1. Introduction
Volatile organic compounds (VOCs) are a wide array of hydrocarbon-based gases and vapors that are known as one of the major contributors to air pollution. VOCs can be found in various industrial exhausts including, but not limited to, transportation, chemical industries, petrochemical plants, and petroleum refineries [
1]. VOCs’ high vapor pressure, low boiling point, and strong reactivity contribute to their photochemical reaction in the atmosphere [
2]. Many negative impacts of VOCs on human health and the environment, such as their contribution to the depletion of the ozone layer, photochemical ozone formation in the tropospheric layer, global greenhouse effect, and contribution to the formation of fine particulate matter (PM
2.5), have been reported [
3,
4]. Some VOCs are known carcinogenic, mutagenic, and neurotoxic chemicals [
5].
Due to the negative effects of VOCs on humans and the environment, and the fact that their concentration in the atmosphere is increasing (e.g., as a result of industrialization), environmental regulations aim to control and decrease the emission of VOCs into the environment. The Goteborg Protocol recommendation is to decrease the world’s VOC emission by 50% by 2020 compared to the base year of 2000 [
4,
6]. Accordingly, in recent years, great efforts have been made to develop new and more efficient techniques for VOC abatement from emission sources.
A wide array of physicochemical technologies, such as thermal and catalytic oxidation, photocatalytic oxidation [
7], biological degradation [
8,
9], condensation, adsorption, and absorption [
3], have been used for capturing VOCs from industrial exhausts [
10]. In comparison to other air pollution control technologies, adsorption-based technologies are being considered as simple, capable to manage multicomponent pollutants, efficient and cost effective process that can be used for capturing and recovering VOCs at different concentrations [
11,
12]. While adsorption can occur at all solid-gas interfaces, however, the capacity of adsorbents to capture the adsorbate molecules depends on the different characteristics of adsorbents including specific surface area, pore size distribution [
13,
14], pore volume, particle size, presence of functional groups, and a preferential affinity for the target adsorbate [
15].
Several categories of materials are known as efficient adsorbents for removal of different air pollutants, in which activated carbon, silica gel, alumina oxide, and several types of zeolites are the most common ones [
16]. While activated carbon is widely used, however, it has several disadvantages including: inflammability risk, low thermal stability, pores blockage, and problems associated with regeneration processes [
2,
11,
12,
17]. Activated carbon is not a selective adsorbent, and we cannot control and tune its pore size because of its amorphous structure [
18]. In order to overcome the problems associated with the commercially-available adsorbents (e.g., activated carbon), development of new porous materials as more efficient adsorbents is an emerging area of research with massive interest.
Metal organic frameworks (MOFs) or organic-inorganic hybrid solids, also known as porous coordination polymers (PCPs), have attracted great attention in recent years because of their unique structural chemistry including, but not limited to, high surface area, their possibility to incorporate functional groups, and tunable porosities [
19,
20]. MOFs can be used as adsorbents [
21,
22], and in catalysis [
23,
24,
25], drug delivery [
26], chemical sensors [
27], and for different energy applications [
28].
Zeolitic imidazole frameworks (ZIFs) are a subgroup of MOFs with a crystal structure similar to zeolites. In ZIF structure, a metal ion (M = Zn
2+ or Co
2+) replaces Si and Al tetrahedral and an imidazolate (Im) linker replaces the bridging oxygen in zeolites. The composed M-Im-M angle is ~145°, which is similar to the Si-O-Si angle of zeolite crystal structure [
29,
30,
31,
32].
Different types of ZIFs are synthesized in which ZIF-8 has gained remarkable attention because of its unique properties. ZIF-8 composed of Zn (II) linked with 2-methylimidazolate bridges forming a sodalite (SOD)-like structure similar to Y and X types of zeolite structures. ZIF-8’s porous structure is composed of large cavities with 1.16 nm diameters, connected through 0.34 nm pore openings [
29,
33,
34]. ZIF-8 has high thermal and chemical stability that cannot be seen in many MOFs and other ZIFs [
29,
31,
35]. ZIF-8 is stable in refluxing benzene, methanol, water, and aqueous sodium hydroxide for seven days at different temperatures, from 25 °C to the boiling point of each solvent [
31]. The crystal structure of ZIF-8 is stable in air for 5 h at 300 °C, in argon for 5 h at 400 °C, and in N
2 at 550 °C [
36,
37], and under pressure [
38].
For the first time, Yaghi et al. synthesized ZIF-8 in 2006 by dissolving 2-methylimidazol and zinc nitrate tetrahydrate in dimethylformamide (DMF) at 140 °C for 24 h [
31]. Trapping of DMF molecules inside the ZIF-8 cavities was one of the disadvantages of Yaghi’s method [
32]. Since then, many other research teams synthesized ZIF-8 using different solvents with smaller kinetic diameters [
32], such as methanol [
39], and straight and branched aliphatic alcohols [
40]. These organic solvents are toxic and expensive. Recently, a great deal of effort has been made to synthesize ZIF-8 in aqueous solution by using metal to ligand ratios of 2 (Zn:Hmim = 2) [
32,
41].
He et al. synthesized ZIF-8 from stoichiometric Zn and Hmim in the presence of ammonium hydroxide at room temperature with Zn:Hmim:NH
3:H
2O molar ratio of 1:2:32:157 [
32].
In this study, we synthesized ZIF-8 using an environmentally-friendly method in aqueous solution at room temperature. ZIF-8 samples were characterized using scanning electron microscopy (SEM), X-ray diffraction (XRD), and FT-IR techniques. The effect of humidity on adsorption properties of toluene on ZIF-8 samples were studied. The effect of humidity on adsorption capacity/efficiency is an important factor for application of sorbents in real conditions. The adsorption experiments were carried out in a fixed-bed adsorption setup. Adsorption behavior of the ZIF-8 samples (as synthesized and thermally-activated) toward toluene was studied at different RH. The experimental data were fitted into the Thomas and Yan mathematical models.
2. Materials and Methods
2.1. Sample Preparation
Zinc nitrate hexahydrate [Zn(NO3)2.6H2O], 2-methylimidazole (Hmim, C4H6N2), and ammonium hydroxide (NH3, 28–30% aqueous solution,) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All chemicals were used without further purification.
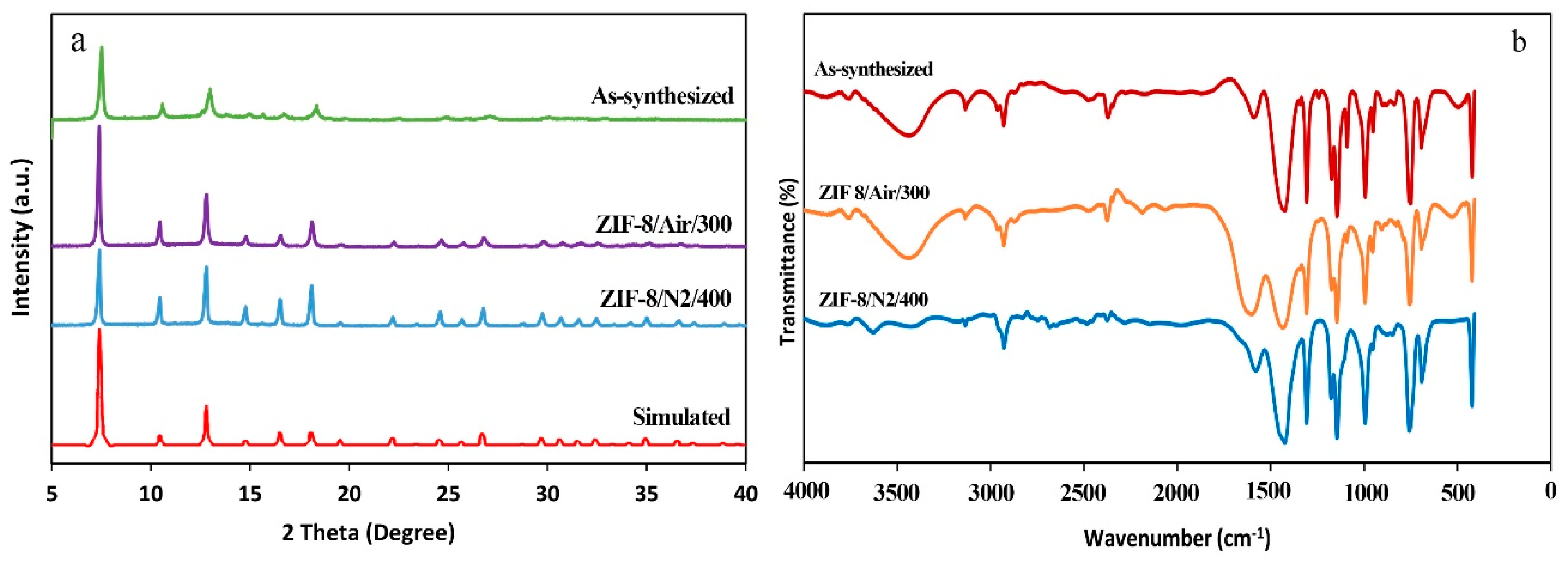
Synthesis of ZIF-8 was carried out according to a procedure reported by He et al. [
32]. Briefly, 1.18 g of Zn(NO
3)
2.6H
2O was dissolved in 6 mL of deionized (DI) water. Then 0.66 g of 2-methylimidazole was dissolved in 8.35 mL of ammonium hydroxide solution. The zinc solution was added slowly to the 2-methylimidazole solution under stirring. The mixture immediately converted to a milky suspension. Then stirred for another 10 min at room temperature. The milky suspension was centrifuged at 4000 rpm for 10 min and the supernatant was separated (decanted). In order to wash the synthesized ZIF-8, the product was dispersed in 60 mL DI water and centrifuged again. Washing was repeated three times. The washed product was dried at 60 °C overnight in an oven prior to conducting characterization analyses.
2.2. Characterization
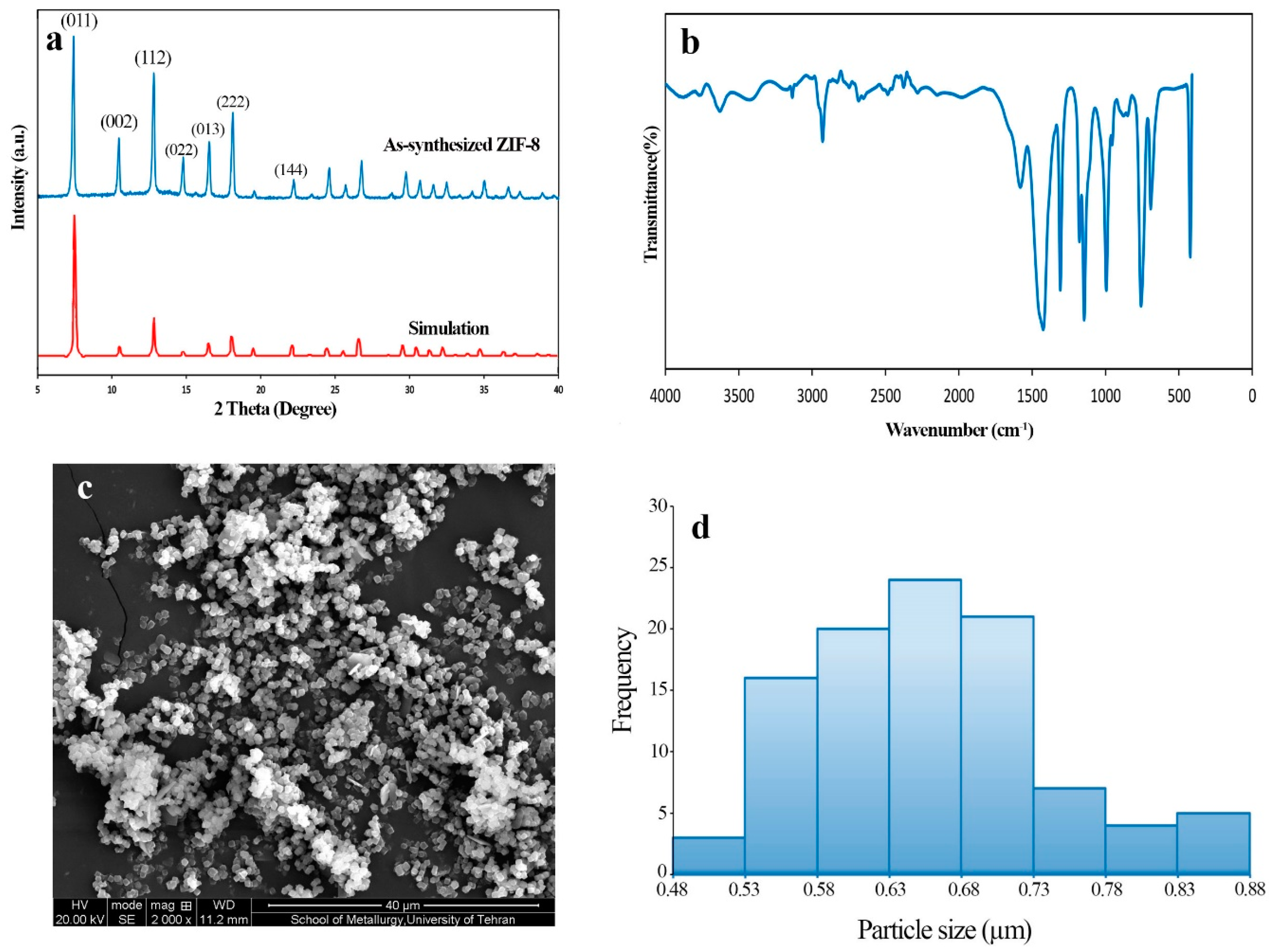
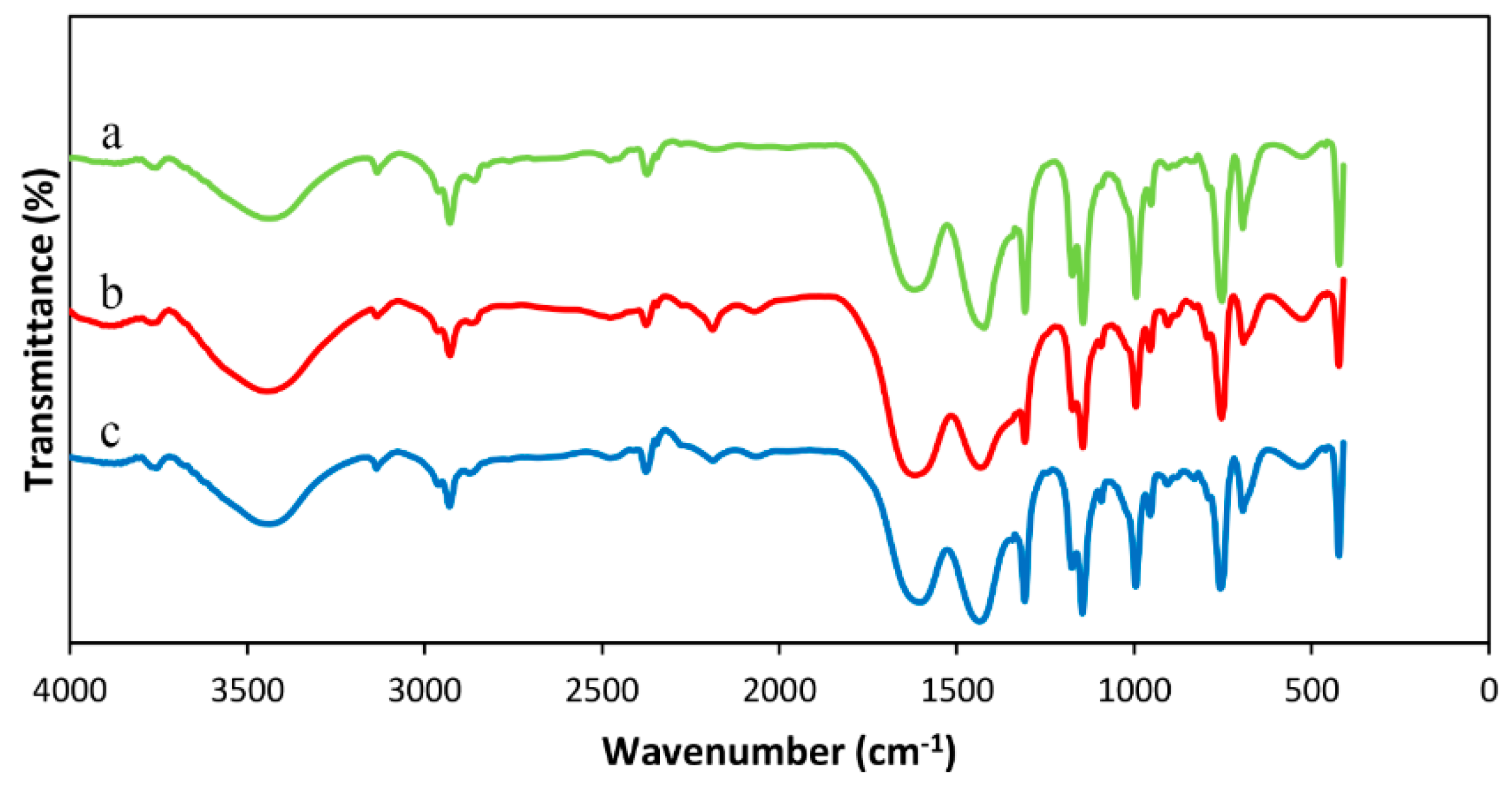
Crystallinity and purity of the synthesized samples were studies using X-ray diffraction (XRD). The XRD patterns were acquired using a D8 Advance instrument (Bruker, Karlsruhe, Germany) (Cu Kα radiation). All the XRD patterns were collected at a scan rate of 2/min and step size of 0.02° between 5 and 40 (2ϴ) degrees. Fourier transform infrared (FT-IR) spectra were examined to confirm the formation of ZIF-8 as well as to compare the effects of various condition on the samples structure. FT-IR spectra were attained using a PerkinElmer-Spectrum RXI infrared spectrometer (PerkinElmer, Waltham, MA, USA). Scanning electron microscopy (SEM) was performed to observe the morphology and determine particle sizes of the synthesized ZIF-8. SEM micrographs were obtained using a CamScan Device MV 2300 (CamScan, Kingston, ON, Canada) at an acceleration voltage of 20 kV. In order to conduct SEM tests, the samples were mounted on conductive carbon double-sided sticky tape and a thin layer of gold was coated on the samples to decrease charging effects. Mean, standard deviation, and the particle size distribution of ZIF-8 was measured manually using Photoshop software from the SEM image. The diameter of about 100 particles in the SEM image were measured to determine these parameters.
2.3. The Experimental Setup
Adsorption parameters of ZIF-8 were determined under various conditions using a fixed bed reactor. Known concentration of toluene vapor was generated by injection pure toluene (Merck, Kenilworth, NJ, USA, 99.99%) with the optimum constant flow rate into the dried and pure N2 (99.999) as the carrier gas. The injection of toluene was performed by a syringe pump (Chymex fusion 100, Houston, TX, USA) and N2 flow rate was controlled by mass flow controller (Alicat MC-Series, Tucson, AZ, USA). The relative humidity was generated by mixing water vapor with the carrier gas. For this purpose, a container with 20 mL distilled water was placed on a constant-temperature silicon oil bath. A PID-type thermostat (Autonics, Busan, Korea) controlled the temperature of the oil bath. In this condition, water vapor was generated with a constant rate. The adsorbent was packed into a quartz tube, with 15 cm length and 0.5 cm internal diameters, using glass wool on both sides to fix it. Toluene concentrations in the influent and effluent were measured using a gas chromatograph equipped with a CP-Sil 5 CB capillary column and a flame ionization detector (GC-FID; Varian CP 3800, Santa Clara, CA, USA).
2.4. Measurement of Toluene Dynamic Adsorption Capacity
Adsorption capacity of ZIF-8 samples toward toluene was measured using breakthrough curves obtained under different experimental conditions. The breakthrough curves are the concentration-time profile, which are expressed in term of
C/
C0 as a function of time (where
C is effluent and
C0 is influent concentration of the toluene). A known concentration of toluene was passed through the fixed bed of the adsorbent, and the concentration of toluene at effluent of the reactor was measured. The concentration of toluene in the feed was controlled at 1000 ppmv by using a 0.02 L∙min
−1 flow rate of the carrier gas (
Q). The amount of adsorbent (
M) in each experiment was 0.05 g, which was packed into the quartz tube. The time of the breakthrough adsorption
tb (min) and time of the equilibrium adsorption
te (min) were determined, while the ratio of influent and effluent toluene concentrations (
C/
C0) were equal to 0.05 and 0.95, respectively. Breakthrough capacity was measured using the following equation [
24,
42,
43]:
where
q is the breakthrough capacity (mg∙g
−1), which is indeed the total mass of toluene adsorbed on ZIF-8 (
M). In Equation (1) the first term is the total toluene in the influent gas relative to the mass of ZIF-8 in the duration of
te and the second term is the total mass of toluene in the effluent gas.
The total adsorption percentage of toluene (
A) was calculated using the following equation [
44]:
where
s is the adsorbed toluene (mg) on ZIF-8 and
x is the total amount of toluene in influent (mg) in the duration of
te.
2.5. Activation of ZIF-8
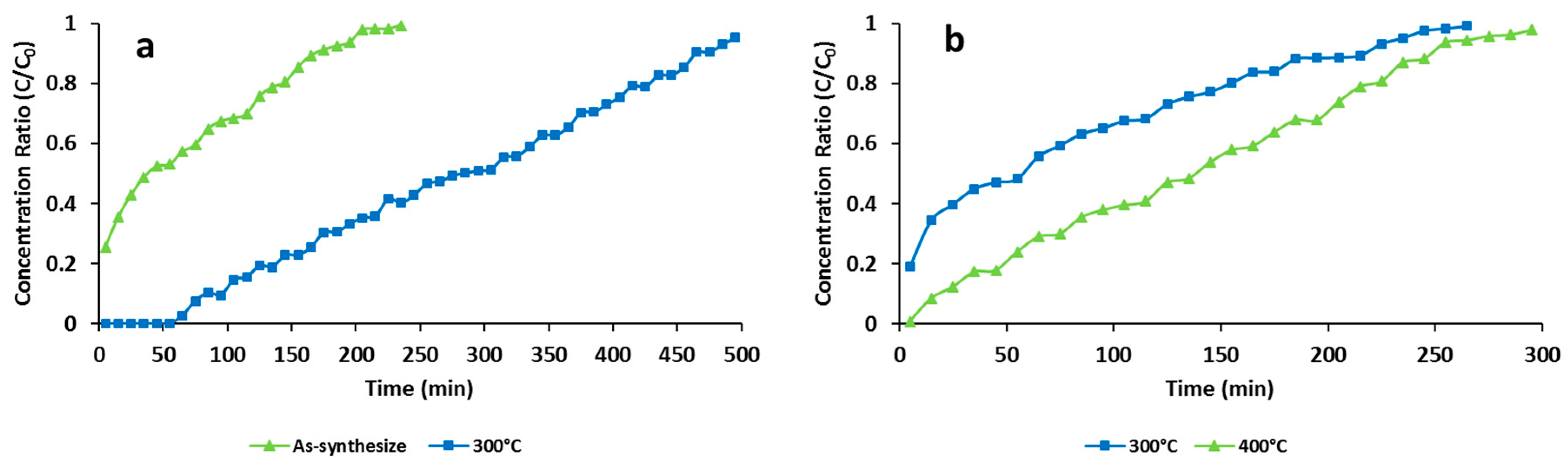
The adsorption performances of ZIF-8 were examined after pretreatment of adsorbents in various conditions and the results were compared with the performances of the as-synthesized ZIF-8. Pretreatment processes were performed in dry air and N
2 atmospheres. In each process 0.05 g of ZIF-8 was packed into the quartz tube and heated to anticipated temperatures under nitrogen or dried air at a flow rate of 0.02 L∙min
−1 for180 min. The adsorbents were heated in dry air at 300 °C, while the N
2 atmosphere was pretreated at 300 °C and 400 °C. Activated ZIF-8 samples were cooled to ambient temperature, then adsorption tests were carried out. The activation temperatures were selected based on the results of previous studies on ZIF-8’s thermal stability [
31,
36,
37,
45].
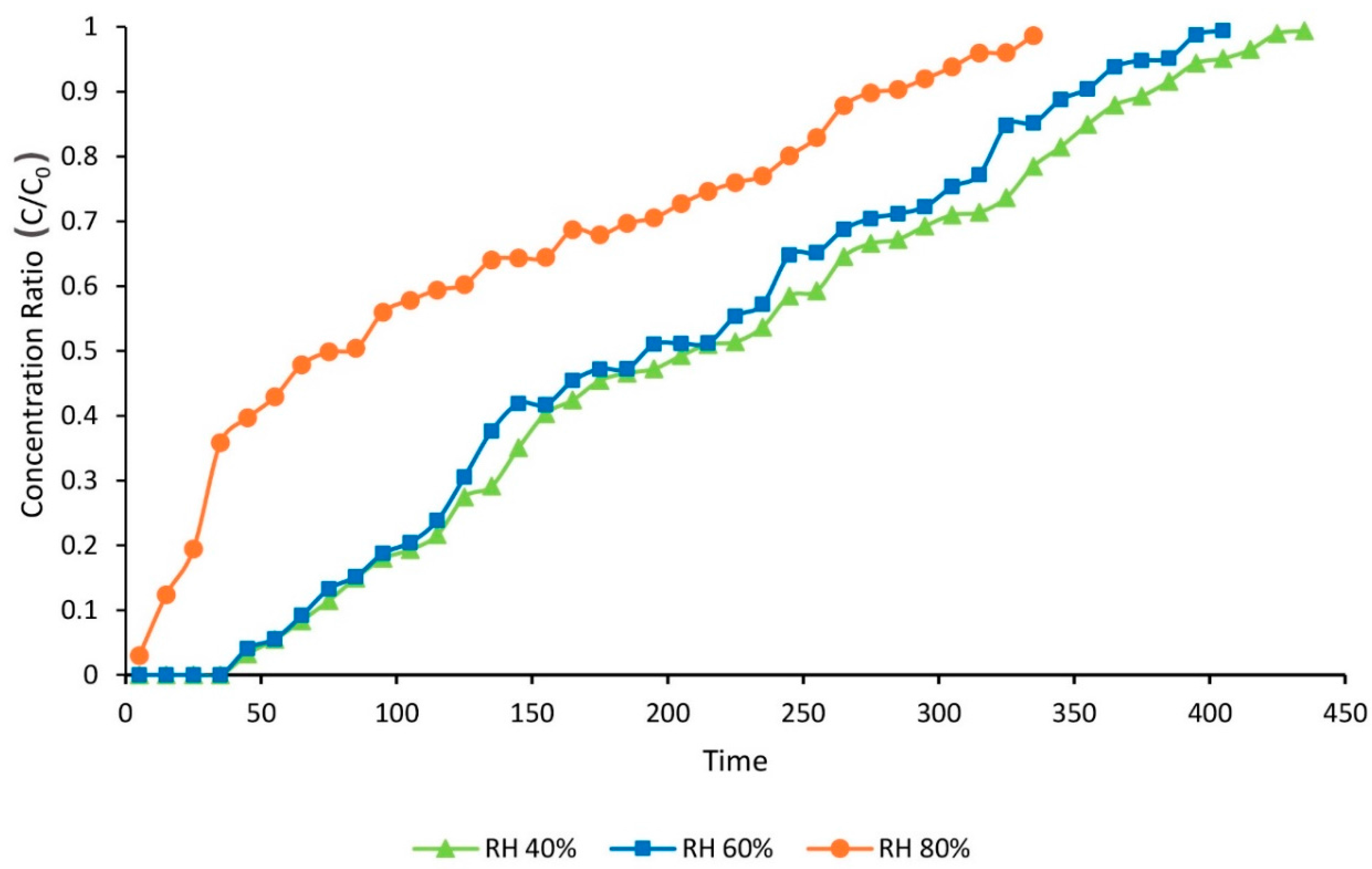
2.6. The Effect of RH
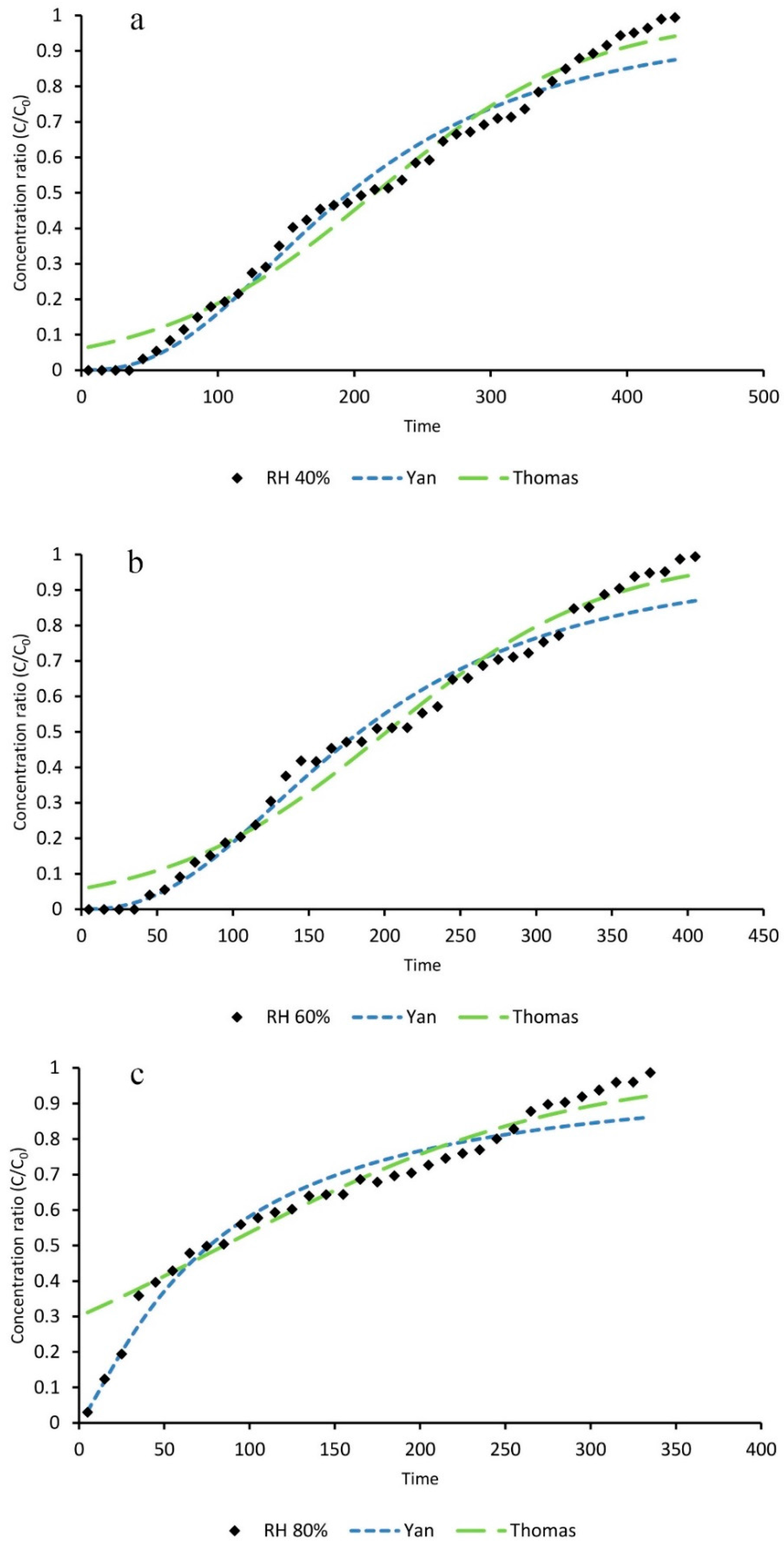
The effect of relative humidity (RH) on adsorption capacity, total adsorption percentage, time of breakthrough, and time of equilibration of toluene adsorption on ZIF-8 were studied. All samples were pretreated in the optimal condition.
The flow rate of carrier gas was set at 0.02 L∙min−1 by mass flow controller (MFC). The temperature of the silicon oil bath that contained a distilled water flask was adjusted so that the desired RH (40%, 60% and 80%) was achieved in the influent gas. A constant concentration of toluene (1000 ppmv) was achieved by adjusting the rate of toluene injection by a syringe pump. GC-FID analysis was used to check the toluene concentration.
4. Conclusions
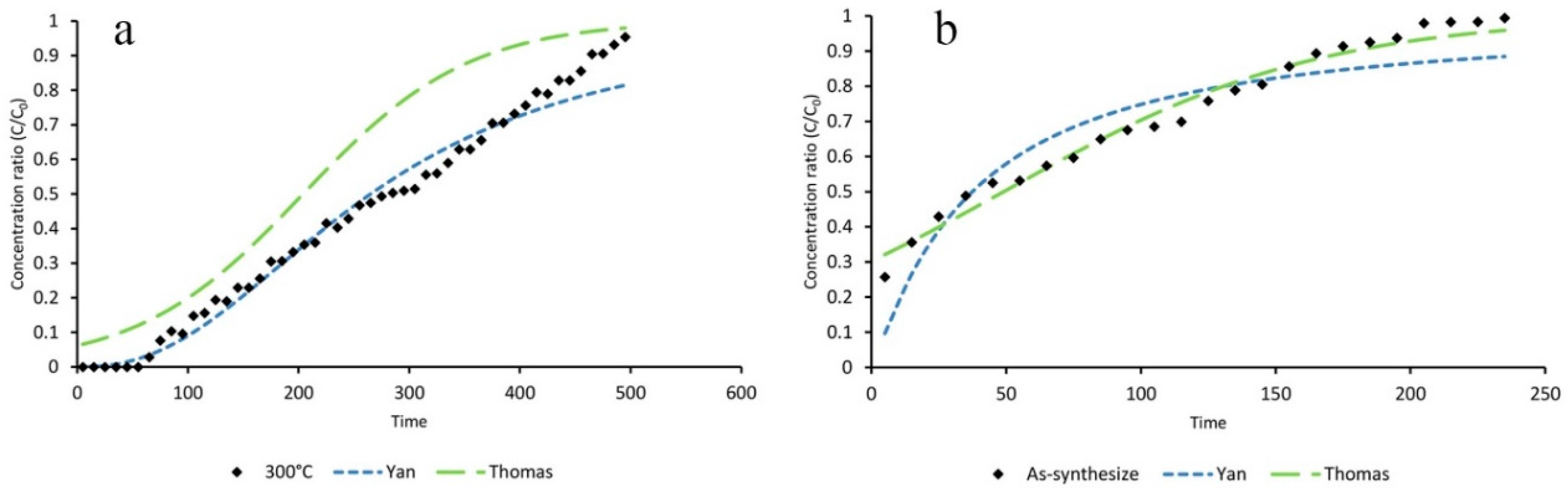
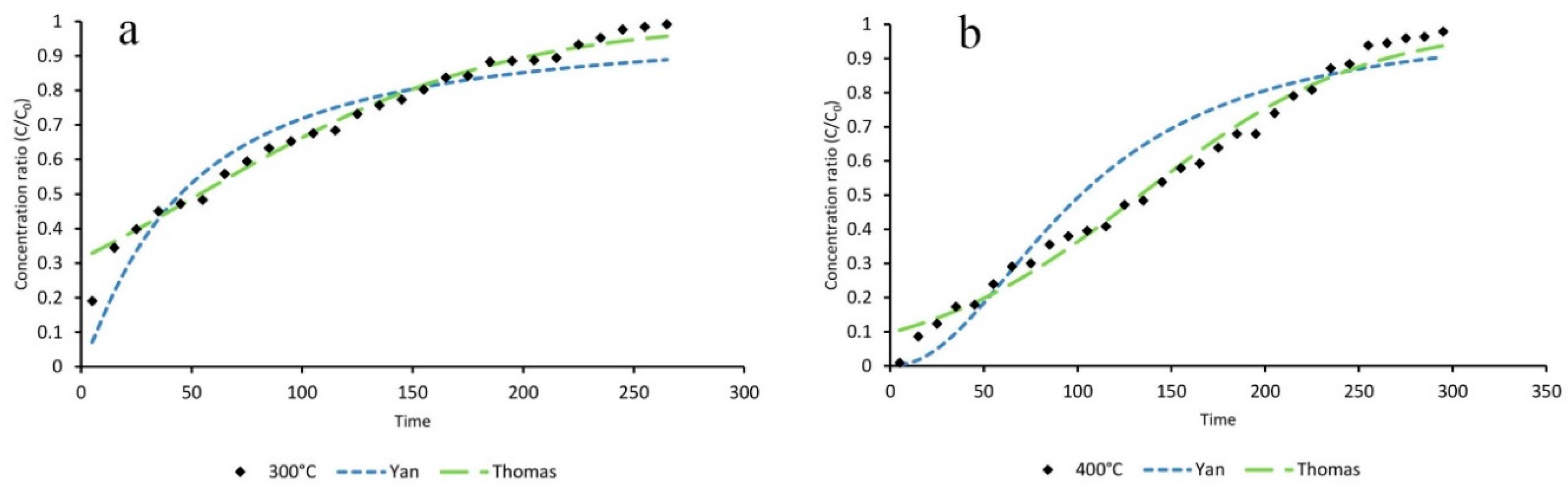
ZIF-8 was synthesized from aqueous solution, and should be considered as a facile and environmentally-friendly method. Adsorption of toluene by the ZIF-8 and its activated form was examined in a continuous mode using a fixed bed reactor. Adsorption parameters were calculated from the obtained breakthrough curves. The results of thermal activation under dry air and N2 atmospheres revealed that an oxidizing condition would result a ZIF-8 adsorbent with higher adsorption capacity for capturing toluene from polluted air streams. ZIF-8 activated under dry airflow at 300 °C for 3 h proved to be the most efficient adsorbent for toluene under the tested conditions.
Experimental data of the dynamic adsorption of toluene showed that toluene removal efficiencies of activated ZIF-8 did not change significantly by increasing the RH from 40% to 60%. However, its adsorption capacity dropped dramatically at RH higher than 80%. It can be concluded that activated ZIF-8 should be considered an efficient adsorbent for toluene removal at low and medium RH (e.g., 40–60%).
The breakthrough curve of toluene adsorption on activated ZIF-8 under different conditions were simulated by fitting the experimental data into the Yan and Thomas mathematical models. The statistical parameters, such as root mean square error (RMSE), sum square error (SSE), squared correlation coefficient (R2), and adjusted squared correlation coefficient (R2adj), were calculated and evaluated. It was concluded that both models could be applicable for curve fitting of toluene dynamic adsorption on activated ZIF-8 at different RH.