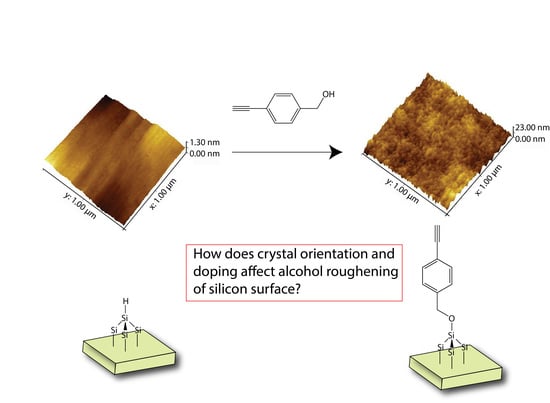
Influences of Doping and Crystal Orientation on Surface Roughening upon Alcohol Grafting onto Silicon Hydride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermal Reaction Protocol
2.3. Contact Angle Measurements
2.4. Atomic Force Microscopy
2.5. X-ray Photoelectron Spectroscopy (XPS)
3. Results
4. Summary and Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Boukherroub, R.; Morin, S.; Bensebaa, F.; Wayner, D.D.M. New synthetic routes to alkyl monolayers on the Si(111) surface. Langmuir 1999, 15, 3831–3835. [Google Scholar] [CrossRef]
- Buriak, J.M. Silicon-carbon bonds on porous silicon surfaces. Adv. Mater. 1999, 11. [Google Scholar] [CrossRef]
- Buriak, J.M.; Stewart, M.P.; Geders, T.W.; Allen, M.J.; Choi, H.C.; Smith, J.; Raftery, D.; Canham, L.T. Lewis acid mediated hydrosilylation on porous silicon surfaces. J. Am. Chem. Soc. 1999, 121, 11491–11502. [Google Scholar] [CrossRef]
- Cleland, G.; Horrocks, B.R.; Houlton, A. Direct functionalization of silicon via the self-assembly of alcohols. J. Chem. Soc. Faraday Trans. 1995, 91, 4001–4003. [Google Scholar] [CrossRef]
- Radi, A.; Leung, K.T. Competitive bonding of amino and hydroxyl groups in ethanolamine on Si(100)2 × 1: Temperature-dependent X-ray photoemission and thermal desorption studies of nanochemistry of a double-chelating agent. Mater. Express 2011, 1, 144–153. [Google Scholar] [CrossRef]
- Khung, Y.L.; Ngalim, S.H.; Scaccabarozi, A.; Narducci, D. Thermal and uv hydrosilylation of alcohol-based bifunctional alkynes on Si(111) surfaces: How surface radicals influence surface bond formation. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Hacker, C.A.; Anderson, K.A.; Richter, L.J.; Richter, C.A. Comparison of Si-O-C interfacial bonding of alcohols and aldehydes on Si(111) formed from dilute solution with ultraviolet irradiation. Langmuir 2005, 21, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Boukherroub, R.; Morin, S.; Sharpe, P.; Wayner, D.D.M.; Allongue, P. Insights into the formation mechanisms of Si-or monolayers from the thermal reactions of alcohols and aldehydes with Si(111)-H. Langmuir 2000, 16, 7429–7434. [Google Scholar] [CrossRef]
- Michalak, D.J.; Amy, S.R.; Esteve, A.; Chabal, Y.J. Investigation of the chemical purity of silicon surfaces reacted with liquid methanol. J. Phys. Chem. C 2008, 112, 11907–11919. [Google Scholar] [CrossRef]
- Michalak, D.J.; Rivillon, S.; Chabal, Y.J.; Esteve, A.; Lewis, N.S. Infrared spectroscopic investigation of the reaction of hydrogen-terminated, (111)-oriented, silicon surfaces with liquid methanol. J. Phys. Chem. B 2006, 110, 20426–20434. [Google Scholar] [CrossRef] [PubMed]
- Sieval, A.B.; Linke, R.; Heij, G.; Meijer, G.; Zuilhof, H.; Sudholter, E.J.R. Amino-terminated organic monolayers on hydrogen-terminated silicon surfaces. Langmuir 2001, 17, 7554–7559. [Google Scholar] [CrossRef]
- Sieval, A.B.; van den Hout, B.; Zuilhof, H.; Sudholter, E.J.R. Molecular modeling of covalently attached alkyl monolayers an the hydrogen-terminated Si(111) surface. Langmuir 2001, 17, 2172–2181. [Google Scholar] [CrossRef]
- Coletti, C.; Marrone, A.; Giorgi, G.; Sgamellotti, A.; Cerofolini, G.; Re, N. Nonradical mechanisms for the uncatalyzed thermal functionalization of silicon surfaces by alkenes and alkynes: A density functional study. Langmuir 2006, 22, 9949–9956. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Ruther, R.E.; Streifer, J.A.; Hamers, R.J. UV-induced grafting of alkenes to silicon surfaces: Photoemission versus excitons. J. Am. Chem. Soc. 2010, 132, 4048–4049. [Google Scholar] [CrossRef] [PubMed]
- Buriak, J.M. Illuminating silicon surface hydrosilylation: An unexpected plurality of mechanisms. Chem. Mater. 2014, 26, 763–772. [Google Scholar] [CrossRef]
- Cai, W.; Lin, Z.; Strother, T.; Smith, L.M.; Hamers, R.J. Chemical modification and patterning of iodine-terminated silicon surfaces using visible light. J. Phys. Chem. B 2002, 106, 2656–2664. [Google Scholar] [CrossRef]
- Khung, Y.L.; Ngalim, S.H.; Meda, L.; Narducci, D. Preferential formation of Si-O-C over Si-C linkage upon thermal grafting on hydrogen-terminated silicon (111). Chem. Eur. J. 2014, 20, 15151–15158. [Google Scholar] [CrossRef] [PubMed]
- Khung, Y.L.; Ngalim, S.H.; Scaccabarozzi, A.; Narducci, D. Formation of stable Si-O-C submonolayers on hydrogen-terminated silicon (111) under low-temperature conditions. Beilstein J. Nanotechnol. 2015, 6, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.D.; Hauser, J.R.; Roulston, D.J. Electron and hole mobilities in silicon as a function of concentration and temperature. IEEE Trans. Electron. Devices 1982, 29, 292–295. [Google Scholar] [CrossRef]
- Fischetti, M.V.; Ren, Z.; Solomon, P.M.; Yang, M.; Rim, K. Six-band k center dot p calculation of the hole mobility in silicon inversion layers: Dependence on surface orientation, strain, and silicon thickness. J. Appl. Phys. 2003, 94, 1079–1095. [Google Scholar] [CrossRef]
- Avila, A.; Montero, I.; Galan, L.; Ripalda, J.M.; Levy, R. Behavior of oxygen doped sic thin films: An X-ray photoelectron spectroscopy study. J. Appl. Phys. 2001, 89, 212–216. [Google Scholar] [CrossRef]
- Li, N.; Hu, P.; Zhang, X.; Liu, Y.; Han, W. Effects of oxygen partial pressure and atomic oxygen on the microstructure of oxide scale of ZrB2-SiC composites at 1500 degrees C. Corros. Sci. 2013, 73, 44–53. [Google Scholar] [CrossRef]
- Thogersen, A.; Selj, J.H.; Marstein, E.S. Oxidation effects on graded porous silicon anti-reflection coatings. J. Electrochem. Soc. 2012, 159, D276–D281. [Google Scholar] [CrossRef]
- Guerrero-Lemus, R.; Moreno, J.D.; Martin-Palma, R.J.; Ben-Hander, F.; Martinez-Duart, J.M.; Fierro, J.L.G.; Gomez-Garrido, P. Influence of oxidation and carbon-containing contamination in the stabilization of the luminescence in porous silicon. Thin Solid Films 1999, 354, 34–37. [Google Scholar] [CrossRef]
- Gunter, P.L.J.; Gijzeman, O.L.J.; Niemantsverdriet, J.W. Surface roughness effects in quantitative XPS: Magic angle for determining overlayer thickness. Appl. Surf. Sci 1997, 115, 342–346. [Google Scholar] [CrossRef]
- Olejnik, K.; Zemek, J. Applicability of magic angle for angle-resolved X-ray photoelectron spectroscopy of corrugated SiO2/Si surfaces: Monte carlo calculations. Surf. Sci. 2008, 602, 2581–2586. [Google Scholar] [CrossRef]
- Zemek, J.; Olejnik, K.; Klapetek, P. Photoelectron spectroscopy from randomly corrugated surfaces. Surf. Sci. 2008, 602, 1440–1446. [Google Scholar] [CrossRef]
- Michalak, D.J.; Amy, S.R.; Aureau, D.; Dai, M.; Esteve, A.; Chabal, Y.J. Nanopatterning Si(111) surfaces as a selective surface-chemistry route. Nat. Mater. 2010, 9, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Gerischer, H.; Mindt, W. The mechanisms of the decomposition of semiconductors by electrochemical oxidation and reduction. Electrochim. Acta 1968, 13, 1329–1341. [Google Scholar] [CrossRef]
- Kolasinski, K.W. Etching of silicon in fluoride solutions. Surf. Sci. 2009, 603, 1904–1911. [Google Scholar] [CrossRef]
- Walsh, R. Bond-dissociation energy values in silicon-containing compounds and some of their implications. Acc. Chem. Res. 1981, 14, 246–252. [Google Scholar] [CrossRef]
- Lehmann, V. The physics of macropore formation in low doped n-type silicon. J. Electrochem. Soc. 1993, 140, 2836–2843. [Google Scholar] [CrossRef]
- Nahm, K.S.; Seo, Y.H.; Lee, H.J. Formation mechanism of stains during Si etching reaction in HF-oxidizing agent H2O solutions. J. Appl. Phys. 1997, 81, 2418–2424. [Google Scholar] [CrossRef]
- Shane, S.F.; Kolasinski, K.W.; Zare, R.N. State-specific study of hydrogen desorption from Si(100)-(2 × 1): Comparison of disilane and hydrogen adsorption. J. Vac. Sci. Technol. A Vac. Surf. Films 1992, 10, 2287–2291. [Google Scholar] [CrossRef]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tung, J.; Khung, Y.L. Influences of Doping and Crystal Orientation on Surface Roughening upon Alcohol Grafting onto Silicon Hydride. Appl. Sci. 2017, 7, 859. https://doi.org/10.3390/app7080859
Tung J, Khung YL. Influences of Doping and Crystal Orientation on Surface Roughening upon Alcohol Grafting onto Silicon Hydride. Applied Sciences. 2017; 7(8):859. https://doi.org/10.3390/app7080859
Chicago/Turabian StyleTung, Joline, and Yit Lung Khung. 2017. "Influences of Doping and Crystal Orientation on Surface Roughening upon Alcohol Grafting onto Silicon Hydride" Applied Sciences 7, no. 8: 859. https://doi.org/10.3390/app7080859