New Insights in the Ion Beam Sputtering Deposition of ZnO-Fluoropolymer Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
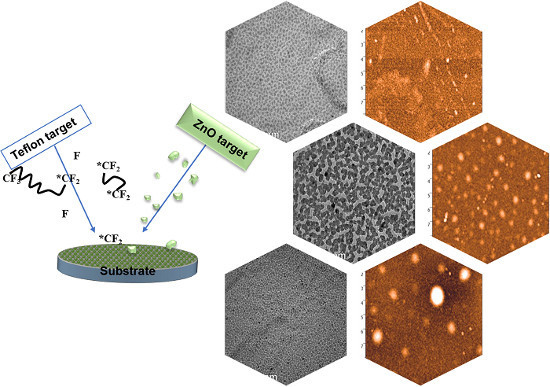
2.2. IBS Deposition of ZnO-CFx Nanocomposites
2.3. Morphological Characterization of ZnO-CFx Nanocomposites
2.4. Surface Analytical Characterization by XPS
2.5. Water Contact Angle (WCA) Measurements
3. Results and Discussion
3.1. Thin Film Deposition and Morphological Characterization
3.2. Surface Analytical Characterization
3.3. Water Contact Angle (WCA) Measurements
3.4. Treatments in Deposition Chamber to Reduce Fluorides Content
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Loh, K.J.; Chang, D. Zinc oxide nanoparticle-polymeric thin films for dynamic strain sensing. J. Mater. Sci. 2010, 46, 228–237. [Google Scholar] [CrossRef]
- D’Agostino, R. Plasma Deposition, Treatment, and Etching of Polymers: The Treatment and Etching of Polymers; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 978-0-323-13908-3. [Google Scholar]
- Quaranta, F.; Valentini, A.; Favia, P.; Lamendola, R.; d’Agostino, R. Ion-beam sputtering deposition of fluoropolymer thin films. Appl. Phys. Lett. 1993, 63, 10–11. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Uno, M.; Tominari, Y.; Takeya, J. Fabrication of high-mobility organic single-crystal field-effect transistors with amorphous fluoropolymer gate insulators. Org. Electron. 2008, 9, 753–756. [Google Scholar] [CrossRef]
- Wu, J.; Xia, J.; Zhang, Y.; Lei, W.; Wang, B. A simple method to fabricate the different extents of superhydrophobic surfaces. Phys. E Low-Dimens. Syst. Nanostruct. 2010, 42, 1325–1328. [Google Scholar] [CrossRef]
- Bayat, A.; Ebrahimi, M.; Nourmohammadi, A.; Moshfegh, A.Z. Wettability properties of PTFE/ZnO nanorods thin film exhibiting UV-resilient superhydrophobicity. Appl. Surf. Sci. 2015, 341, 92–99. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Jafari, R.; Farzaneh, M. An optimization of superhydrophobic polyvinylidene fluoride/zinc oxide materials using Taguchi method. Appl. Surf. Sci. 2014, 288, 229–237. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Wang, E.; Zhang, X.; Yuan, R.; Zhu, Y. Superhydrophobic poly(vinylidene fluoride) membranes with controllable structure and tunable wettability prepared by one-step electrospinning. Polymer 2016, 82, 105–113. [Google Scholar] [CrossRef]
- Bahgat, A.; Mohamed, A.M.A.; Abdullah, A.M.; Almaadeed, M. Superhydrophobic and Corrosion Behavior of Electrospun PVDF-ZnO Coating. ECS Trans. 2015, 64, 57–67. [Google Scholar] [CrossRef]
- Luan, B.C.D.; Huang, J.; Zhang, J. Enhanced thermal conductivity and wear resistance of polytetrafluoroethylene composites through boron nitride and zinc oxide hybrid fillers. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Zha, J.W.; Dang, Z.M.; Zhao, K.; Zheng, X.Q.; Li, S.T. Prominent nonlinear electrical conduction characteristic in T-ZnOw/PTFE composites with low threshold field. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 567–573. [Google Scholar] [CrossRef]
- Cioffi, N.; Losito, I.; Torsi, L.; Farella, I.; Valentini, A.; Sabbatini, L.; Zambonin, P.G.; Bleve-Zacheo, T. Analysis of the Surface Chemical Composition and Morphological Structure of Vapor-Sensing Gold—Fluoropolymer Nanocomposites. Chem. Mater. 2002, 14, 804–811. [Google Scholar] [CrossRef]
- Cioffi, N.; Ditaranto, N.; Torsi, L.; Picca, R.A.; Sabbatini, L.; Valentini, A.; Novello, L.; Tantillo, G.; Bleve-Zacheo, T.; Zambonin, P.G. Analytical characterization of bioactive fluoropolymer ultra-thin coatings modified by copper nanoparticles. Anal. Bioanal. Chem. 2005, 381, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, A.Y.; Deng, X.; Onyshchenko, I.; Vujosevic, D.; Vuksanovic, V.; Cvelbar, U.; De Geyter, N.; Morent, R.; Leys, C. Atmospheric pressure plasma deposition of antimicrobial coatings on non-woven textiles. Eur. Phys. J. Appl. Phys. 2016, 75, 24710. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Picca, R.A.; Cioffi, N. Nano-Antimicrobials Based on Metals. In Novel Antimicrobial Agents and Strategies; Phoenix, D.A., Harris, F., Dennison, S.R., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 181–218. ISBN 978-3-527-67613-2. [Google Scholar]
- Pollini, M.; Paladini, F.; Sannino, A.; Picca, R.A.; Sportelli, M.C.; Cioffi, N.; Nitti, M.A.; Valentini, M.; Valentini, A. Nonconventional routes to silver nanoantimicrobials: Technological issues, bioactivity, and applications. In Nanotechnology in Diagnosis, Treatment and Prophylaxis of Infectious Diseases; Kon, M.R., Ed.; Academic Press: Boston, MA, USA, 2015; Chapter 6; pp. 87–105. ISBN 978-0-12-801317-5. [Google Scholar]
- Sportelli, M.C.; Picca, R.A.; Cioffi, N. Recent advances in the synthesis and characterization of nano-antimicrobials. TrAC Trends Anal. Chem. 2016, 84 Pt A, 131–138. [Google Scholar] [CrossRef]
- Zaporojtchenko, V.; Chakravadhanula, V.S.K.; Faupel, F.; Tamulevičius, S.; Andrulevičius, M.; Tamulevičienė, A.; Augulis, L. Residual stress in polytetrafluoroethylene-metal nanocomposite films prepared by magnetron sputtering. Thin Solid Films 2010, 518, 5944–5949. [Google Scholar] [CrossRef]
- Farella, I.; Valentini, A.; Cioffi, N.; Torsi, L. Dual ion-beam sputtering deposition of palladium-fluoropolymer nano-composites. Appl. Phys. A 2005, 80, 791–795. [Google Scholar] [CrossRef]
- Faupel, F.; Zaporojtchenko, V.; Greve, H.; Schürmann, U.; Chakravadhanula, V.S.K.; Hanisch, C.; Kulkarni, A.; Gerber, A.; Quandt, E.; Podschun, R. Deposition of Nanocomposites by Plasmas. Contrib. Plasma Phys. 2007, 47, 537–544. [Google Scholar] [CrossRef]
- Faupel, F.; Zaporojtchenko, V.; Strunskus, T.; Elbahri, M. Metal-Polymer Nanocomposites for Functional Applications. Adv. Eng. Mater. 2010, 12, 1177–1190. [Google Scholar] [CrossRef]
- Wang, X.; Hall, J.E.; Böhm, G.G.A.; Lin, C.J. Nano-Sized Polymer-Metal Composites. U.S. Patent 7,112,369, 26 September 2006. [Google Scholar]
- Michalczyk, M.J.; Sharp, K.G.; Stewart, C.W. Fluoropolymer Nanocomposites. U.S. Patent 5,726,247, 10 March 1998. [Google Scholar]
- Roy, R.A.; Messier, R.; Krishnaswamy, S.V. Preparation and properties of r.f.-sputtered polymer-metal thin films. Thin Solid Films 1983, 109, 27–35. [Google Scholar] [CrossRef]
- See, K.C.; Spicer, J.B.; Brupbacher, J.; Zhang, D.; Vargo, T.G. Modeling Interband Transitions in Silver Nanoparticle—Fluoropolymer Composites. J. Phys. Chem. B 2005, 109, 2693–2698. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Eilers, H. Electrical conductivity of thin-film composites containing silver nanoparticles embedded in a dielectric fluoropolymer matrix. Thin Solid Films 2008, 517, 575–581. [Google Scholar] [CrossRef]
- Rahachou, A.V.; Rogachev, A.A.; Yarmolenko, M.A.; Jiang, X.-H.; Liu, Z.B. Molecular structure and optical properties of PTFE-based nanocomposite polymer–metal coatings. Appl. Surf. Sci. 2012, 258, 1976–1980. [Google Scholar] [CrossRef]
- Liu, C.; Fairhurst, R.; Ren, L.; Green, S.; Tong, J.; Arnell, R. Co-deposition of titanium/polytetrafluoroethylene films by unbalanced magnetron sputtering. Surf. Coat. Technol. 2002, 149, 143–150. [Google Scholar] [CrossRef]
- Pihosh, Y.; Biederman, H.; Slavinska, D.; Kousal, J.; Choukourov, A.; Trchova, M.; Mackova, A.; Boldyryeva, A. Composite SiOx/fluorocarbon plasma polymer films prepared by r.f. magnetron sputtering of SiO2 and PTFE. Vacuum 2006, 81, 38–44. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98. [Google Scholar] [CrossRef]
- Tan, W.K.; Razak, K.A.; Ibrahim, K.; Lockman, Z. Formation of ZnO nanorod arrays on polytetraflouroethylene (PTFE) via a seeded growth low temperature hydrothermal reaction. J. Alloys Compd. 2011, 509, 820–826. [Google Scholar] [CrossRef]
- Srivastava, M.; Basu, B.B.J.; Rajam, K.S. Improving the Hydrophobicity of ZnO by PTFE Incorporation. J. Nanotechnol. 2011, 2011, 392754. [Google Scholar] [CrossRef]
- Li, F.; Hu, K.; Li, J.; Zhao, B. The friction and wear characteristics of nanometer ZnO filled polytetrafluoroethylene. Wear 2001, 249, 877–882. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, Y.; Li, C.; Gao, X.; Cao, X.; Li, J. The structure and photoluminescence properties of RF-sputtered films of ZnO on Teflon substrate. Mater. Lett. 2008, 62, 2907–2909. [Google Scholar] [CrossRef]
- Wei, Q.; Yu, L.; Hou, D.; Huang, F. Surface characterization and properties of functionalized nonwoven. J. Appl. Polym. Sci. 2008, 107, 132–137. [Google Scholar] [CrossRef]
- Fahlteich, J.; Steiner, C.; Schiller, N.; Miesbauer, O.; Noller, K.; Deichmann, K.-J.; Mirza, M.; Amberg-Schwab, S. Roll-to-roll thin film coating on fluoropolymer webs—Status, challenges and applications. Surf. Coat. Technol. 2017, 314, 160–168. [Google Scholar] [CrossRef]
- Neelakantan, N.K.; Weisensee, P.B.; Overcash, J.W.; Torrealba, E.J.; King, W.P.; Suslick, K.S. Spray-on omniphobic ZnO coatings. RSC Adv. 2015, 5, 69243–69250. [Google Scholar] [CrossRef]
- Liu, Y.; Zang, Y.; Wei, G.; Li, J.; Fan, X.-L.; Cheng, C. Stress and structural studies of ZnO thin films on polymer substrate under different RF powered conditions. Mater. Lett. 2009, 63, 2597–2599. [Google Scholar] [CrossRef]
- Guo, Q.X.; Mitsuishi, Y.; Tanaka, T.; Nishio, M.; Ogawa, H.; Huang, Y.Z. Microfabrication of ZnO on a PTFE Template Patterned by Using Synchrotron Radiation. J. Korean Phys. Soc. 2008, 53, 2796. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Ji, Q.; Wang, X. RF Sputtering of Composite Fluorocarbon/ZnO Films and their Basic Properties. Key Eng. Mater. 2008, 373–374, 159–162. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Ji, Q.; Liu, R.; Liu, Z.; Qi, H. Structure and washing fastness of composite fluorocarbon/ZnO films prepared by RF sputtering. Surf. Coat. Technol. 2009, 203, 3405–3409. [Google Scholar] [CrossRef]
- Zhang, Y.H. Composite fluorocarbon/ZnO films prepared by RF magnetron sputtering of Zn and PTFE. Surf. Coat. Technol. 2008, 202, 2612–2615. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Ji, Q.; Liu, Z.J. Preparation and UV Absorption Property of Hybrid Fluorocarbon/ZnO Thin Films Deposited by RF Magnetron Sputtering. Adv. Mater. Res. 2010, 143, 792–796. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Nitti, M.A.; Valentini, M.; Picca, R.A.; Bonerba, E.; Sabbatini, L.; Tantillo, G.; Cioffi, N.; Valentini, A. Ion Beam Sputtering Deposition and Characterization of ZnO-Fluoropolymer Nano-Antimicrobials. Sci. Adv. Mater. 2014, 6, 1019–1025. [Google Scholar] [CrossRef]
- Cioffi, N.; Farella, I.; Torsi, L.; Valentini, A.; Sabbatini, L.; Zambonin, P.G. Ion-beam sputtered palladium-fluoropolymer nano-composites as active layers for organic vapours sensors. Sens. Actuators B 2003, 93, 181–186. [Google Scholar] [CrossRef]
- Schön, G. Auger and direct electron spectra in X-ray photoelectron studies of zinc, zinc oxide, gallium and gallium oxide. J. Electron Spectrosc. Relat. Phenom. 1973, 2, 75–86. [Google Scholar] [CrossRef]
- Wagner, C.D.; Joshi, A. The auger parameter, its utility and advantages: A review. J. Electron. Spectrosc. Relat. Phenom. 1988, 47, 283–313. [Google Scholar] [CrossRef]
- NIST XPS Database. Available online: http://srdata.nist.gov/xps (accessed on 3 December 2017).
- Luckenbach, R. Beilstein Handbook of Organic Chemistry: Covering the Literature from 1960 through 1979; 5th Supplementary Series; Springer: New York, NY, USA, 1982; ISBN 978-3-540-11341-6. [Google Scholar]
- Palumbo, F.; Mundo, R.D. Wettability: Significance and measurement. In Polymer Surface Characterization; De Gruyter: Berlin, Germany; Boston, MA, USA, 2014; Chapter 7; ISBN 978-3-11-028811-7. [Google Scholar]
- Zinc Fluoride. Available online: https://toxnet.nlm.nih.gov/ (accessed on 3 December 2017).
- Iannone, F.; Casiello, M.; Monopoli, A.; Cotugno, P.; Sportelli, M.C.; Picca, R.A.; Cioffi, N.; Dell’Anna, M.M.; Nacci, A. Ionic liquids/ZnO nanoparticles as recyclable catalyst for polycarbonate depolymerization. J. Mol. Catal. A Chem. 2017, 426, 107–116. [Google Scholar] [CrossRef]






| φ | Sample Region | % F | % Zn | F/Zn |
|---|---|---|---|---|
| 0.05 | Cluster | n.d. | n.d. | - |
| Background | n.d. | n.d. | - | |
| Total | 7.2 | 0.2 | 36.0 | |
| 0.10 | Cluster | 2.0 | 0.6 | 3.3 |
| Background | 3.3 | 0.8 | 4.1 | |
| Total | 3.4 | 0.8 | 4.2 | |
| 0.15 | Cluster | 2.4 | 1.7 | 1.4 |
| Background | 5.6 | 1.5 | 3.7 | |
| Total | 5.3 | 1.5 | 3.5 |
| φ | % C | % F | % O | % Zn |
|---|---|---|---|---|
| 0.05 | 46.3 ± 0.3 | 3.4 ± 0.2 | 51.6 ± 0.4 | 1.4 ± 0.2 |
| 0.10 | 33.5 ± 1.4 | 8.8 ±0.2 | 43.6 ± 0.8 | 14.2 ± 0.4 |
| 0.15 | 35.6 ± 0.4 | 8.9 ±0.2 | 40.9 ± 0.2 | 14.1 ± 0.5 |
| φ | θ Advancing/Degree | θ Receding/Degree |
|---|---|---|
| 0.05 | 112 ± 1 | 73 ± 1 |
| 0.10 | 108 ± 1 | 28 ± 2 |
| 0.15 | 111 ± 1 | 28 ± 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sportelli, M.C.; Valentini, M.; Picca, R.A.; Milella, A.; Nacci, A.; Valentini, A.; Cioffi, N. New Insights in the Ion Beam Sputtering Deposition of ZnO-Fluoropolymer Nanocomposites. Appl. Sci. 2018, 8, 77. https://doi.org/10.3390/app8010077
Sportelli MC, Valentini M, Picca RA, Milella A, Nacci A, Valentini A, Cioffi N. New Insights in the Ion Beam Sputtering Deposition of ZnO-Fluoropolymer Nanocomposites. Applied Sciences. 2018; 8(1):77. https://doi.org/10.3390/app8010077
Chicago/Turabian StyleSportelli, Maria Chiara, Marco Valentini, Rosaria Anna Picca, Antonella Milella, Angelo Nacci, Antonio Valentini, and Nicola Cioffi. 2018. "New Insights in the Ion Beam Sputtering Deposition of ZnO-Fluoropolymer Nanocomposites" Applied Sciences 8, no. 1: 77. https://doi.org/10.3390/app8010077