Changes in Pore Structure of Coal Associated with Sc-CO2 Extraction during CO2-ECBM
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coal Samples
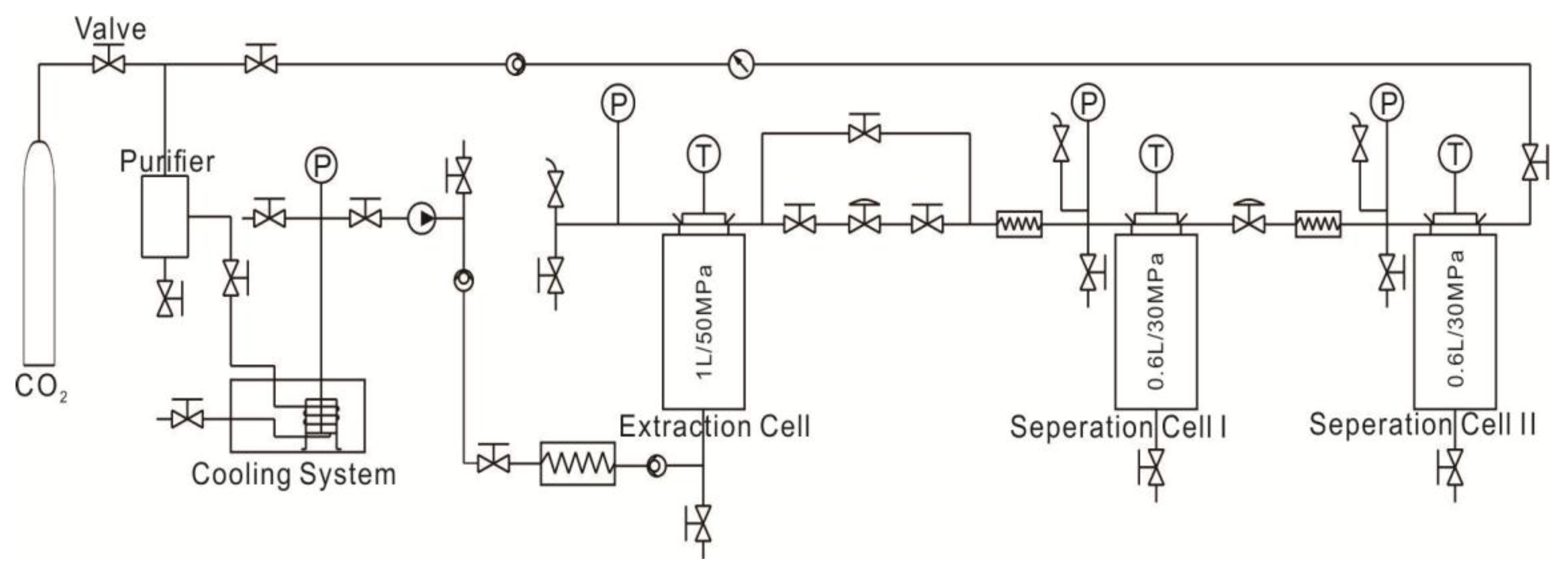
2.2. Sc-CO2 Extraction
2.3. Mercury Porosimetry
3. Results and Discussion
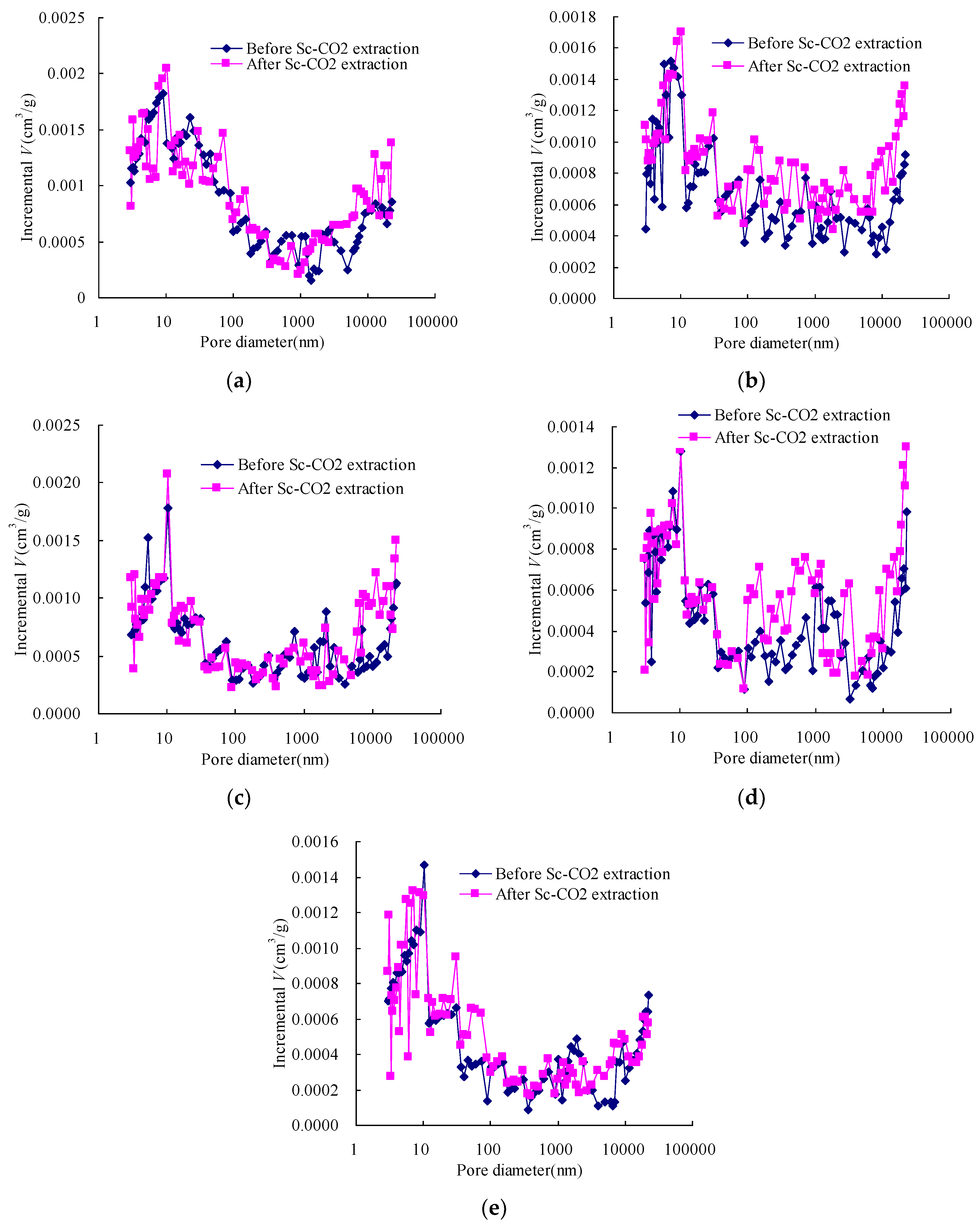
3.1. Pore Size Distribution of Coal before and after Sc-CO2 Extraction
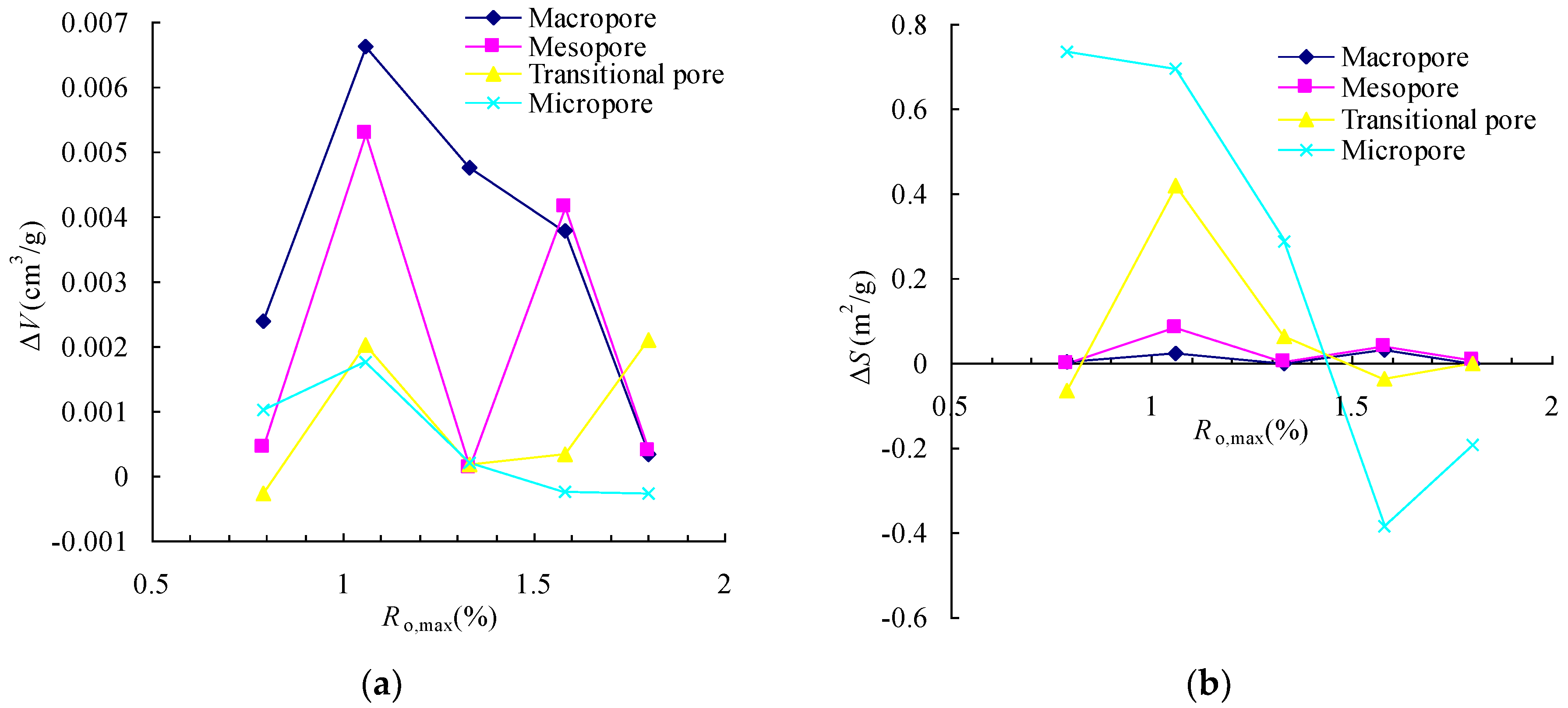
3.2. The Changes in Pore Volume and Surface Area
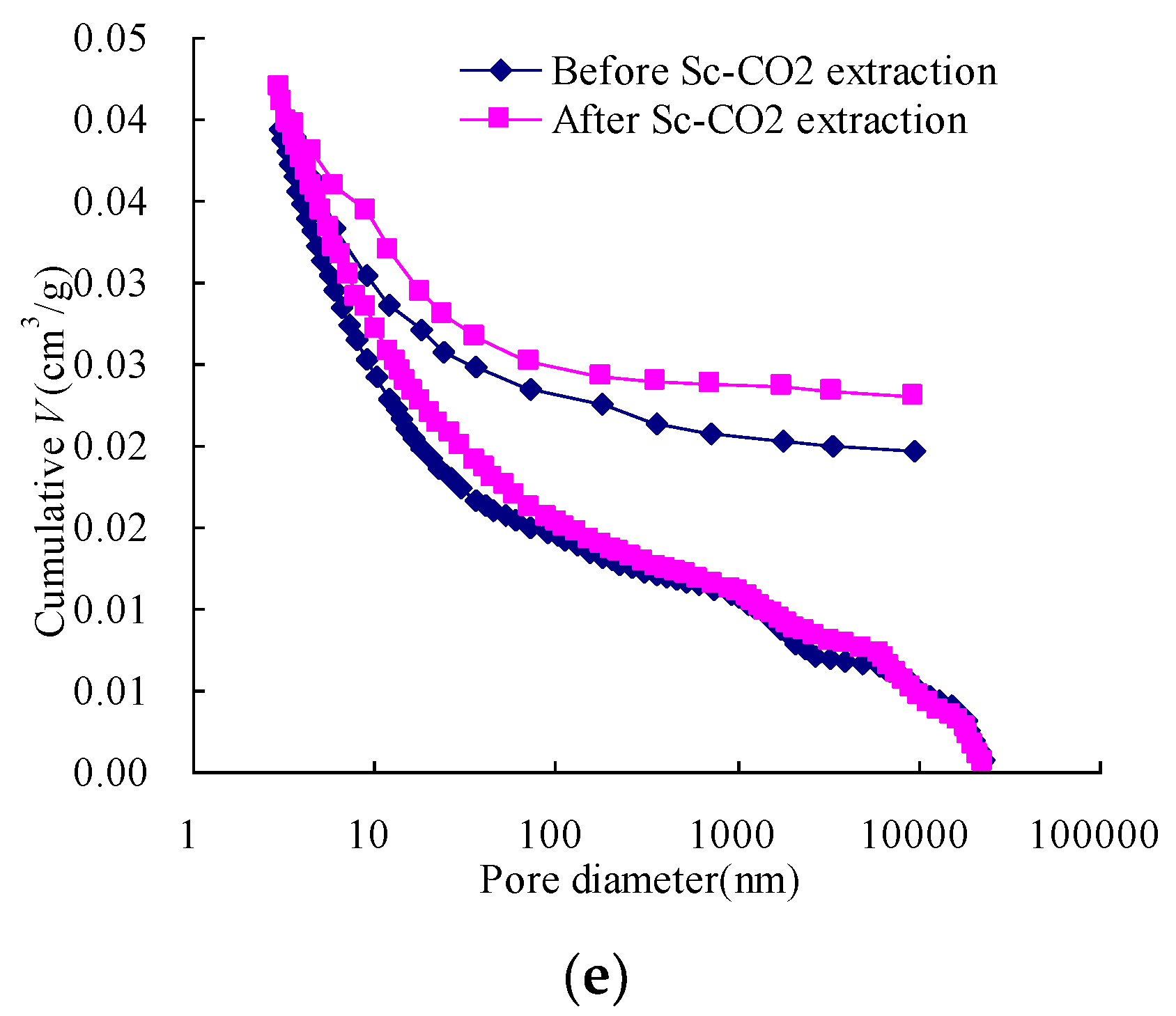
3.3. The Changes in Pore Connectivity
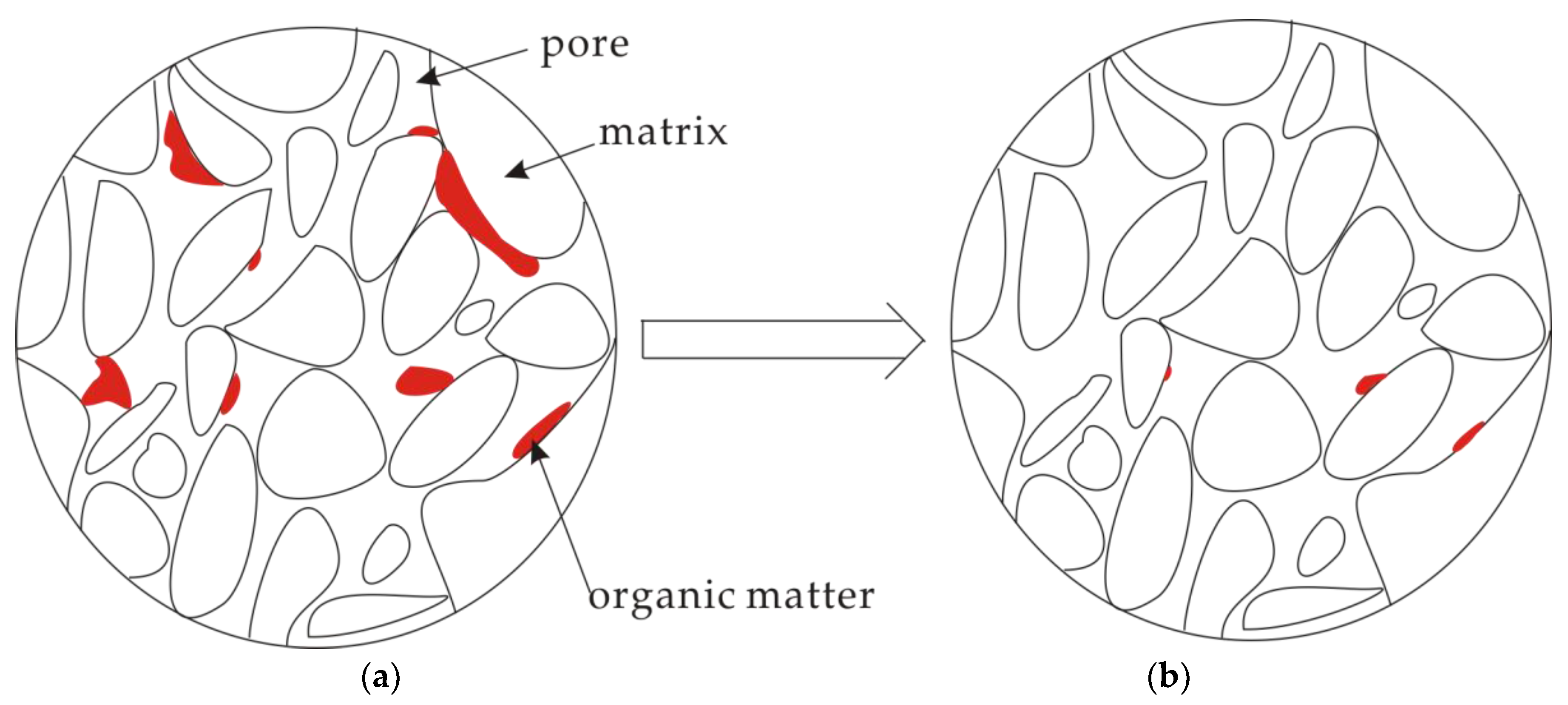
3.4. A Conceptual Model for Pore Structure Evolution
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). IPCC Special Report on Carbon Dioxide Capture and Storage; Prepared by working group III of the intergovernmental panel on climate change [Metz, B.; Davidson, O.; Coninck, de H.C.; Loos, m.; Meyer, L.A. (des.)]; Cambridge University: Cambridge, UK; New York, NY, USA, 2005; ISBN 13-978-0-521-86643-9. [Google Scholar]
- Johnson, W.E.; Macfarlane, R.M.; Breston, J.N.; Neil, D.C. Laboratory experiments with carbonated water and liquid carbon dioxide as oil recovery agents. Prod. Mon. 1952, 17, 18–22. [Google Scholar]
- Busch, A.; Gensterblum, Y.; Krooss, B.M. Methane and CO2 sorption and desorption measurements on dry Argonne premium coals: Pure components and mixtures. Int. J. Coal Geol. 2003, 55, 205–224. [Google Scholar] [CrossRef]
- Ampomah, W.; Balch, R.; Cather, M.; Rose-Coss, D.; Dai, Z.; Heath, J.; Dewers, T.; Mozley, P. Evaluation of CO2 storage mechanisms in CO2 enhanced oil recovery sites: Application to Morrow sandstone reservoir. Energy Fuels 2016, 30, 8545–8555. [Google Scholar] [CrossRef]
- Qin, Y. Mechanism of CO2 enhanced CBM recovery in China: A review. J. China Univ. Min. Technol. 2008, 18, 406–412. [Google Scholar] [CrossRef]
- Gale, J.J. Using coal seams for CO2 sequestration. Geol. Belg. 2004, 7, 99–103. [Google Scholar]
- Fu, X.H.; Qin, Y.; Wei, C.T. Coalbed Methane Geology; China University of Mining and Technology Press: Xuzhou, China, 2007; pp. 46–47. (In Chinese) [Google Scholar]
- Rogers, R.; Ramurthy, K.; Rodvelt, G.; Mullen, M. Coalbed Methane: Principles and Practices, 2nd ed.; Oktibbeha Publishing Co., LLC.: Starkville, MS, USA, 2007; pp. 126–128. ISBN 978-0-9794084-1-0. [Google Scholar]
- Su, X.B.; Chen, R.; Lin, X.Y.; Li, J.H. Desorption characteristic curves of carbon dioxide and methane in coal and their application. Nat. Gas Ind. 2008, 28, 17–20, (In Chinese with English abstract). [Google Scholar]
- Busch, A.; Gensterblum, Y.; Krooss, M.B.; Siemons, N. Investigation of high-pressure selective adsorption/desorption behaviour of CO2 and CH4 on coals: An experimental study. Int. J. Coal Geol. 2006, 66, 53–68. [Google Scholar] [CrossRef]
- Day, S.; Sakurovs, R.; Weir, S. Supercritical gas sorption on moist coals. Int. J. Coal Geol. 2008, 74, 203–214. [Google Scholar] [CrossRef]
- Fitzgerald, J.E.; Sudibandriyo, M.Z.; Pan, R.L.; Robinson, R.L.; Gasem, K.A.M. Modeling the adsorption of pure gases on coals with the SLD model. Carbon 2003, 41, 2203–2216. [Google Scholar] [CrossRef]
- Gathitu, B.B.; Chen, W.; McClure, M. Effects of coal interaction with supercritical CO2: Physical structure. Ind. Eng. Chem. Res. 2009, 48, 5024–5034. [Google Scholar] [CrossRef]
- He, J.Y.; Shi, Y.; Ahn, S.; Kang, J.W.; Lee, C.H. Adsorption and desorption of CO2 on Korean coal under subcritical to supercritical conditions. J. Phys. Chem. B 2010, 114, 4854–4861. [Google Scholar] [CrossRef]
- Day, S.; Fry, R.; Sakurovs, R. Swelling of Australian coals in supercritical CO2. Int. J. Coal Geol. 2008, 74, 41–52. [Google Scholar] [CrossRef]
- Cui, R.M.B.; Chikatamarla, L. Adsorption-induced coal swelling and stress: Implications for methane production and acid gas sequestration into coal seams. J. Geophys. Res. 2007, 112, B10202. [Google Scholar] [CrossRef]
- Pan, Z.; Connell, L.D. A theoretical model for gas adsorption-induced coal swelling. Int. J. Coal Geol. 2007, 69, 243–252. [Google Scholar] [CrossRef]
- Chen, R.; Qin, Y.; Wei, C.T.; Wang, L.L.; Wang, Y.Y.; Zhang, P.F. New discovery on supercritical CO2-H2O treated coal: Pore structure and methane adsorption. Acta Geol. Sin. (Engl. Ed.) 2017, 91, 1509–1510. [Google Scholar] [CrossRef]
- Wang, G.X.; Massarotto, P.; Rudolph, V. An improved permeability model of coal for coalbed methane recovery and CO2 geosequestration. Int. J. Coal Geol. 2009, 77, 127–136. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Goodman, A.L.; Rosenbaum, E. Characterization of coal before and after supercritical CO2 exposure via feature relocation using field-emission scanning electron microscopy. Fuel 2013, 107, 777–786. [Google Scholar] [CrossRef]
- Xu, D.Q. Supercritical fluid extraction of soluble organic matter in hydrocarbon source rocks and its application. Nat. Gas Geosci. 2011, 22, 235–239. [Google Scholar]
- Wang, Y.L. Application of Supercritical Fluid Extraction in Organic Geochemistry; Lanzhou Institute of Geology, Chinese Academy of Science: Lanzhou, China, 2002. [Google Scholar]
- He, Z.X. Evolution and Oil Gas of the Ordos Basin; Petroleum Industry Press: Beijing, China, 2003; pp. 1–77. [Google Scholar]
- Zhang, Y.K.; Zhou, L.F.; Dang, B.; Sun, W. Relationship between the mesozoic and enozoic tectonic stress fields and the hydrocarbon accumulation in the Ordos basin. Pet. Geol. Exp. 2006, 28, 215–219. [Google Scholar]
- Qin, Y.; Song, D.Y. The Coalification and Its Palaeogeothermal System in Southern Shanxi and the Geological Mechanism of Gas Controlling in Coalication; Geological Publishing House: Beijing, China, 1998; pp. 1–90. ISBN 9787116025349. [Google Scholar]
- Berkowitz, N. An Introduction to Coal Technology; Academic Press: New York, NY, USA, 1979; p. 31. [Google Scholar]
- Wang, X.H.; Wang, Y.B.; Gao, S.S. Differences in pore structures and absorptivity between tectonically deformed and undeformed coals. Geol. J. Chin. Univ. 2012, 18, 528–532. [Google Scholar]
- Washburn, E.W. The dynamics of capillary flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Hodot, B.B. Coalbed and Gas Outburst; (Trans by Song Shizhao, Wang Youan, In Chinese); Coal Industry Press: Beijing, China, 1966. [Google Scholar]
- Liu, C.J.; Wang, G.X.; Sang, S.X.; Rudolh, V. Changes in pore structure of anthracite coal associated with CO2 sequestration process. Fuel 2010, 89, 2665–2672. [Google Scholar] [CrossRef]
- Salmas, C.; Androutsopoulos, G. Mercury porosimetry: Contact angle hysteresis of materials with controlled pore structure. J. Colloid Interface Sci. 2001, 239, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Tsakiroglou, C.D.; Payatakes, A.C. Mercury intrusion and retraction in model porous media. Adv. Colloid Interface Sci. 1998, 75, 215–253. [Google Scholar] [CrossRef]


| Sample | Qiyi Coal | Liulin Coal | Dengjiazhuang Coal | Shuangliu Coal | Maozequ Coal |
|---|---|---|---|---|---|
| Ro,max (%) | 0.79 | 1.06 | 1.33 | 1.58 | 1.80 |
| Vitrinite (%) | 78.40 | 78.48 | 67.41 | 56.84 | 63.37 |
| Inertinite (%) | 14.87 | 19.16 | 25.18 | 37.71 | 30.43 |
| Exinite (%) | 5.48 | 2.36 | 4.94 | 3.13 | 4.72 |
| Mineral (%) | 1.25 | 10.0 | 2.47 | 2.32 | 1.48 |
| Mad (%) | 4.34 | 1.94 | 1.09 | 0.83 | 0.98 |
| Ad (%) | 6.57 | 10.51 | 8.47 | 11.36 | 7.69 |
| Vad (%) | 31.05 | 27.59 | 26.85 | 23.52 | 20.31 |
| Samples | Pore Volumes (10−2 cm3/g) | Pore Surface Area (m2/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| VMa | VMe | VTr | VMi | VCu | SMa | SMe | STr | SMi | SCu | ||
| Qiyi coal | Before extraction | 1.62 | 0.80 | 2.28 | 2.45 | 7.15 | 0.016 | 0.091 | 4.182 | 17.442 | 21.731 |
| After extraction | 1.86 | 0.84 | 2.26 | 2.55 | 7.51 | 0.018 | 0.092 | 4.120 | 18.179 | 22.409 | |
| Liulin coal | Before extraction | 1.64 | 0.82 | 1.35 | 1.75 | 5.56 | 0.018 | 0.116 | 3.066 | 14.028 | 17.228 |
| After extraction | 2.30 | 1.35 | 1.56 | 1.93 | 7.14 | 0.044 | 0.199 | 3.486 | 14.724 | 18.453 | |
| Dengjiazhuang coal | Before extraction | 1.65 | 0.63 | 1.29 | 1.59 | 5.16 | 0.014 | 0.076 | 2.763 | 14.829 | 17.682 |
| After extraction | 2.13 | 0.64 | 1.30 | 1.61 | 5.68 | 0.015 | 0.080 | 2.825 | 15.116 | 18.036 | |
| Shuangliu coal | Before extraction | 1.21 | 0.47 | 0.83 | 1.33 | 3.84 | 0.011 | 0.047 | 1.765 | 13.086 | 14.909 |
| After extraction | 1.59 | 0.89 | 0.86 | 1.30 | 4.64 | 0.043 | 0.088 | 1.729 | 12.701 | 14.561 | |
| Maozequ coal | Before extraction | 1.07 | 0.38 | 0.97 | 1.51 | 3.93 | 0.007 | 0.075 | 1.495 | 8.837 | 10.414 |
| After extraction | 1.10 | 0.42 | 1.18 | 1.49 | 4.19 | 0.007 | 0.082 | 1.497 | 8.646 | 10.232 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Qin, Y.; Wei, C.; Wang, L.; Wang, Y.; Zhang, P. Changes in Pore Structure of Coal Associated with Sc-CO2 Extraction during CO2-ECBM. Appl. Sci. 2017, 7, 931. https://doi.org/10.3390/app7090931
Chen R, Qin Y, Wei C, Wang L, Wang Y, Zhang P. Changes in Pore Structure of Coal Associated with Sc-CO2 Extraction during CO2-ECBM. Applied Sciences. 2017; 7(9):931. https://doi.org/10.3390/app7090931
Chicago/Turabian StyleChen, Run, Yong Qin, Chongtao Wei, Linlin Wang, Youyang Wang, and Pengfei Zhang. 2017. "Changes in Pore Structure of Coal Associated with Sc-CO2 Extraction during CO2-ECBM" Applied Sciences 7, no. 9: 931. https://doi.org/10.3390/app7090931





