Effect of HCV Core Antigen and RNA Clearance during Therapy with Direct Acting Antivirals on Hepatic Stiffness Measured with Shear Wave Elastography in Patients with Chronic Viral Hepatitis C
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment Regimens
2.3. Study Design
2.4. HCV RNA and HCVcAg Measurement
2.5. Other Laboratory Measures
2.6. Liver Stiffness
2.7. Statistical Analysis
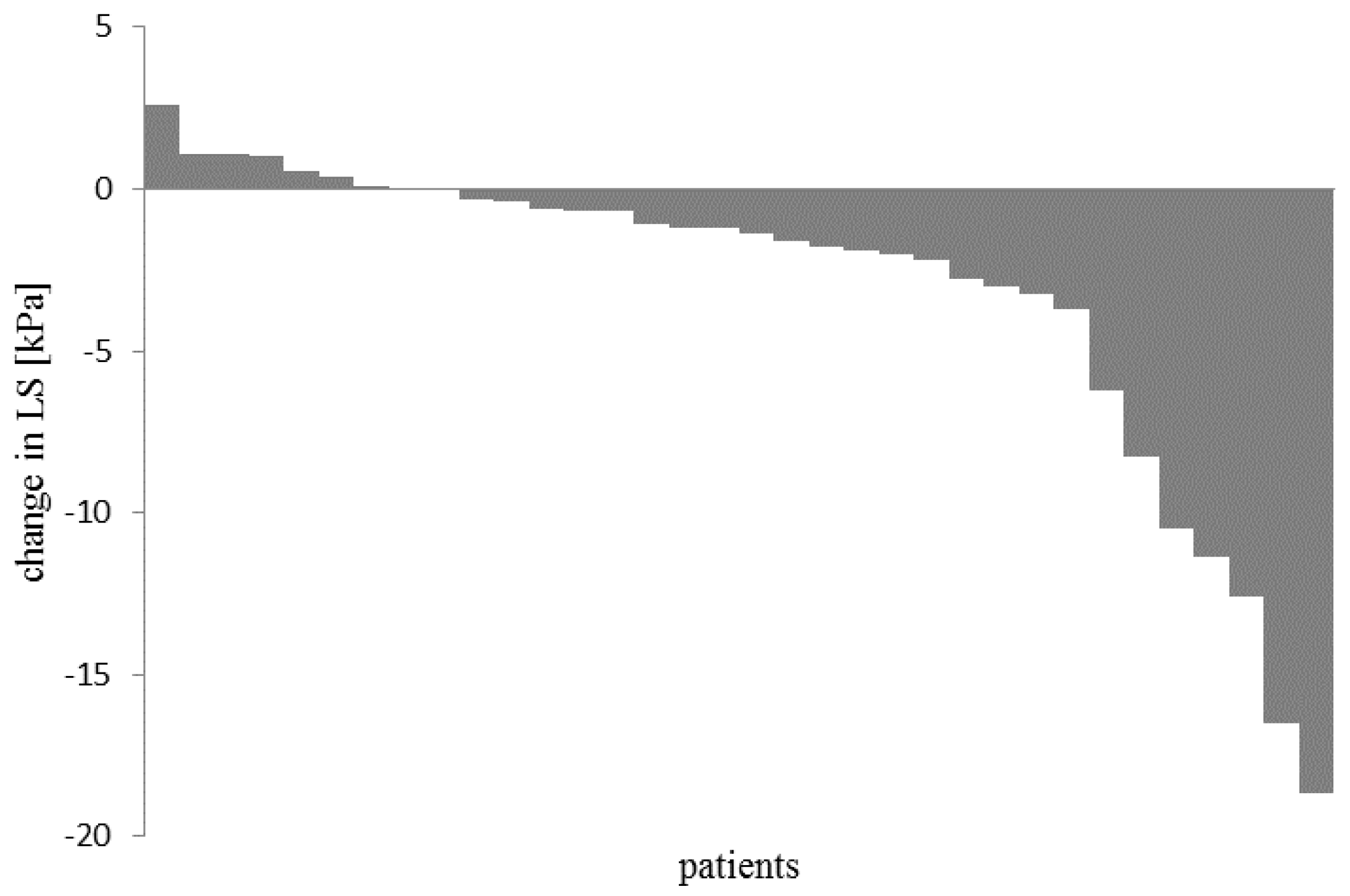
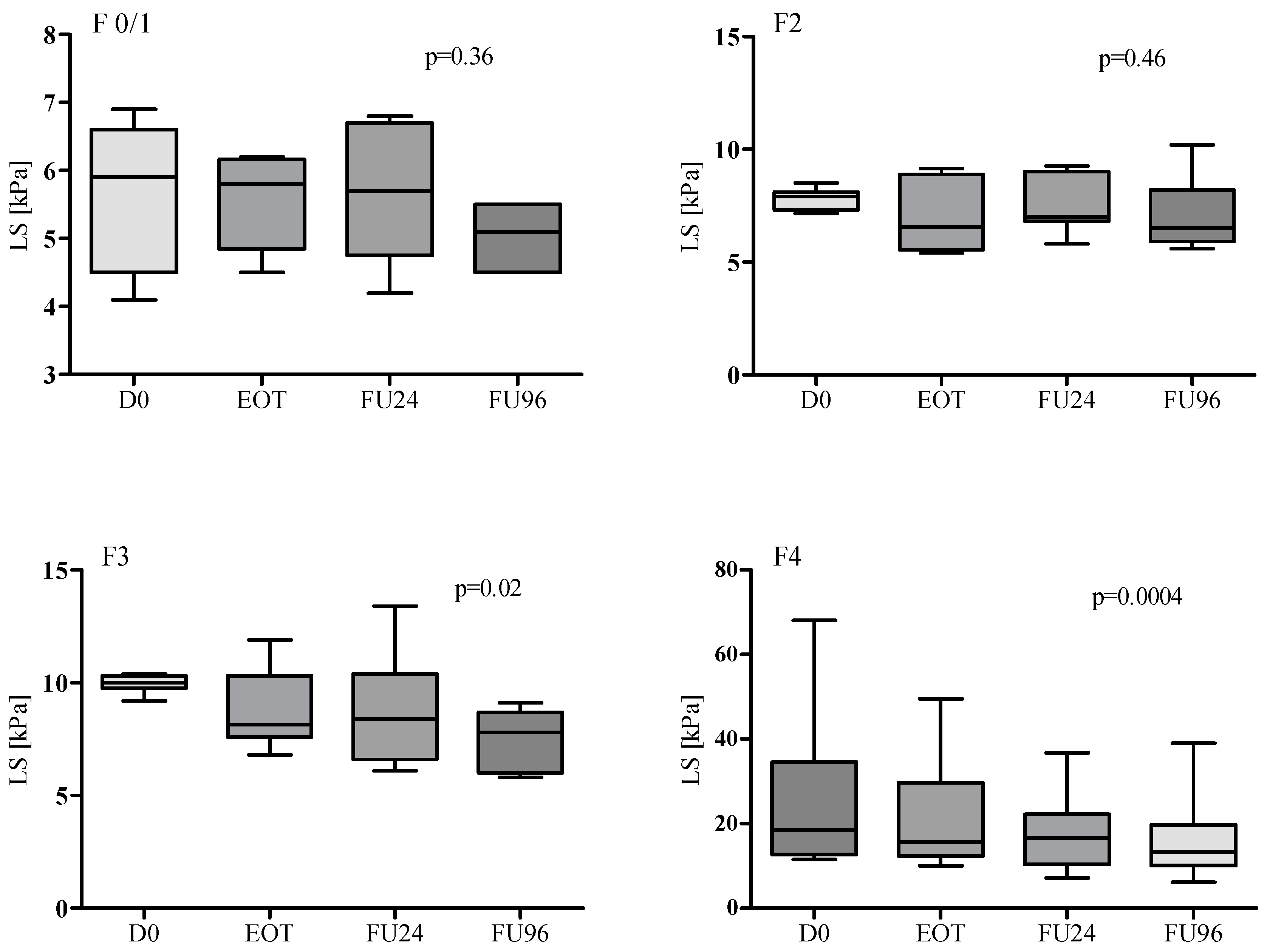
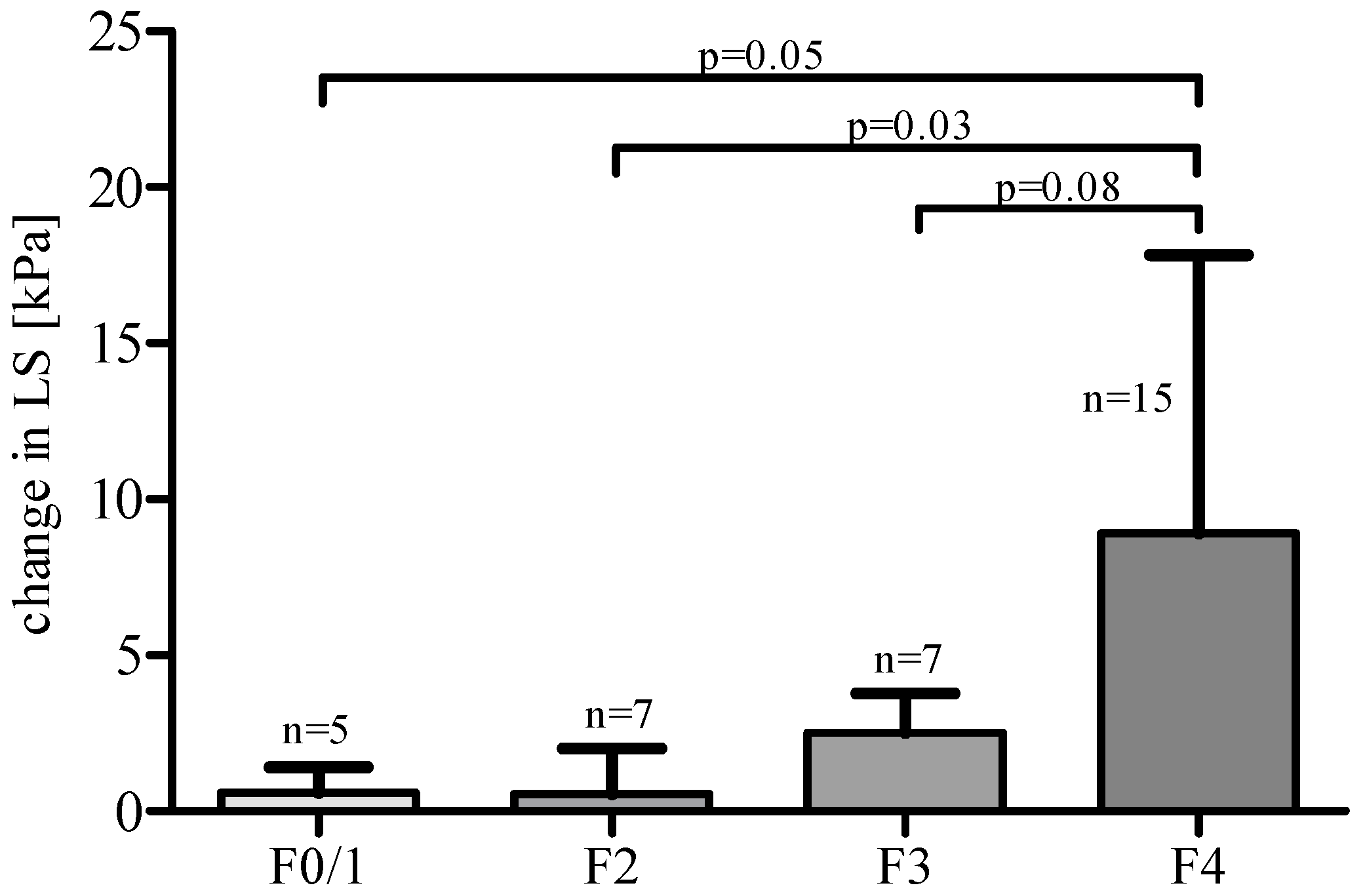
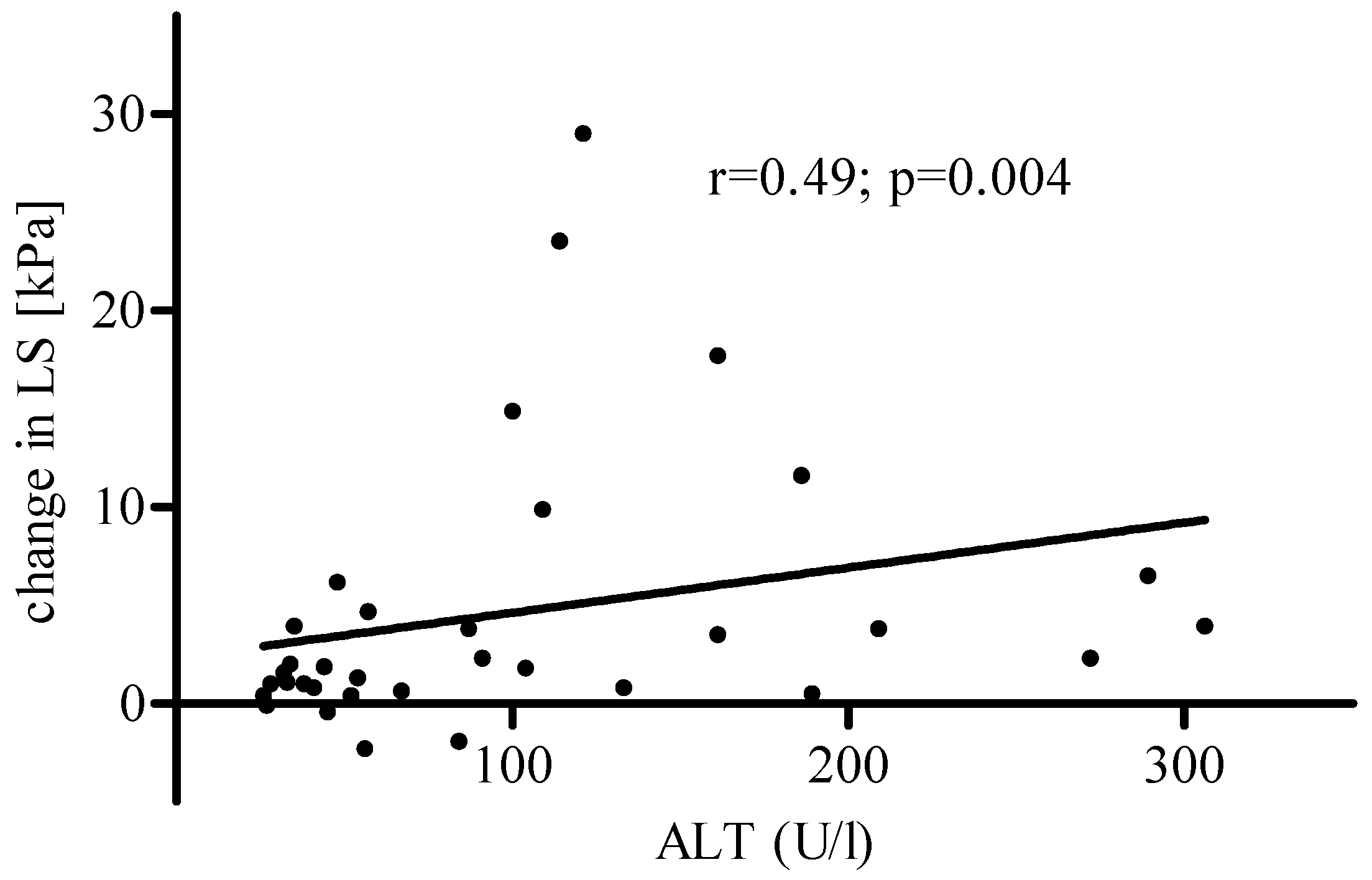
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blach, S.; Zeuzem, S.; Manns, M. Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef]
- Alkhouri, N.; Lawitz, E.; Poordad, F. Novel treatments for chronic hepatitis C: Closing the remaining gaps. Curr. Opin. Pharmacol. 2017, 37, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.R.; Servidone, M.; Easterbrook, P.; Linas, B.P. Economic evaluation of HCV testing approaches in low and middle income countries. BMC Infect. Dis. 2017, 17 (Suppl. 1), 697. [Google Scholar] [CrossRef] [PubMed]
- Jülicher, P.; Galli, C. Identifying cost-effective screening algorithms for active hepatitis C virus infections in a high prevalence setting. J. Med. Econ. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hullegie, S.J.; GeurtsvanKessel, C.H.; Van der Eijk, A.A.; Ramakers, C.; Rijnders, B.J.A. HCV antigen instead of RNA testing to diagnose acute HCV in patients treated in the Dutch Acute HCV in HIV Study. J. Int. AIDS 2017, 20, 21621. [Google Scholar] [CrossRef] [PubMed]
- Duchesne, L.; Njouom, R.; Lissock, F.; Tamko-Mella, G.F.; Rallier, S.; Poiteau, L.; Soulier, A.; Chevaliez, S.; Vernet, G.; Rouveau, N.; et al. HCV Ag quantification as a one-step procedure in diagnosing chronic hepatitis C infection in Cameroon: The ANRS 12336 study. J. Int. AIDS Soc. 2017, 20, 21446. [Google Scholar] [CrossRef] [PubMed]
- Łucejko, M.; Flisiak, R. Quantitative measurement of HCV core antigen for management of interferon-free therapy in HCV infected patients. Antivir. Ther. 2017. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Recommendations on Treatment of Hepatitis C 2016. J. Hepatol. 2017, 66, 153–194. [Google Scholar] [CrossRef]
- Bravo, A.A.; Sheth, S.G.; Chopra, S. Liver biopsy. N. Engl. J. Med. 2001, 344, 495–500. [Google Scholar] [CrossRef] [PubMed]
- The French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 1994, 20, 15–20. [Google Scholar]
- Bedossa, P.; Dargere, D.; Paradis, V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003, 38, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Piccinino, F.; Sagnelli, E.; Pasquale, G.; Giusti, G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J. Hepatol. 1986, 2, 165–173. [Google Scholar] [CrossRef]
- Friedrich-Rust, M.; Poynard, T.; Castera, L. Critical comparison of elastography methods to assess chronic liver disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Castera, L.; Chan, H.L.Y.; Arrese, M.; The Clinical Practice Guideline Panel. EASL-ALEH clinical practice guidelines: Noninvasive tests for evaluation of liver disease severity and prognosis. J. Hepatol. 2015, 63, 237–264. [Google Scholar]
- Sandrin, L.; Fourquet, B.; Hasquenoph, J.M. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 2003, 29, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Bercoff, J.; Tanter, M.; Fink, M. Supersonic shear imaging: A new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Bavu, E.; Gennisson, J.-L.; Couade, M.; Bercoff, J.; Mallet, V.; Fink, M.; Badel, A.; Vallet-Pichard, A.; Nalpas, B.; Tanter, M.; et al. Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: A clinical study on 113 hepatitis C virus patients. Ultrasound Med. Biol. 2011, 37, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.A.; Gurusamy, K.S.; Ntaoula, S.; Cholongitas, E.; Davidson, B.R.; Burroughs, A.K. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: A meta-analysis of diagnostic accuracy. J. Hepatol. 2011, 54, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Deffieux, T.; Gennisson, J.L.; Bousquet, L.; Corouge, M.; Cosconea, S.; Amroun, D.; Tripon, S.; Terris, B.; Mallet, V.; Sogni, P.; et al. Investigating liver stiffness and viscosity for fibrosis, steatosis and activity staging using shear wave elastography. J. Hepatol. 2015, 62, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, N.; Kobayashi, M.; Akuta, N.; Kominami, Y.; Fujiyama, S.; Kawamura, Y.; Sezaki, H.; Hosaka, T.; Suzuki, F.; Saitoh, S.; et al. Serial changes in liver stiffness and controlled attenuation parameter following direct-acting antiviral therapy against hepatitis C virus genotype 1b. J. Med. Virol. 2018, 90, 313–331. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, A.; Alem, S.A.; Fouad, R.; El Raziky, M.; El Akel, W.; Abdo, M.; Tantawi, O.; AbdAllah, M.; Bourliere, M.; Esmat, G. Changes in liver stiffness measurements and fibrosis scores following sofosbuvir based treatment regimens without interferon. J. Gastroenterol. Hepatol. 2017, 32, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Cassinotto, C.; Lapuyade, B.; Mouries, A.; Hiriart, J.B.; Vergniol, J.; Gaye, D.; Castain, C.; Le Bail, B.; Chermak, F.; Foucher, J.; et al. Non-invasive assessment of liver fibrosis with impulse elastography: Comparison of supersonic shear imaging with ARFI and FibroScanVR. J. Hepatol. 2014, 61, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Guibal, A.; Renosi, G.; Rode, A.; Scoazec, J.Y.; Guillaud, O.; Chardon, L.; Munteanu, M.; Dumortier, J.; Collin, F.; Lefort, T.; et al. Shear wave elastography: An accurate technique to stage liver fibrosis in chronic liver diseases. Diagn. Interv. Imaging 2016, 97, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, R.; Pogorzelska, J.; Berak, H.; Horban, A.; Orłowska, I.; Simon, K.; Tuchendler, E.; Madej, G.; Piekarska, A.; Jabłkowski, M.; et al. Prevalence of HCV genotypes in Poland—The EpiTer study. Clin. Exp. Hepatol. 2016, 2, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Polish Group of HCV Experts; Halota, W.; Flisiak, R.; Boroń-Kaczmarska, A.; Juszczyk, J.; Małkowski, P.; Pawłowska, M.; Simon, K.; Tomasiewicz, K. Recommendations for the treatment of hepatitis C issued by the Polish Group of HCV Experts—2016. Clin. Exp. Hepatol. 2016, 2, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Tinelli, C.; Dal Bello, B.; Zicchetti, M.; Filice, G.; Filice, C. Liver Fibrosis Study Group. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: A pilot study. Hepatology 2012, 56, 2125–2133. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Gennisson, J.-L.; Deffieux, T.; Tanter, M.; Fink, M. Quantitative viscoelasticity mapping of human liver using supersonic shear imaging: Preliminary in vivo feasibility study. Ultrasound Med. Biol. 2009, 35, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Sarvazyan, A.P. A new approach to remote ultrasonic evaluation of viscoelastic properties of tissues for diagnostics and healing monitoring. In Proceedings of the ARPA/ONR Medical Ultrasonic Imaging Technology Workshop, Landsdowne, VA, USA, 24–26 January 1995. [Google Scholar]
- Rudenko, O.V.; Sarvazyan, A.P.; Emelianov, S.Y. Acoustic radiation force and streaming induced by focused nonlinear ultrasound in a dissipative medium. J. Acoust. Soc. Am. 1996, 99, 2791–2798. [Google Scholar] [CrossRef]
- Poynard, T.; Pham, T.; Perazzo, H.; Munteanu, M.; Luckina, E.; Elaribi, D.; Ngo, Y.; Bonyhay, L.; Seurat, N.; Legroux, M.; et al. Real-Time Shear Wave versus Transient Elastography for Predicting Fibrosis: Applicability, and Impact of Inflammation and Steatosis. A Non-Invasive Comparison. PLoS ONE 2016, 11, e0163276. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.T.; Lim, S.M.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.H.; Chon, C.Y.; Cho, M.; Lee, J.W.; Kim, S.U. Liver stiffness measurement using acoustic radiation force impulse (ARFI) elastography and effect of necroinflammation. Dig. Dis. Sci. 2012, 57, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Arena, U.; Vizzutti, F.; Corti, G.; Ambu, S.; Stasi, C.; Bresci, S.; Moscarella, S.; Boddi, V.; Petrarca, A.; Laffi, G.; et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 2008, 47, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Coco, B.; Oliveri, F.; Maina, A.M.; Ciccorossi, P.; Sacco, R.; Colombatto, P.; Bonino, F.; Brunetto, M.R. Transient elastography: A new surrogate marker of liver fibrosis influenced by major changes of transaminases. J. Viral Hepat. 2007, 14, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Chekuri, S.; Nickerson, J.; Bichoupan, K.; Sefcik, R.; Doobay, K.; Chang, S.; DelBello, D.; Harty, A.; Dieterich, D.T.; Perumalswami, P.V.; et al. Liver Stiffness Decreases Rapidly in Response to Successful Hepatitis C Treatment and Then Plateaus. PLoS ONE 2016, 11, e0159413. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Changchien, C.S.; Hung, C.H.; Tung, W.C.; Kee, K.M.; Chen, C.H.; Hu, T.H.; Lee, C.M.; Lu, S.N. Liver stiffness decrease after effective antiviral therapy in patients with chronic hepatitis C: Longitudinal study using FibroScan. J. Gastroenterol. Hepatol. 2010, 25, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Kawabe, N.; Hashimoto, S.; Harata, M.; Nitta, Y.; Murao, M.; Nakano, T.; Shimazaki, H.; Kobayashi, K.; Ichino, N.; et al. Reduction of liver stiffness by interferon treatment in the patients with chronic hepatitis, C. Hepatol. Res. 2010, 40, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Hezode, C.; Castera, L.; Roudot-Thoraval, F.; Bouvier-Alias, M.; Rosa, I.; Roulot, D.; Leroy, V.; Mallat, A.; Pawlotsky, J.M. Liver stiffness diminishes with antiviral response in chronic hepatitis, C. Aliment. Pharmacol. Ther. 2011, 34, 656–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ANRS CO13 HEPAVIH Cohort. Regression of liver stiffness after sustained hepatitis C virus (HCV) virological responses among HIV/HCV—Coinfected patients. Aids 2015, 29, 1821–1830. [Google Scholar] [CrossRef]
- Tada, T.; Kumada, T.; Toyoda, H.; Mizuno, K.; Sone, Y.; Kataoka, S.; Hashinokuchi, S. Improvement of liver stiffness in patients with hepatitis C virus infection who received direct-acting antiviral therapy and achieved sustained virological response. J. Gastroenterol. Hepatol. 2017, 32, 198–1988. [Google Scholar] [CrossRef] [PubMed]
- Tachi, Y.; Hirai, T.; Kojima, Y.; Ishizu, Y.; Honda, T.; Kuzuya, T.; Hayashi, K.; Ishigami, M.; Goto, H. Liver stiffness reduction correlates with histological characteristics of hepatitis C patients with sustained virological response. Liver Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, Y.; Imazeki, F.; Moriyama, M.; Yano, M.; Arakawa, Y.; Yokosuka, O.; Kuroki, T.; Nishiguchi, S.; Sata, M.; Yamada, G.; et al. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann. Intern. Med. 2000, 132, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Veldt, B.J.; Saracco, G.; Boyer, N.; Cammà, C.; Bellobuono, A.; Hopf, U.; Castillo, I.; Weiland, O.; Nevens, F.; Hansen, B.E.; et al. Long term clinical outcome of chronic hepatitis C patients with sustained virological response to interferon monotherapy. Gut 2004, 53, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Toccaceli, F.; Laghi, V.; Capurso, L.; Koch, M.; Sereno, S.; Scuderi, M. Long-term liver histology improvement in patients with chronic hepatitis C and sustained response to interferon. J. Viral Hepat. 2003, 10, 126–133. [Google Scholar] [CrossRef] [PubMed]
- George, S.L.; Bacon, B.R.; Brunt, E.M.; Mihindukulasuriya, K.L.; Hoffmann, J.; Di Bisceglie, A.M. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: A 5-year follow-up of 150 patients. Hepatology 2009, 49, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Miyaki, E.; Imamura, M.; Hiraga, N.; Murakami, E.; Kawaoka, T.; Tsuge, M.; Hiramatsu, A.; Kawakami, Y.; Aikata, H.; Hayes, C.N.; et al. Daclatasvir and asunaprevir treatment improves liver function parameters and reduces liver fibrosis markers in chronic hepatitis C patients. Hepatol. Res. 2016, 46, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Deterding, K.; Honer Zu Siederdissen, C.; Port, K.; Solbach, P.; Sollik, L.; Kirschner, J.; Mix, C.; Cornberg, J.; Worzala, D.; Mix, H.; et al. Improvement of liver function parameters in advanced HCV-associated liver cirrhosis by IFN-free antiviral therapies. Aliment. Pharmacol. Ther. 2015, 42, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Tachi, Y.; Hirai, T.; Ishizu, Y.; Honda, T.; Kuzuya, T.; Hayashi, K.; Ishigami, M.; Goto, H. α-fetoprotein levels after interferon therapy predict regression of liver fibrosis in patients with sustained virological response. J. Gastroenterol. Hepatol. 2016, 31, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Patton, H.M.; Patel, K.; Behling, C.; Bylund, D.; Blatt, L.M.; Vallee, M.; Heaton, S.; Conrad, A.; Pockros, P.J.; McHutchison, J.G.; et al. The impact of steatosis on disease progression and early and sustained treatment response in chronic hepatitis C patients. J. Hepatol. 2004, 40, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, K.; Ohue, C.; Iida, K.; Kimura, T.; Tanaka, E.; Kiyosawa, K.; Yagi, S. Development of a simple and highly sensitive enzyme immunoassay for hepatitis C virus core antigen. J. Clin. Microbiol. 1999, 37, 1802–1808. [Google Scholar] [PubMed]
- Mederacke, I.; Potthoff, A.; Meyer-Olson, D.; Meier, M.; Raupach, R.; Manns, M.P.; Wedemeyer, H.; Tillmann, H.L. HCV core antigen testing in HIV- and HBV-coinfected patients, and in HCV-infected patients on hemodialysis. J. Clin. Virol. 2012, 53, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Łucejko, M.; Grzeszczuk, A.; Jaroszewicz, J.; Flisiak, R. Serum HCV core antigen concentration in HCV monoinfection and HCV/HIV coinfection. Pol. Merkur. Lekarski 2013, 35, 72–76. [Google Scholar] [PubMed]
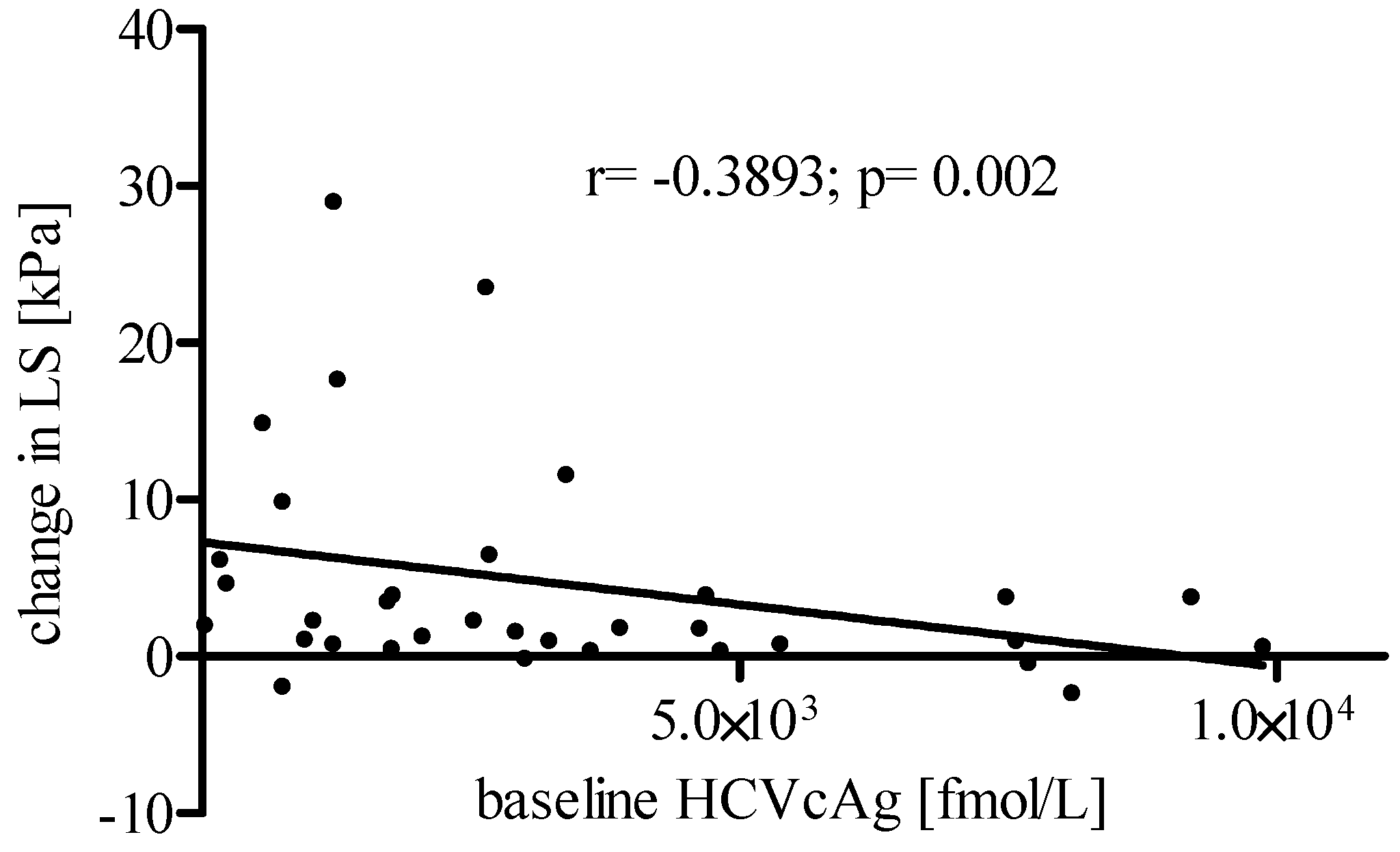
| Characteristics | All; N = 34 |
|---|---|
| Age, years [median (IQR)] | 50 (41.5–58.5) |
| Males [n; %] | 10; 59 |
| BMI, kg/m2 [median (IQR)] | 26.2 (23.2–28.6) |
| HCV RNA, log10 IU/mL [median (IQR)] | 5.9 (5.6–6.3) |
| HCVcAg, fmol/L [median (IQR)] | 2653 (1168–4716) |
| HCV genotype 1a/1b/3a/4 [%] | 6/88/0/6 |
| Prior HCV treatment history | - |
| null response [n; %] | 8; 24 |
| partial response [n; %] | 4; 12 |
| relapse [n; %] | 9; 26 |
| naive [n; %] | 12; 35 |
| unknown [n; %] | 1; 3 |
| PLT—× 103 cell/mm3 [median (IQR)] | 154 (91–201) |
| Hemoglobin—g/dL [median (IQR)] | 15.1 (13.73–15.9) |
| Albumin—mg/dL [median (IQR)] | 4.3 (3.9–4.7) |
| ALT—IU/mL [median (IQR)] | 75.5 (40.3–140) |
| Bilirubin—mg/dL [median (IQR)] | 0.7 (0.5–0.9) |
| AFP—ng/dL [median (IQR)] | 6.2 (2.8–20.2) |
| Liver stiffness—kPa [median (IQR)] | 10.2 (7.8–17.9) |
| Liver cirrhosis [n;%] | 14; 42 |
| MELD score [median (min–max)] | 7 (6–15) |
| Child Pugh score [median (min–max)] | 5 (5–9) |
| N = 34 | Baseline | EOT | p | FU24 | p | FU96 | p |
|---|---|---|---|---|---|---|---|
| [me dian; IQR] | - | - | baseline vs. EOT | - | baseline vs. FU24 | - | baseline vs. FU96 |
| HCV RNA log10 IU/mL | 5.9 (5.6–6.3) | 0 | <0.0001 | 0 | <0.0001 | 0 | <0.0001 |
| HCVcAg fmol/L | 2653 (1168–4716) | 0 | <0.0001 | 0 | <0.0001 | - | - |
| Albumin mg/dL | 4.3 (3.9–4.7) | 4.5 (4.2–4.8) | 0.05 | 4.8 (4.4–5) | <0.0001 | 4.7 (4.5–4.9) | <0.0001 |
| Bilirubin mg/dL | 0.7 (0.5–0.9) | 0.7 (0.4–1.2) | ns | 0.6 (0.4–0.8) | 0.008 | 0.6 (0.4–0.9) | 0.05 |
| Creatinine mg/dL | 0.685 (0.585–0.765) | 0.715 (0.610–0.810) | 0.08 | 0.725 (0.623–0.803) | 0.03 | 0.820 (0.745–0.890) | <0.0001 |
| Liver stiffness kPa | 10.2 (7.8–17.9) | 9.8 (6.5–13.9) | 0.008 | 9.1 (6.8–15.6) | 0.008 | 8.2 (5.9–13.2) | <0.0001 |
| ALT IU/mL | 75.5 (40.25–140) | 24 (16–30.25) | <0.0001 | 22 (15.75–32.25) | <0.0001 | - | - |
| PLT × 103 cell/mm3 | 154 (91–201) | 167 (108.3–219.5) | 0.04 | 155 (97.8–207.5) | ns | - | - |
| Hemoglobin g/dL | 15.1 (13.7–15.9) | 14.1 (12.5–15.6) | 0.009 | 15.1 (14.1–16.4) | ns | - | - |
| AFP ng/dL | 6.2 (2.8–20.2) | 3.3 (2.3–4.7) | <0.0001 | 3.4 (2.4–4.8) | <0.0001 | - | - |
| p-Values | Baseline | EOT | FU24 | FU96 |
|---|---|---|---|---|
| baseline | x | x | x | x |
| EOT | 0.008 | x | x | x |
| FU24 | 0.008 | 0.09 | x | x |
| FU96 | <0.0001 | 0.0002 | 0.005 | x |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łucejko, M.; Flisiak, R. Effect of HCV Core Antigen and RNA Clearance during Therapy with Direct Acting Antivirals on Hepatic Stiffness Measured with Shear Wave Elastography in Patients with Chronic Viral Hepatitis C. Appl. Sci. 2018, 8, 198. https://doi.org/10.3390/app8020198
Łucejko M, Flisiak R. Effect of HCV Core Antigen and RNA Clearance during Therapy with Direct Acting Antivirals on Hepatic Stiffness Measured with Shear Wave Elastography in Patients with Chronic Viral Hepatitis C. Applied Sciences. 2018; 8(2):198. https://doi.org/10.3390/app8020198
Chicago/Turabian StyleŁucejko, Mariusz, and Robert Flisiak. 2018. "Effect of HCV Core Antigen and RNA Clearance during Therapy with Direct Acting Antivirals on Hepatic Stiffness Measured with Shear Wave Elastography in Patients with Chronic Viral Hepatitis C" Applied Sciences 8, no. 2: 198. https://doi.org/10.3390/app8020198




