Facile Synthesis of Two-Dimensional Porous MgCo2O4 Nanosheets as Anode for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of MgCo2O4 Nanosheets
2.2. Material Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
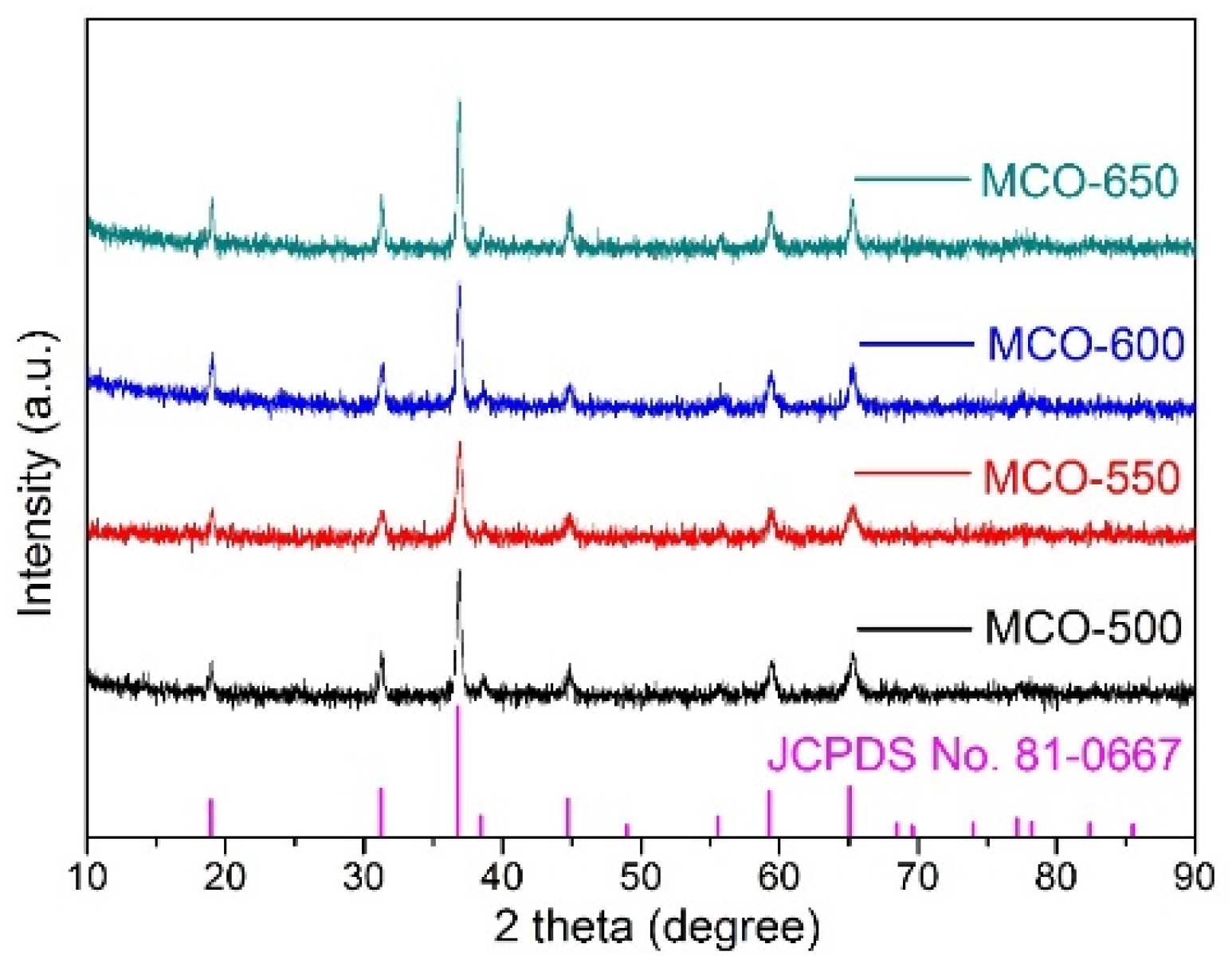
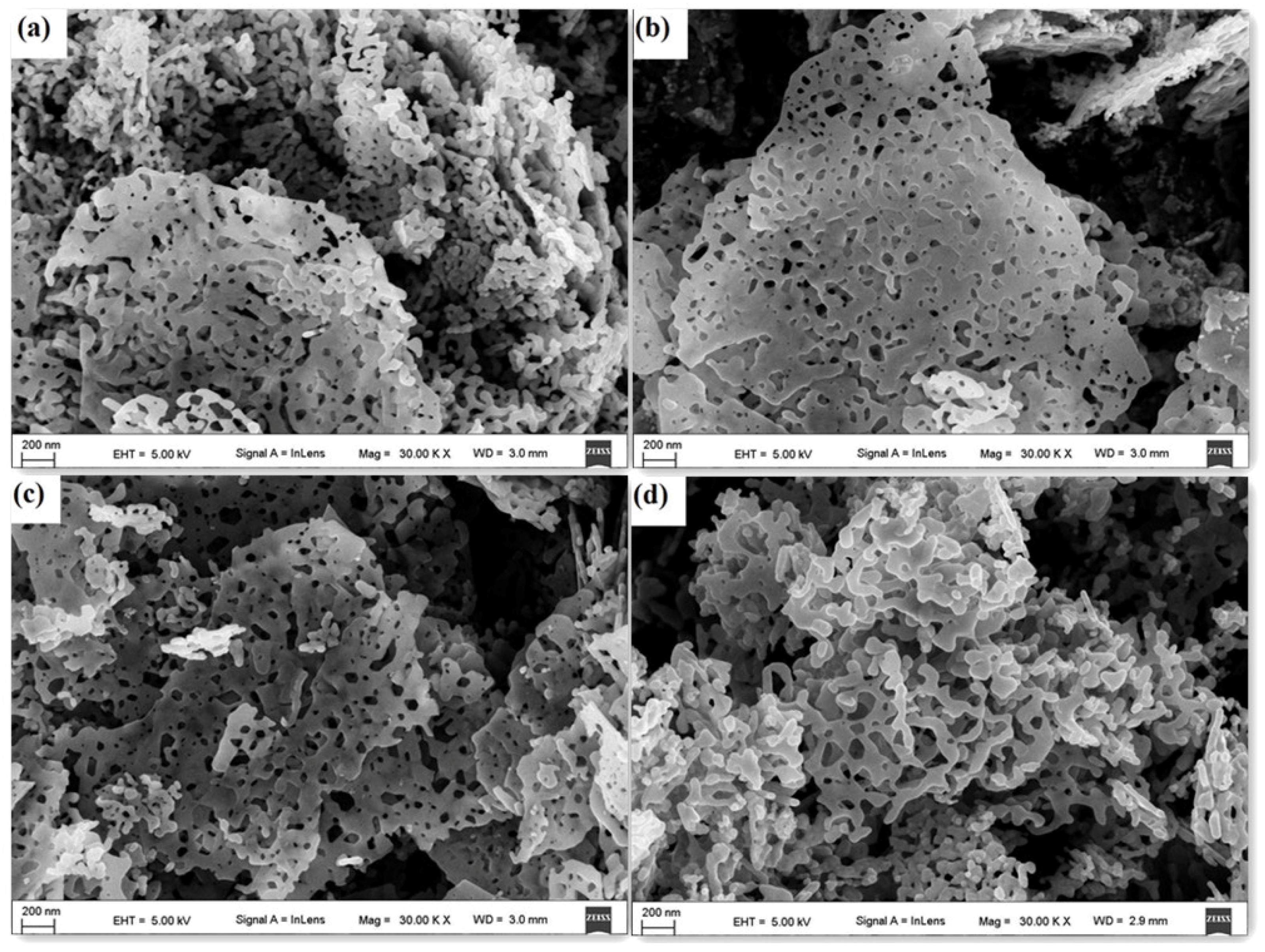
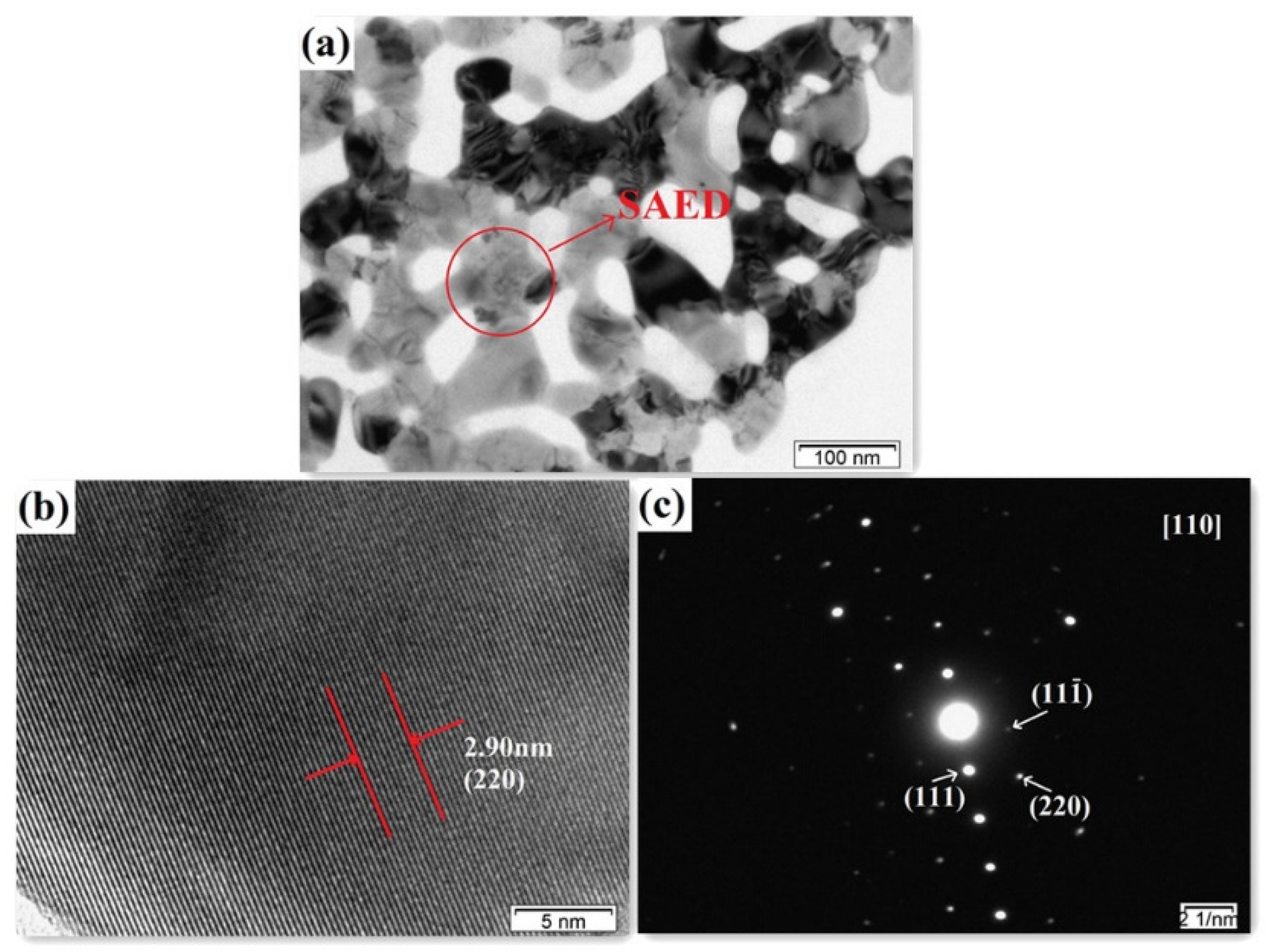
3.1. Structure and Morphology
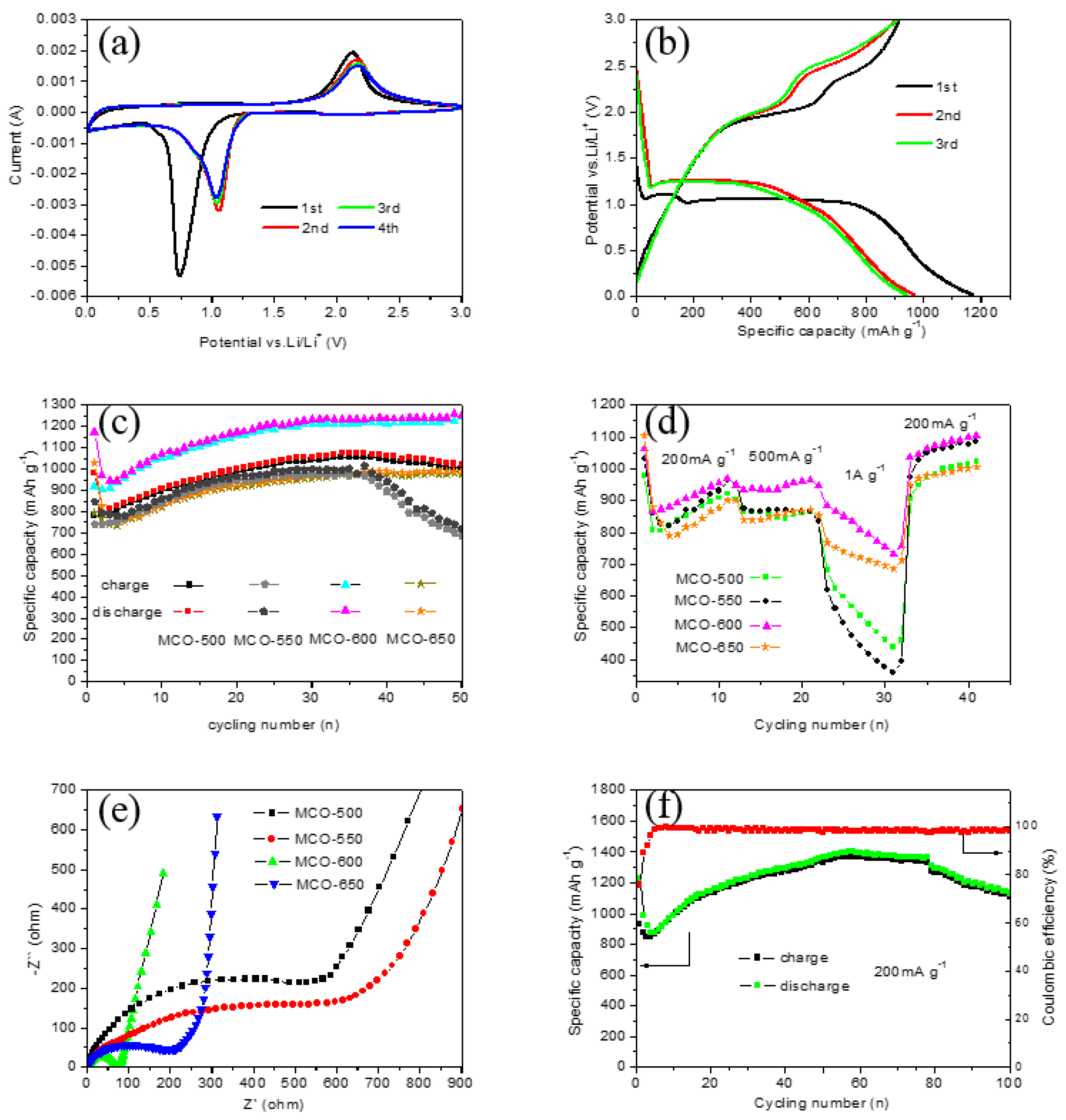
3.2. Electrochemical Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.M. Nanomaterials for rechargeable lithium batteries. Angew. Chem.-Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Han, C.H.; Yang, J.W.; Su, J.; Xu, X.M.; Li, S.; Xu, L.; Fang, R.P.; Jiang, H.; Zou, X.D.; et al. Stable Alkali Metal Ion Intercalation Compounds as Optimized Metal Oxide Nanowire Cathodes for Lithium Batteries. Nano Lett. 2015, 15, 2180–2185. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ou, X.; Xiong, X.; Zheng, F.; Hu, R.; Chen, Y.; Liu, M.; Huang, K. V5S8-graphite hybrid nanosheets as a high rate-capacity and stable anode material for sodium-ion batteries. Energy Environ. Sci. 2016, 10. [Google Scholar] [CrossRef]
- Ou, X.; Yang, C.; Xiong, X.; Zheng, F.; Pan, Q.; Jin, C.; Liu, M.; Huang, K. A New rGO-Overcoated Sb2Se3 Nanorods Anode for Na+ Battery: In Situ X-ray Diffraction Study on a Live Sodiation/Desodiation Process. Adv. Funct. Mater. 2017, 27, 1606242. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, C.; Wang, G.; Lin, Y.; Ou, X.; Wang, J.; Zhao, B.; Liu, M.; Lin, Z.; Huang, K. SnS nanoparticles electrostatically anchored on three-dimensional N-doped graphene as an active and durable anode for sodium-ion batteries. Energy Environ. Sci. 2017, 10, 1757–1763. [Google Scholar] [CrossRef]
- Zhou, J.; Tao, Q.; Na, X.; Wang, M.; Ni, X.; Liu, X.; Shen, X.; Yan, C. Selenium-Doped Cathodes for Lithium–Organosulfur Batteries with Greatly Improved Volumetric Capacity and Coulombic Efficiency. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.B.; Chen, J.S.; Hng, H.H.; Lou, X.W. Nanostructured metal oxide-based materials as advanced anodes for lithium-ion batteries. Nanoscale 2012, 4, 2526–2542. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.S. New energy storage devices for post lithium-ion batteries. Energy Environ. Sci. 2013, 6, 2256. [Google Scholar] [CrossRef]
- Chang, H.X.; Wu, H.K. Graphene-based nanocomposites: Preparation, functionalization, and energy and environmental applications. Energy Environ. Sci. 2013, 6, 3483–3507. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.F.; Yan, B.; Xiong, D.B.; Li, D.J.; Lawes, S.; Sun, X.L. Recent Developments and Understanding of Novel Mixed Transition-Metal Oxides as Anodes in Lithium Ion Batteries. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.L.; Li, J.C.; Xiao, X.C.; Lott, A.; Lu, W.Q.; Sheldon, B.W.; Wu, J. Silicon-Based Nanomaterials for Lithium-Ion Batteries: A Review. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Yuan, C.Z.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed Transition-Metal Oxides: Design, Synthesis, and Energy-Related Applications. Angew. Chem.-Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Sharma, N.; Rao, G.V.S.; Chowdari, B.V.R. Nanophase ZnCo2O4 as a high performance anode material for Li-ion batteries. Adv. Energy Mater. 2007, 17, 2855–2861. [Google Scholar] [CrossRef]
- Pendashteh, A.; Moosavifard, S.E.; Rahmanifar, M.S.; Wang, Y.; El-Kady, M.F.; Kaner, R.B.; Mousavi, M.F. Highly Ordered Mesoporous CuCo2O4 Nanowires, a Promising Solution for High-Performance Supercapacitors. Chem. Mater. 2015, 27, 3919–3926. [Google Scholar] [CrossRef]
- Sharma, Y.; Sharma, N.; Rao, G.V.S.; Chowdari, B.V.R. Studies on spinel cobaltites, FeCo2O4 and MgCo2O4 as anodes for Li-ion batteries. Solid State Ion. 2008, 179, 587–597. [Google Scholar] [CrossRef]
- Zhu, H.F.; Sun, Y.F.; Zhang, X.; Tang, L.; Guo, J.X. Evaporation-induced self-assembly synthesis of mesoporous FeCo2O4 octahedra with large and fast lithium storage properties. Mater. Lett. 2016, 166, 1–4. [Google Scholar] [CrossRef]
- Bai, J.; Li, X.G.; Liu, G.Z.; Qian, Y.T.; Xiong, S.L. Unusual Formation of ZnCo2O4 3D Hierarchical Twin Microspheres as a High-Rate and Ultralong-Life Lithium-Ion Battery Anode Material. Adv. Funct. Mater. 2014, 24, 3012–3020. [Google Scholar] [CrossRef]
- Wu, H.; Lou, Z.; Yang, H.; Shen, G.Z. A flexible spiral-type supercapacitor based on ZnCo2O4 nanorod electrodes. Nanoscale 2015, 7, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Lee, W.J. Multi-shelled MgCo2O4 hollow microspheres as anodes for lithium ion batteries. J. Mater. Chem. A 2016, 4, 12263–12272. [Google Scholar] [CrossRef]
- Wang, X.J.; Zhai, G.H.; Wang, H. Facile synthesis of MgCo2O4 nanowires as binder-free flexible anode materials for high-performance Li-ion batteries. J. Nanopart. Res. 2015, 17. [Google Scholar] [CrossRef]
- Zhu, Y.; Cao, C.; Zhang, J.; Xu, X. Two-Dimensional Ultrathin ZnCo2O4 Nanosheets: General Formation and Lithium Storage Application. J. Mater. Chem. A 2015, 3, 9556–9564. [Google Scholar] [CrossRef]
- Darbar, D.; Reddy, M.V.; Sundarrajan, S.; Pattabiraman, R.; Ramakrishna, S.; Chowdari, B.V.R. Anodic electrochemical performances of MgCo2O4 synthesized by oxalate decomposition method and electrospinning technique for Li-ion battery application. Mater. Res. Bull. 2016, 73, 369–376. [Google Scholar] [CrossRef]
- Mo, Y.; Ru, Q.; Song, X.; Guo, L.; Chen, J.; Hou, X.; Hu, S. The sucrose-assisted NiCo2O4@C composites with enhanced lithium-storage properties. Carbon 2016, 109, 616–623. [Google Scholar] [CrossRef]
- Huang, J.; Fang, G.; Liu, K.; Zhou, J.; Tang, X.; Cai, K.; Liang, S. Controllable synthesis of highly uniform cuboid-shape MOFs and their derivatives for lithium-ion battery and photocatalysis applications. Chem. Eng. J. 2017, 322, 281–292. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Liu, Y.; Zhao, Y.; Wang, Y.; Wang, Z.; Zhang, W.; Ren, F. Facile Synthesis of Two-Dimensional Porous MgCo2O4 Nanosheets as Anode for Lithium-Ion Batteries. Appl. Sci. 2018, 8, 22. https://doi.org/10.3390/app8010022
Wang F, Liu Y, Zhao Y, Wang Y, Wang Z, Zhang W, Ren F. Facile Synthesis of Two-Dimensional Porous MgCo2O4 Nanosheets as Anode for Lithium-Ion Batteries. Applied Sciences. 2018; 8(1):22. https://doi.org/10.3390/app8010022
Chicago/Turabian StyleWang, Fei, Yong Liu, Yuanfang Zhao, Yue Wang, Zhijie Wang, Wanhong Zhang, and Fengzhang Ren. 2018. "Facile Synthesis of Two-Dimensional Porous MgCo2O4 Nanosheets as Anode for Lithium-Ion Batteries" Applied Sciences 8, no. 1: 22. https://doi.org/10.3390/app8010022




