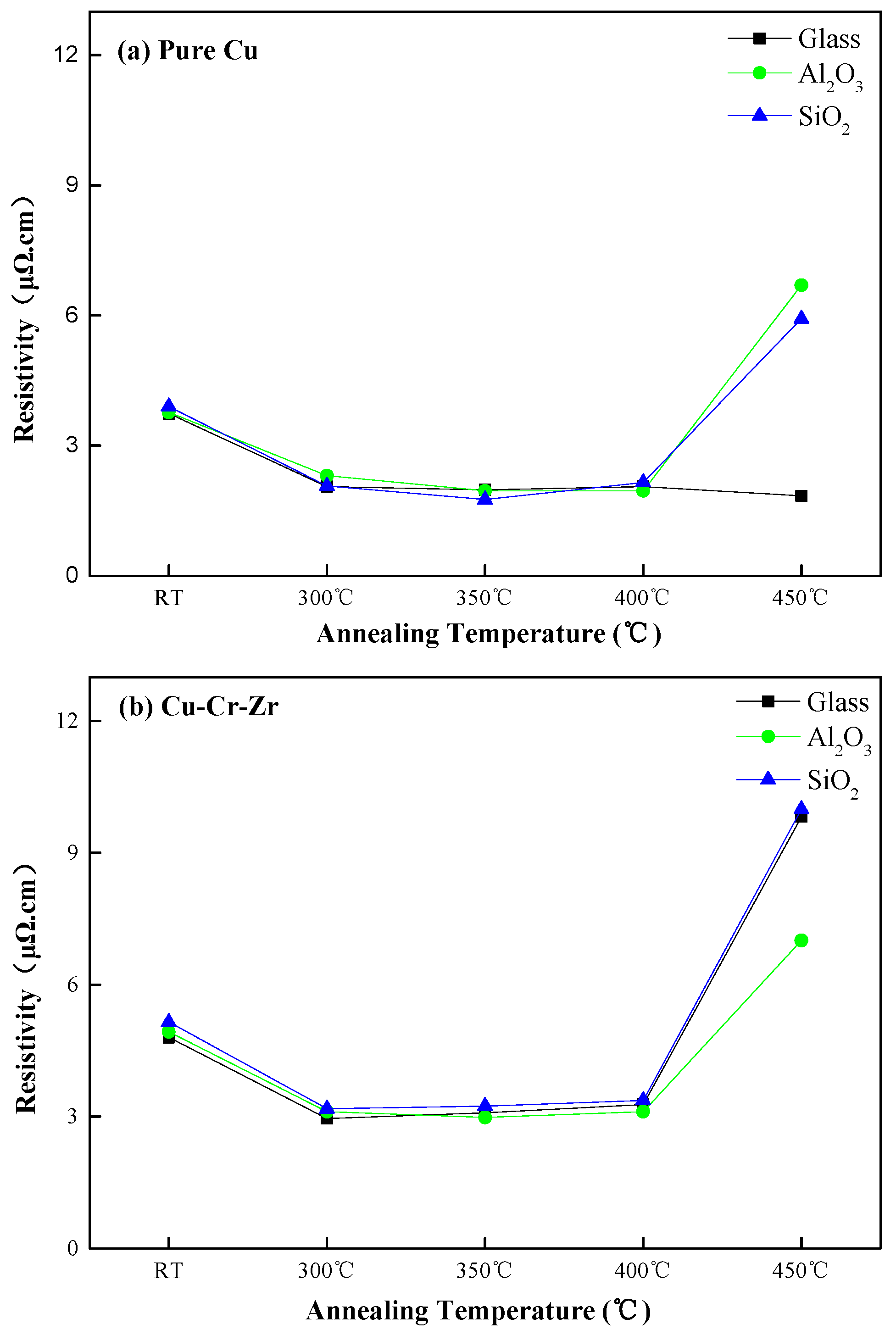
3.1. Resistivity
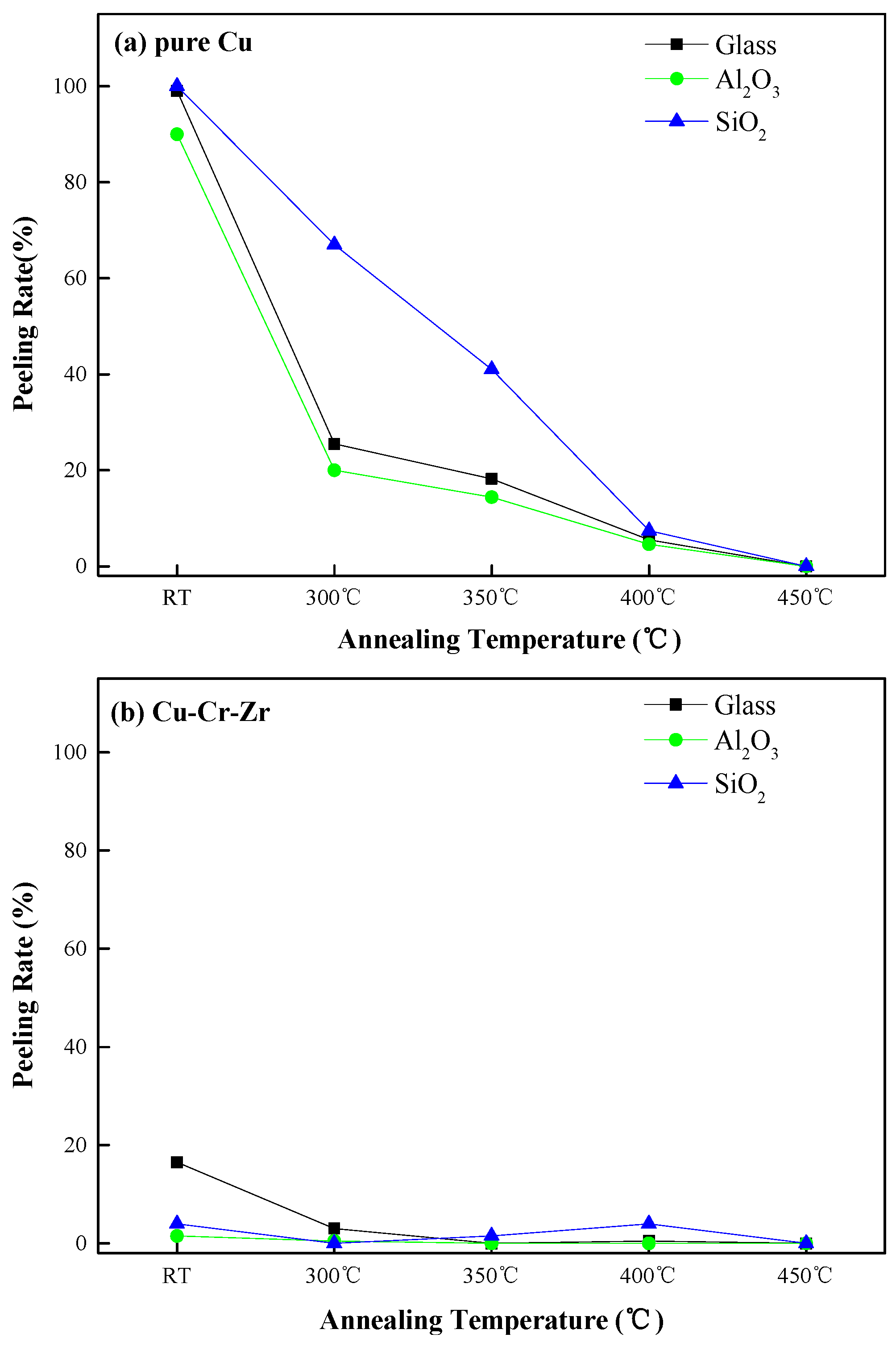
3.2. Adhesion
3.3. X-ray Photoelectron Spectroscopy (XPS) Results
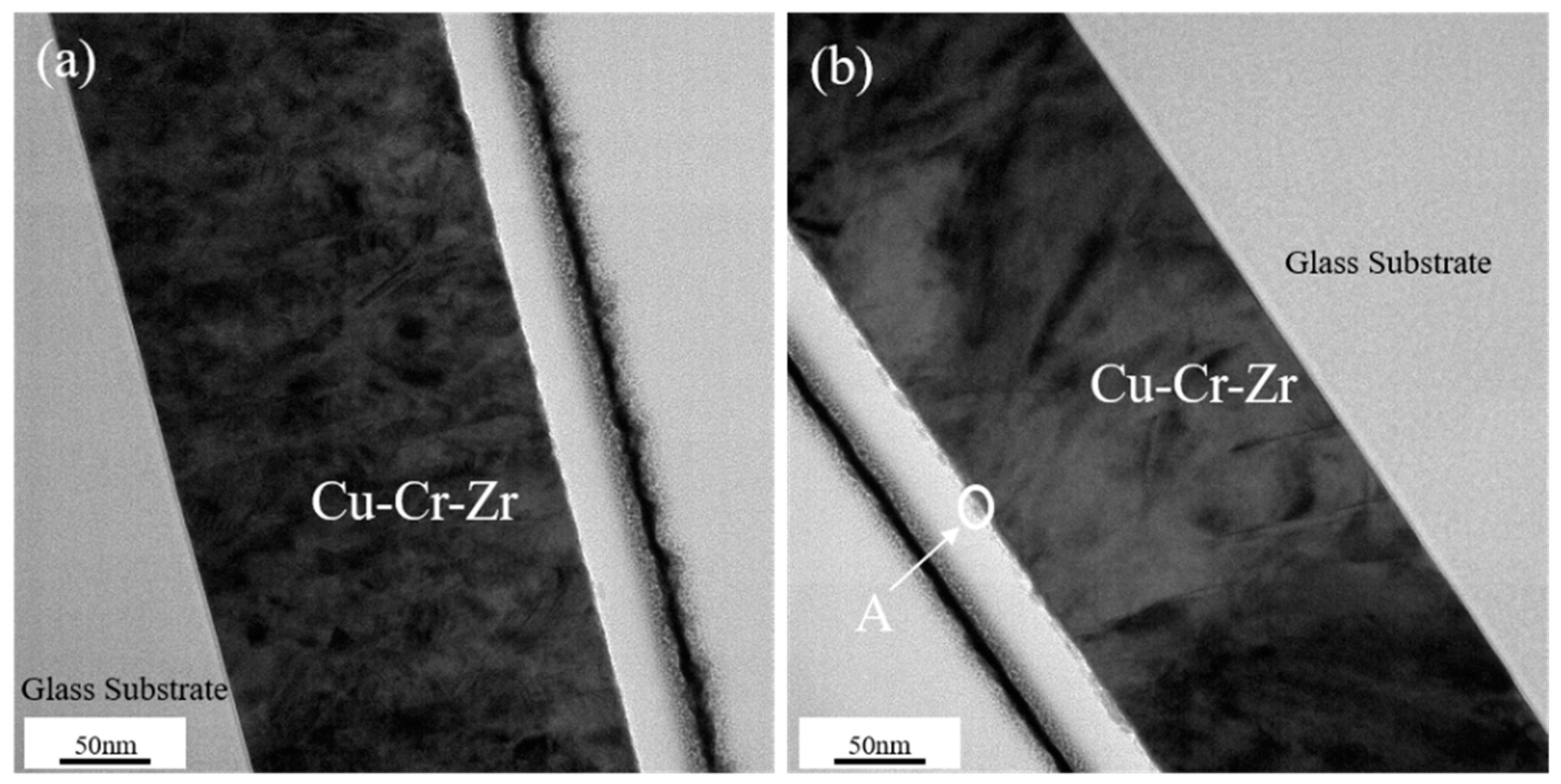
3.4. Transmission Electron Microscopy (TEM) Results
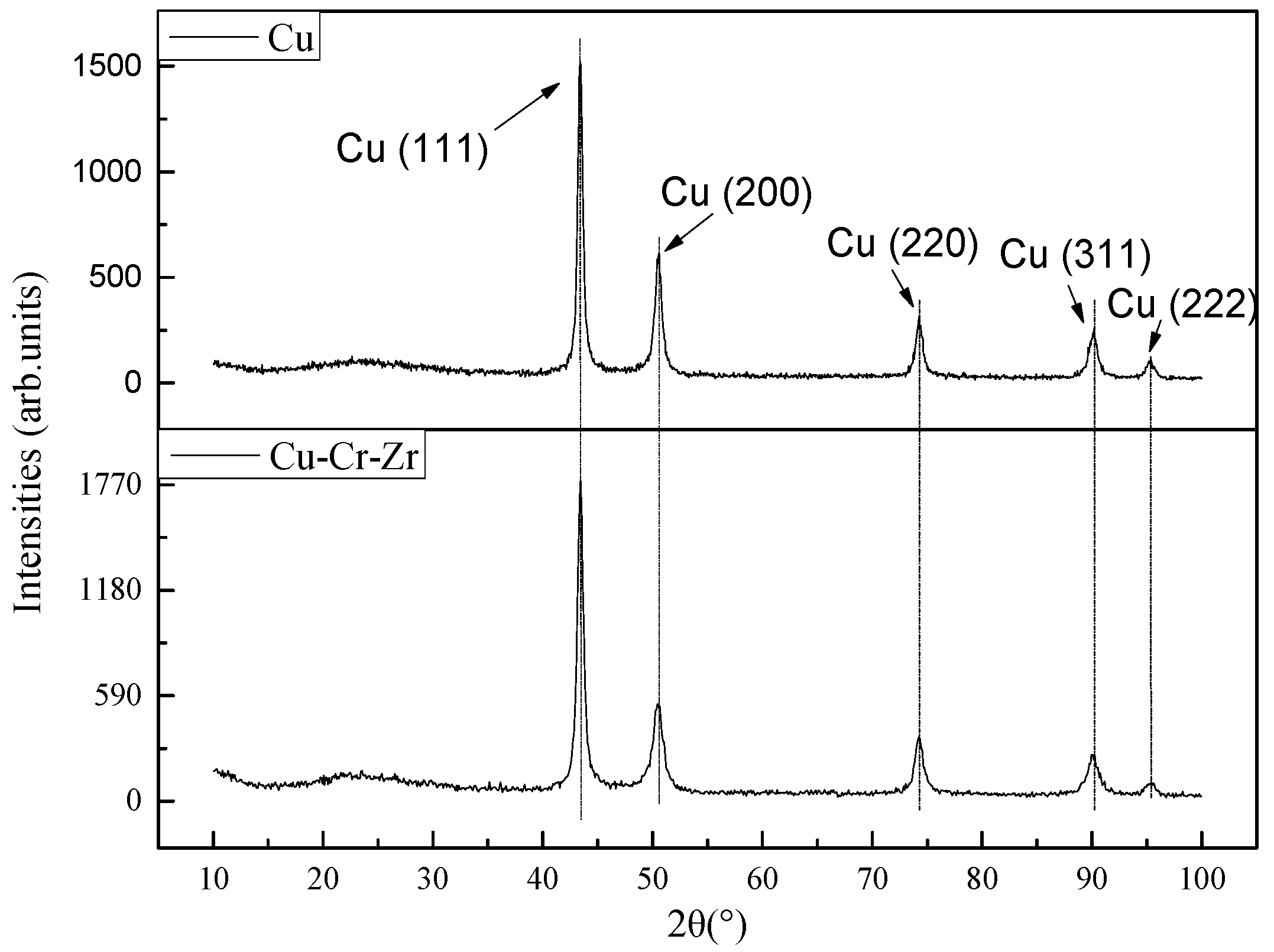
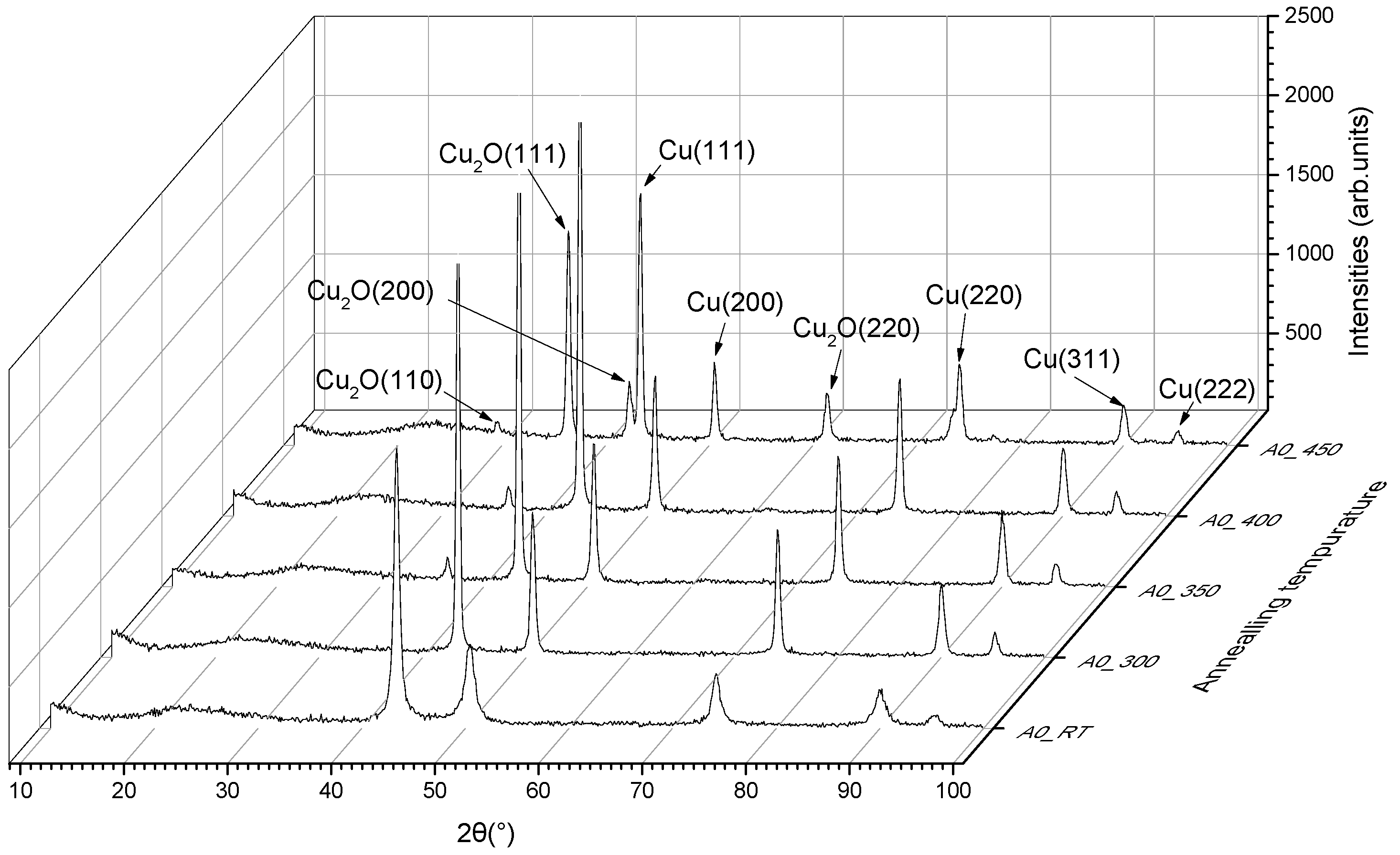
3.5. X-ray Diffraction Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Murarka, S.P.; Hymes, S.W. Copper metallization for ULSL and beyond. Crit. Rev. Solid State Mater. Sci. 1995, 20, 87–124. [Google Scholar] [CrossRef]
- Seah, C.H.; Mridha, S. Adhesive strength of electroplated copper films. J. Mater. Process. Technol. 2001, 114, 252–256. [Google Scholar] [CrossRef]
- Lee, B.S.; Lee, I. A study on the galvanic reaction between Cu and Mo as well as MoW for TFT-LCDs by using a zero-Resistance ammeter. SID Symp. Dig. Tech. Pap. 2009, 40, 1320–1323. [Google Scholar] [CrossRef]
- Nagao, K.; Neaton, J.B. First-principles study of adhesion at Cu/SiO2 interfaces. Phys. Rev. B 2003, 68, 1–9. [Google Scholar] [CrossRef]
- Mcbrayer, J.D.; Swanson, R.M. Diffusion of Metals in Silicon Dioxide. J. Electrochem. Soc. 1986, 133, 1242–1246. [Google Scholar] [CrossRef]
- Igarashi, Y.; Yamanobe, T. Thermal stability of copper interconnects fabricated by dry-etching process. Thin Solid Films 1995, 262, 124–128. [Google Scholar] [CrossRef]
- Hwang, S.; Jung, S. The electric field dependence of Cu migration induced dielectric failure in interlayer dielectric for integrated circuits. J. Appl. Phys. 2007, 101, 074501. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, T.S. Ti/Cu bilayer electrodes for SiNx-passivated Hf-In-Zn-O thin film transistors: Device performance and contact resistance. Appl. Phys. Lett. 2010, 97, 162105. [Google Scholar] [CrossRef]
- Lee, Y.W.; Kim, S. Effect of Ti/Cu Source/Drain on an Amorphous IGZO TFT Employing SiNx Passivation for Low Data-Line Resistance. Electrochem. Solid State Lett. 2012, 15, H126–H129. [Google Scholar] [CrossRef]
- Ahrens, C.; Depta, D. Electrical characterization of conductive and non-conductive barrier layers for Cu-metallization. Appl. Surf. Sci. 1995, 91, 285–290. [Google Scholar] [CrossRef]
- Yun, P.S.; Koike, J. Metal Reaction Doping and Ohmic Contact with Cu-Mn Electrode on Amorphous In-Ga-Zn-O Semiconductor. J. Electrochem. Soc. 2011, 158, H1034–H1040. [Google Scholar] [CrossRef]
- Liu, K.; Chang, T. Investigation of channel width-dependent threshold voltage variation in a-InGaZnO thin-film transistors. Appl. Phys. Lett. 2014, 104, 133503. [Google Scholar] [CrossRef]
- Seo, B.; Lee, S. Effect of nitric acid on wet etching behavior of Cu/Mo for TFT application. Curr. Appl. Phys. 2011, 11, S262–S265. [Google Scholar] [CrossRef]
- Kim, H.; Koseki, T. Cu Wettability and Diffusion Barrier Property of Ru Thin Film for Cu Metallization. J. Electrochem. Soc. 2005, 152, G594–G600. [Google Scholar] [CrossRef]
- Wang, M.; Chang, T.C. Cu/CuMg Gate Electrode for the Application of Hydrogenated Amorphous Silicon Thin-Film Transistors. Electrochem. Solid State Lett. 2007, 10, J83–J85. [Google Scholar] [CrossRef]
- Wang, M.; Chang, T.C. Suppression of Schottky leakage current in island-in amorphous silicon thin film transistors with the Cu/CuMg as source/drain metal. Appl. Phys. Lett. 2007, 91, 062103. [Google Scholar] [CrossRef]
- Yu, Z.; Ren, R. The role of oxygen in the deposition of copper-calcium thin film as diffusion barrier for copper metallization. Appl. Surf. Sci. 2015, 328, 374–379. [Google Scholar] [CrossRef]
- Mori, S.; Kawai, A. Low-Resistivity and Adhesive Sputter-Deposited Cu-Ca Films with an Intermediate Oxide Layer. Jpn. J. Appl. Phys. 2010, 49, 075804. [Google Scholar] [CrossRef]
- Takasawa, S.; Ishibashi, S. Lower Resistivity Wiring Process for TFT Source/Drain Electrodes by Oxygen-Mixture Sputtering of Cu-Ca Alloy. SID Symp. Dig. Tech. Pap. 2009, 40, 1313–1316. [Google Scholar] [CrossRef]
- Koike, J.; Wada, M. Self-forming diffusion barrier layer in Cu-Mn alloy metallization. Appl. Phys. Lett. 2005, 87, 041911. [Google Scholar] [CrossRef]
- Haneda, M.; Iijima, J. Growth behavior of self-formed barrier at Cu-Mn/SiO2 interface at 250–450 °C. Appl. Phys. Lett. 2007, 90, 252107. [Google Scholar] [CrossRef]
- Lozano, J.; Lozanoperez, S. Interdiffusion and barrier layer formation in thermally evaporated Mn/Cu heterostructures on SiO2 substrates. Appl. Phys. Lett. 2011, 98, 123112. [Google Scholar] [CrossRef]
- Koike, J.; Hirota, K. Cu-Mn Electrodes for a-Si TFT and Its Electrical Characteristics. SID Symp. Dig. Tech. Pap. 2010, 41, 1343–1346. [Google Scholar] [CrossRef]
- Tsukimoto, S.; Kabe, T. Effect of Annealing Ambient on the Self-Formation Mechanism of Diffusion Barrier Layers Used in Cu(Ti) Interconnects. J. Electron. Mater. 2007, 36, 258–265. [Google Scholar] [CrossRef]
- Ding, P.J.; Lanford, W. Oxidation resistant high conductivity copper films. Appl. Phys. Lett. 1994, 64, 2897–2899. [Google Scholar] [CrossRef]
- ASTM D 3359. Standard Test Methods for Measuring Adhesion by Tape Test. Available online: http://www.astm.org/cgi-bin/resolver.cgi?D3359 (accessed on 9 August 2017).

| Substrates | Sputtering Mode | Sputtering Power (W) | Ar Pressure (mTorr) | Thickness (Å) |
|---|---|---|---|---|
| Glass | ||||
| Al2O3/Glass | RF | 120 | 1 | 300 |
| SiO2/Glass | RF | 80 | 1 | 250 |
| Percent Area Removed | Classification |
|---|---|
| 0% | 5B |
| Less than 5% | 4B |
| 5–15% | 3B |
| 15–35% | 2B |
| 35–65% | 1B |
| Greater than 65% | 0B |
| Substrates | Room Temperature (RT) | 300 °C | 350 °C | 400 °C | 450 °C |
|---|---|---|---|---|---|
| Glass | 2B | 4B | 5B | 4B | 5B |
| Al2O3/Glass | 4B | 4B | 5B | 5B | 5B |
| SiO2/Glass | 4B | 5B | 4B | 4B | 5B |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; Lu, K.; Hu, S.; Fang, Z.; Ning, H.; Wei, J.; Zhu, Z.; Zhou, Y.; Wang, L.; Yao, R.; et al. High Conductivity and Adhesion of Cu-Cr-Zr Alloy for TFT Gate Electrode. Appl. Sci. 2017, 7, 820. https://doi.org/10.3390/app7080820
Peng J, Lu K, Hu S, Fang Z, Ning H, Wei J, Zhu Z, Zhou Y, Wang L, Yao R, et al. High Conductivity and Adhesion of Cu-Cr-Zr Alloy for TFT Gate Electrode. Applied Sciences. 2017; 7(8):820. https://doi.org/10.3390/app7080820
Chicago/Turabian StylePeng, Junbiao, Kuankuan Lu, Shiben Hu, Zhiqiang Fang, Honglong Ning, Jinglin Wei, Zhennan Zhu, Yicong Zhou, Lei Wang, Rihui Yao, and et al. 2017. "High Conductivity and Adhesion of Cu-Cr-Zr Alloy for TFT Gate Electrode" Applied Sciences 7, no. 8: 820. https://doi.org/10.3390/app7080820







