Effect of Enzymatic Beech Fagus Sylvatica Wood Hydrolysate on Chlorella Biomass, Fatty Acid and Pigment Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Wood Alkaline Pretreatment and Enzymatic Hydrolysis
2.3. Microalgae Cultivation in Medium Supplemented with Organic Carbon
2.4. Growth Rate Measurement
2.5. FAME and Pigment Determination in Microalgae Biomass
3. Results and Discussion
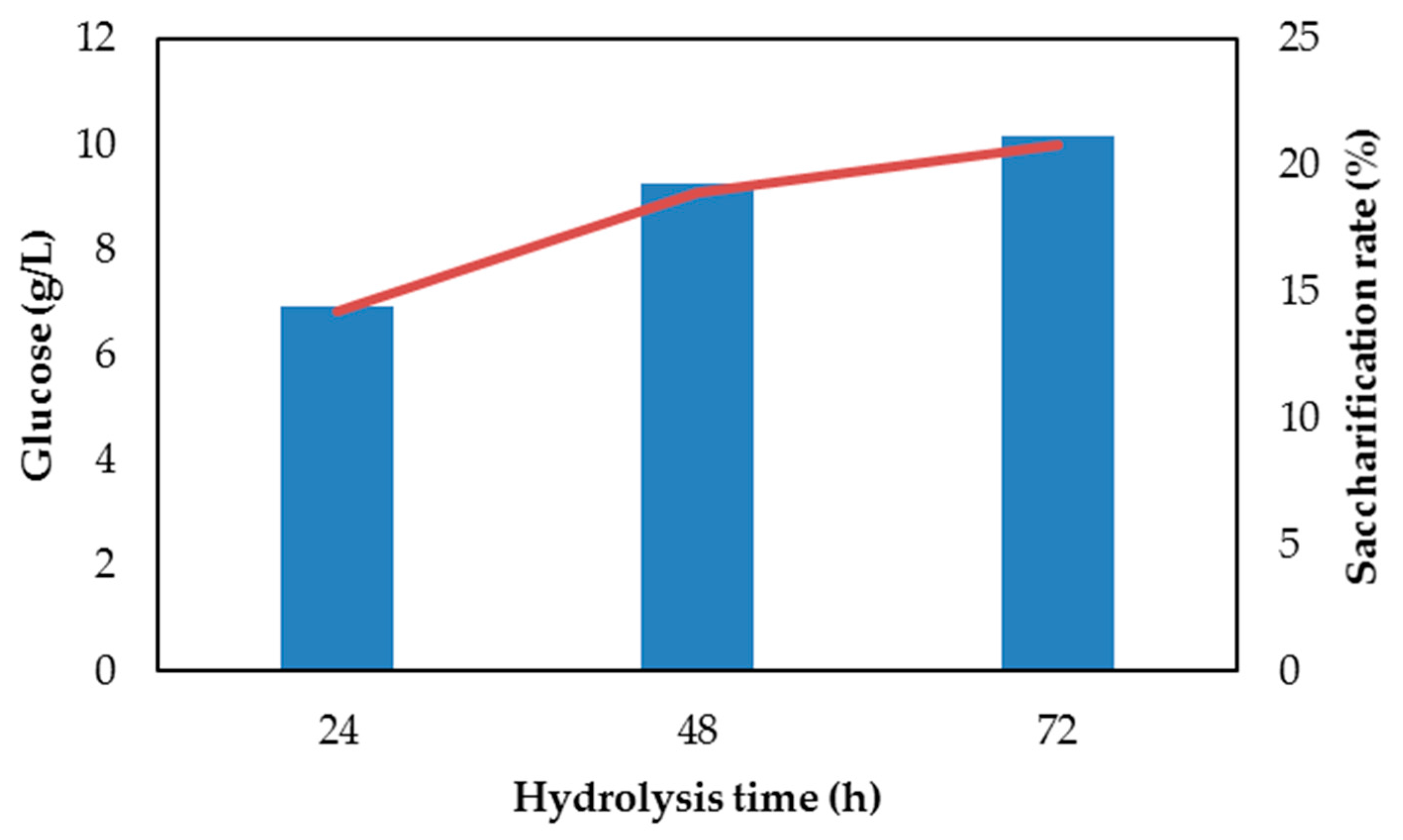
3.1. Pretreatment and Enzymatic Hydrolysis of Wood
3.2. Effect of Neutralized Citrate Buffer on Chlorella Growth
3.3. Effect of Enzymatic Hydrolysate on Chlorella Biomass, Fatty Acid and Pigment Productivity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TMP | TRIS minimal medium. |
| TGP | TMP medium containing synthetic glucose (1 g/L). |
| TGP-Enz10 | TMP medium supplemented with enzymatic hydrolysate at a 10% loading, containing 1 g/L glucose (from hydrolysate) and neutralized citrate buffer at a 10% loading (from hydrolysate). |
| TGP-Cit10 | TMP medium containing synthetic glucose (1 g/L) and neutralized citrate buffer at 10% loading, where 100% citrate buffer equals to 0.05 M. |
| L-Light | cultivation conditions under light irradiance: 75 µE m−2 s−1. |
| D-Dark | cultivation conditions without light supplied: 0 µE m−2 s−1. |
| Cit0 | neutralized citrate buffer at a 0% loading. |
| Cit0.5 | neutralized citrate buffer at a 0.5% loading. |
| Cit1 | neutralized citrate buffer at a 1% loading. |
| Cit2 | neutralized citrate buffer at a 2% loading. |
| Cit5 | neutralized citrate buffer at a 5% loading. |
| Cit10 | neutralized citrate buffer at a 10% loading. |
| Cit15 | neutralized citrate buffer at a 15% loading. |
| Cit20 | neutralized citrate buffer at a 20% loading. |
References
- Hammed, A.M.; Prajapati, S.K.; Simsek, S.; Simsek, H. Growth regime and environmental remediation of microalgae. Algae 2016, 31, 189–204. [Google Scholar] [CrossRef]
- Miazek, K.; Kratky, L.; Sulc, R.; Jirout, T.; Aguedo, M.; Richel, A.; Goffin, D. Effect of Organic Solvents on Microalgae Growth, Metabolism and Industrial Bioproduct Extraction: A Review. Int. J. Mol. Sci. 2017, 18, 1429. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. Commercial production of microalgae: Ponds, tanks, tubes and fermenters. J. Biotechnol. 1999, 70, 313–321. [Google Scholar] [CrossRef]
- Lowrey, J.; Brooks, M.S.; McGinn, P.J. Heterotrophic and mixotrophic cultivation of microalgae for biodiesel production in agricultural wastewaters and associated challenges—A critical review. J. Appl. Phycol. 2015, 27, 1485–1498. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, Z.; Zhang, R. Overview of biomass pretreatment for cellulosic ethanol production. Int. J. Agric. Biol. Eng. 2009, 2, 51–68. [Google Scholar]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Hu, F.; Ragauskas, A. Pretreatment and lignocellulosic chemistry. Bioenergy Res. 2012, 5, 1043–1066. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Slininger, P.J.; Dien, B.S.; Waghmode, S.; Moser, B.R.; Orjuela, A.; Sousa, L.C.; Balan, V. Microbial lipid-based lignocellulosic biorefinery: Feasibility and challenges. Trends Biotechnol. 2015, 33, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Miazek, K.; Remacle, C.; Richel, A.; Goffin, D. Effect of lignocellulose related compounds on microalgae growth and product biosynthesis: A review. Energies 2014, 7, 4446–4481. [Google Scholar] [CrossRef]
- Latte, N.; Perin, J.; Kint, V.; Lebourgeois, F.; Claessens, H. Major Changes in Growth Rate and Growth Variability of Beech (Fagus sylvatica L.) Related to Soil Alteration and Climate Change in Belgium. Forests 2016, 7, 174. [Google Scholar] [CrossRef]
- Simon, M.; Brostaux, Y.; Vanderghem, C.; Jourez, B.; Paquot, M.; Richel, A. Optimization of a formic/acetic acid delignification treatment on beech wood and its influence on the structural characteristics of the extracted lignins. J. Chem. Technol. Biotechnol. 2014, 89, 128–136. [Google Scholar] [CrossRef]
- Miazek, K.; Remacle, C.; Richel, A.; Goffin, D. Beech wood Fagus sylvatica dilute-acid hydrolysate as a feedstock to support Chlorella sorokiniana biomass, fatty acid and pigment production. Bioresour. Technol. 2017, 230, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, K.; Zhu, S.; Feng, J.; Shang, C.; Wang, Z.; Yuan, Z.; Zhuang, X.; Hu, L. Cell growth and fatty acid production of heterotrophic microalgae Chlorella sp. cultivated in enzymatic hydrolyzate of sugarcane bagasse. CIESC J. 2016, 67, 1549–1556. [Google Scholar]
- Joe, M.H.; Kim, J.Y.; Lim, S.; Kim, D.H.; Bai, S.; Park, H.; Lee, S.G.; Han, S.J.; Choi, J.I. Microalgal lipid production using the hydrolysates of rice straw pretreated with gamma irradiation and alkali solution. Biotechnol. Biofuels 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Vanderghem, C.; Brostaux, Y.; Jacquet, N.; Blecker, C.; Paquot, M. Optimization of formic/acetic acid delignification of Miscanthus×giganteus for enzymatic hydrolysis using response surface methodology. Ind. Crops Prod. 2012, 35, 280–286. [Google Scholar] [CrossRef]
- Lehto, J.; Alen, R. Alkaline pre-treatment of hardwood chips prior to delignification. J. Wood Chem. Technol. 2013, 33, 77–91. [Google Scholar] [CrossRef]
- Oka, D.; Kobayashi, K.; Isobe, N.; Ogawa, Y.; Yokoyama, T.; Kimura, S.; Kim, U.J.; Tokuyasu, K.; Wada, M. Enzymatic hydrolysis of wood with alkaline treatment. J. Wood. Sci. 2013, 59, 484–488. [Google Scholar] [CrossRef]
- Li, H.Y.; Chen, X.; Wang, C.Z.; Sun, S.N.; Sun, R.C. Evaluation of the two-step treatment with ionic liquids and alkali for enhancing enzymatic hydrolysis of Eucalyptus: Chemical and anatomical changes. Biotechnol. Biofuels 2016, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Skrivanova, E.; Marounek, M.; Benda, V.; Brezina, P. Susceptibility of Escherichia coli, Salmonella sp. and Clostridium perfringens to organic acids and monolaurin. Vet. Med. 2006, 51, 81–88. [Google Scholar]
- Kang, H.C.; Park, Y.H.; Go, S.J. Growth inhibition of a phytopathogenic fungus, Colletotrichum species by acetic acid. Microbiol. Res. 2003, 158, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zeng, R.; Zhang, S.X.; Yang, Z.H.; Huang, H. Production of microalgal lipids as biodiesel feedstock with fixation of CO2 by Chlorella vulgaris. Food Technol. Biotechnol. 2014, 52, 285–291. [Google Scholar]
- Luo, W.Y.; Du, W.Y.; Su, Y.; Hui, J.J.; Zhuang, J.; Liu, L.L. Growth Characteristic of the Oleaginous Microalga Chlorella ellipsoidea SD-0701 with Lipid Accumulation. Nat. Resour. 2015, 6, 130–139. [Google Scholar]
- Talebi, A.F.; Tabatabaei, M.; Mohtashami, S.K.; Tohidfar, M.; Moradi, F. Comparative salt stress study on intracellular ion concentration in marine and salt-adapted freshwater strains of microalgae. Not. Sci. Biol. 2013, 5, 309–315. [Google Scholar]
- Perez, M.; Nolasco, N.A.; Vasavada, A.; Johnson, M.; Kuehnle, A. Algae-Mediated Valorization of Industrial Waste Streams. Ind. Biotechnol. 2015, 11, 229–234. [Google Scholar] [CrossRef]
- Llano, T.; Quijorna, N.; Coz, A. Detoxification of a Lignocellulosic Waste from a Pulp Mill to Enhance Its Fermentation Prospects. Energies 2017, 10, 348. [Google Scholar] [CrossRef]
- Coz, A.; Llano, T.; Cifrian, E.; Viguri, J.; Maican, E.; Sixta, H. Physico-Chemical Alternatives in Lignocellulosic Materials in Relation to the Kind of Component for Fermenting Purposes. Materials 2016, 9, 574. [Google Scholar] [CrossRef] [PubMed]
- Bumbak, F.; Cook, S.; Zachleder, V.; Hauser, S.; Kovar, K. Best practices in heterotrophic high-cell-density microalgal processes: Achievements, potential and possible limitations. Appl. Microbiol. Biotechnol. 2011, 91, 31–46. [Google Scholar] [CrossRef] [PubMed]

| Fatty Acid Production | TGP (36 h) | TGP-Enz10 (42 h) | TGP-Cit10 (52 h) |
|---|---|---|---|
| Biomass productivity (mg L−1 d−1) A | 403 ± 20 | 345 ± 20 | 279 ± 15 |
| Total fatty acid content (% d.w.) | 3.6 ± 0.05 | 3.5 ± 0.1 | 3.7 ± 0.1 |
| Fatty acid productivity (mg L−1 d−1) | 14.5 ± 0.72 | 12.1 ± 0.6 | 10.32 ± 0.52 |
| Fatty acid (FA) composition (% of total fatty acids) | |||
| C14:0 | 1.00 ± 0.1 | 1.27 ± 0.1 | 1.26 ± 0.23 |
| C16:0 | 29 ± 1.5 | 29.15 ± 0.63 | 28.3 ± 0.56 |
| C18:1 | 5.85 ± 0.5 | 8.31 ± 0.56 | 8.75 ± 0.78 |
| C18:2 | 26.1 ± 0.5 | 41.28 ± 0.39 | 45.33 ± 0.52 |
| C18:3 | 38.4 ± 1.2 | 19.85 ± 1.62 | 16.25 ± 0.49 |
| Pigment Production | TGP (36 h) | TGP-Enz10 (42 h) | TGP-Cit10 (52 h) |
|---|---|---|---|
| Biomass productivity (mg L−1 d−1) A | 403 ± 20 | 345 ± 20 | 279 ± 15 |
| ChlTotal content (% d.w.) | 1.215 ± 0.065 | 1.24 ± 0.03 | 1.288 ± 0.108 |
| ChTotal productivity (mg L−1 d−1) | 4.89 ± 0.25 | 4.278 ± 0.21 | 3.59 ± 0.18 |
| Chl a/b ratio (-) | 3.67 ± 0.09 | 3.645 ± 0.085 | 3.857 ± 0.337 |
| CarTotal content (% d.w.) | 0.297 ± 0.016 | 0.313 ± 0.008 | 0.337 ± 0.03 |
| CarTotal productivity (mg L−1 d−1) | 1.19 ± 0.06 | 1.08 ± 0.054 | 0.94 ± 0.047 |
| ChlTotal/CarTotal ratio (-) | 4.09 ± 0.05 | 3.96 ± 0.11 | 3.82 ± 0.18 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miazek, K.; Remacle, C.; Richel, A.; Goffin, D. Effect of Enzymatic Beech Fagus Sylvatica Wood Hydrolysate on Chlorella Biomass, Fatty Acid and Pigment Production. Appl. Sci. 2017, 7, 871. https://doi.org/10.3390/app7090871
Miazek K, Remacle C, Richel A, Goffin D. Effect of Enzymatic Beech Fagus Sylvatica Wood Hydrolysate on Chlorella Biomass, Fatty Acid and Pigment Production. Applied Sciences. 2017; 7(9):871. https://doi.org/10.3390/app7090871
Chicago/Turabian StyleMiazek, Krystian, Claire Remacle, Aurore Richel, and Dorothee Goffin. 2017. "Effect of Enzymatic Beech Fagus Sylvatica Wood Hydrolysate on Chlorella Biomass, Fatty Acid and Pigment Production" Applied Sciences 7, no. 9: 871. https://doi.org/10.3390/app7090871





