Surface Decorated Zn0.15Cd0.85S Nanoflowers with P25 for Enhanced Visible Light Driven Photocatalytic Degradation of Rh-B and Stability
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrothermal Synthesis of Pure CdS Nanoparticles and Zn0.15Cd0.85S Nanoflowers
2.3. Hydrothermal Synthesis of P25/Zn0.15Cd0.85S Nanocomposite
2.4. Photocatalytic Degradation of Rhodamine B (Rh-B) under Visible Light
2.5. Characterization
3. Results and Discussion
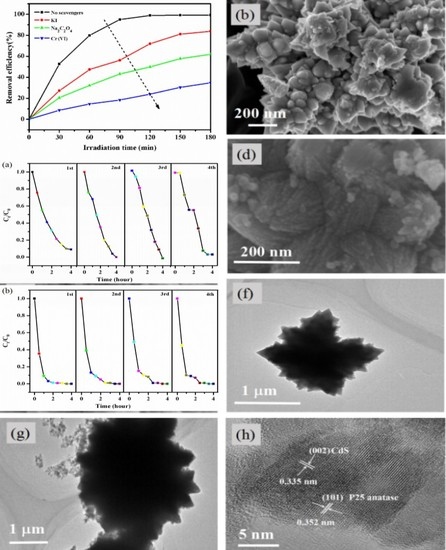
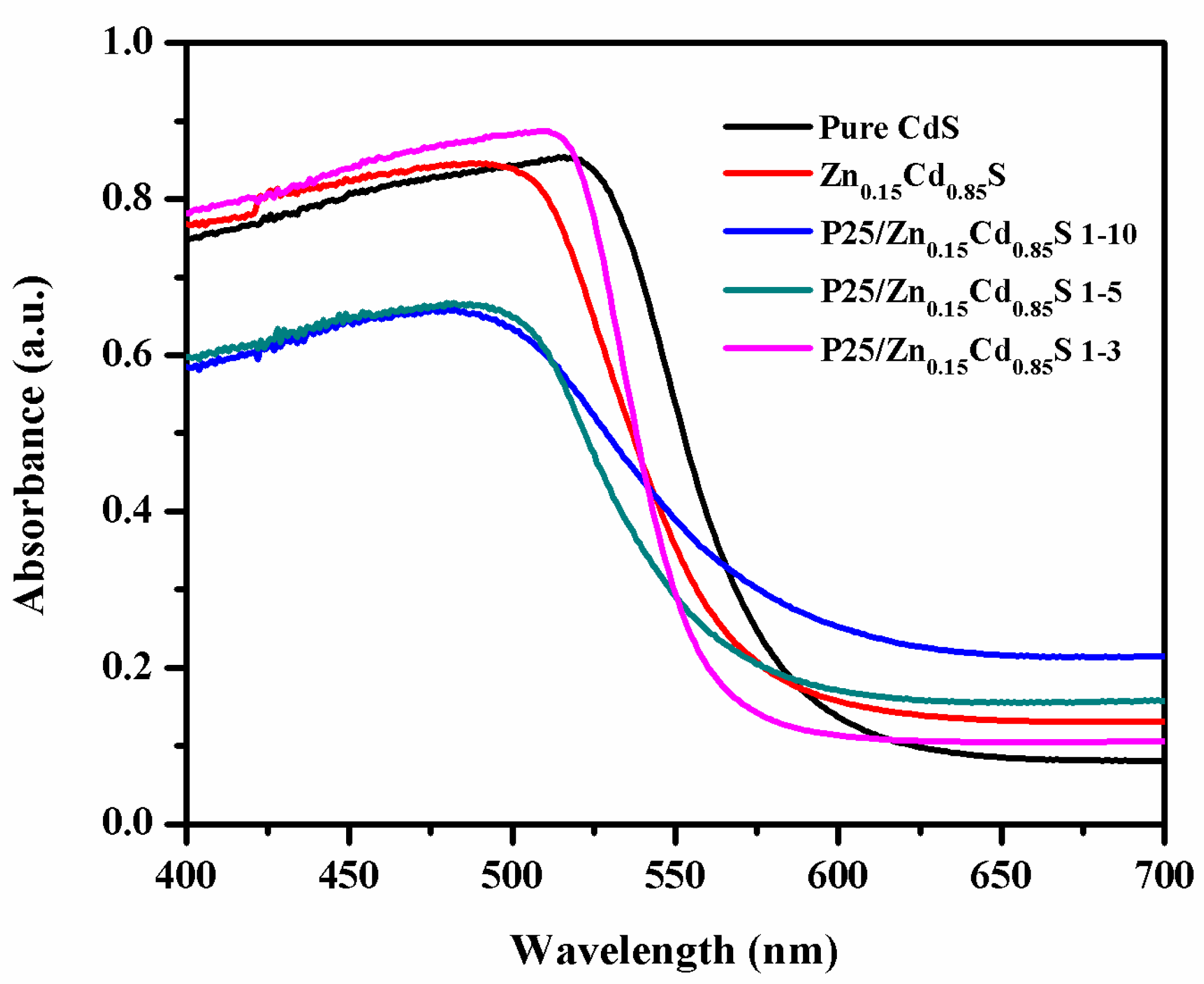
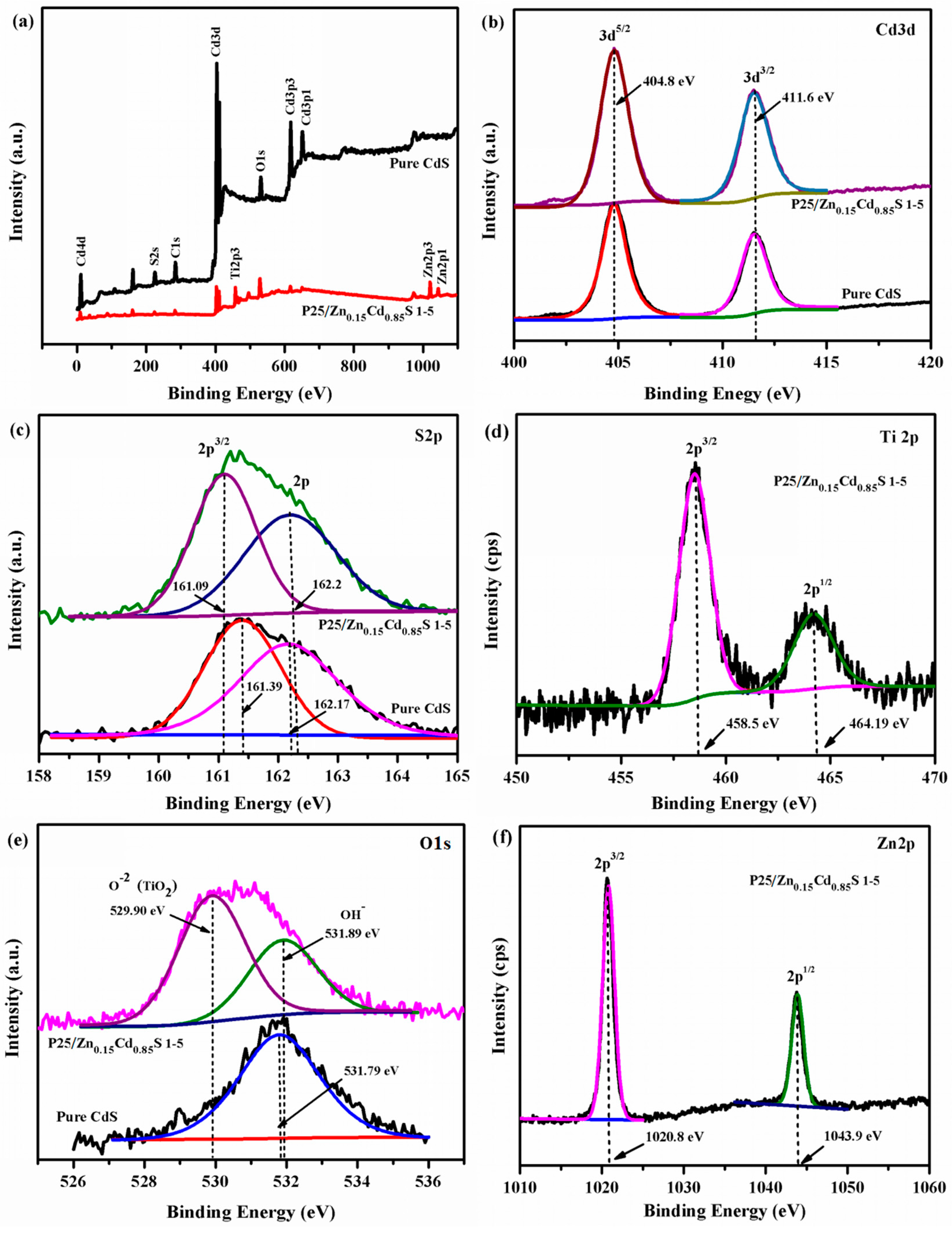
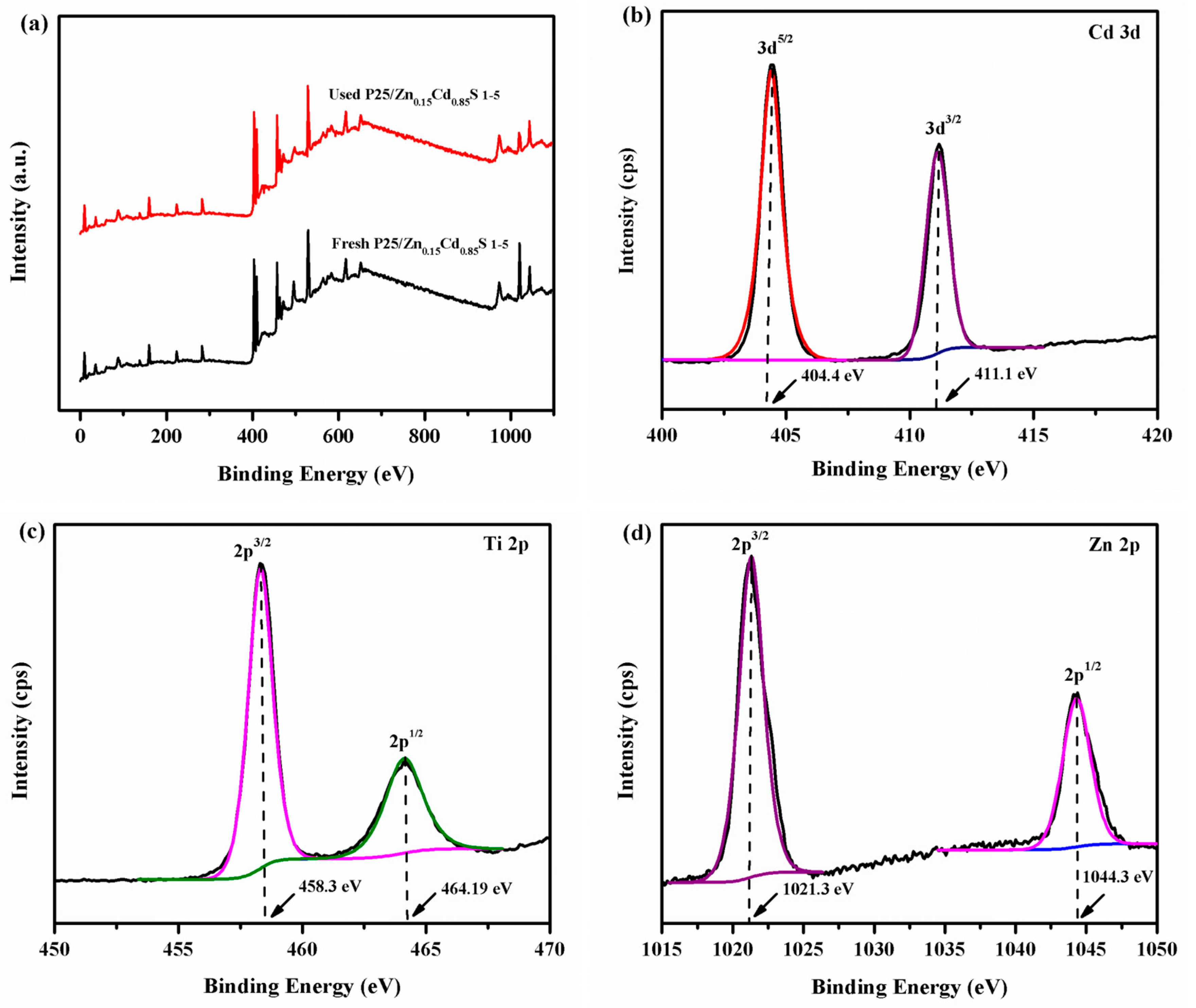
3.1. Structure, Morphology, Surface and Spectroscopic Studies
3.2. Photocatalytic Activity
3.3. Scavengers Method
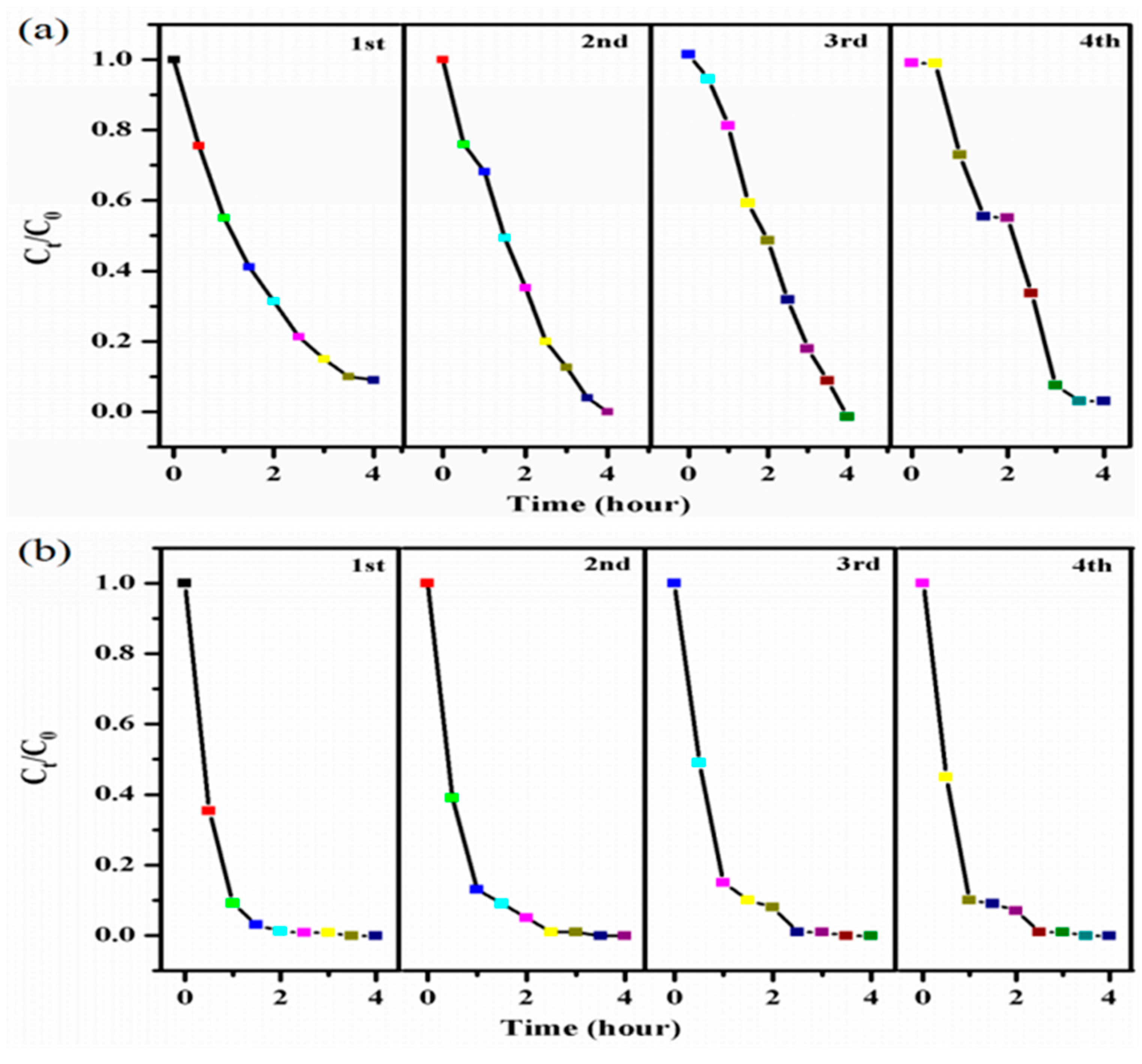
3.4. Recyclability of Photocatalyst
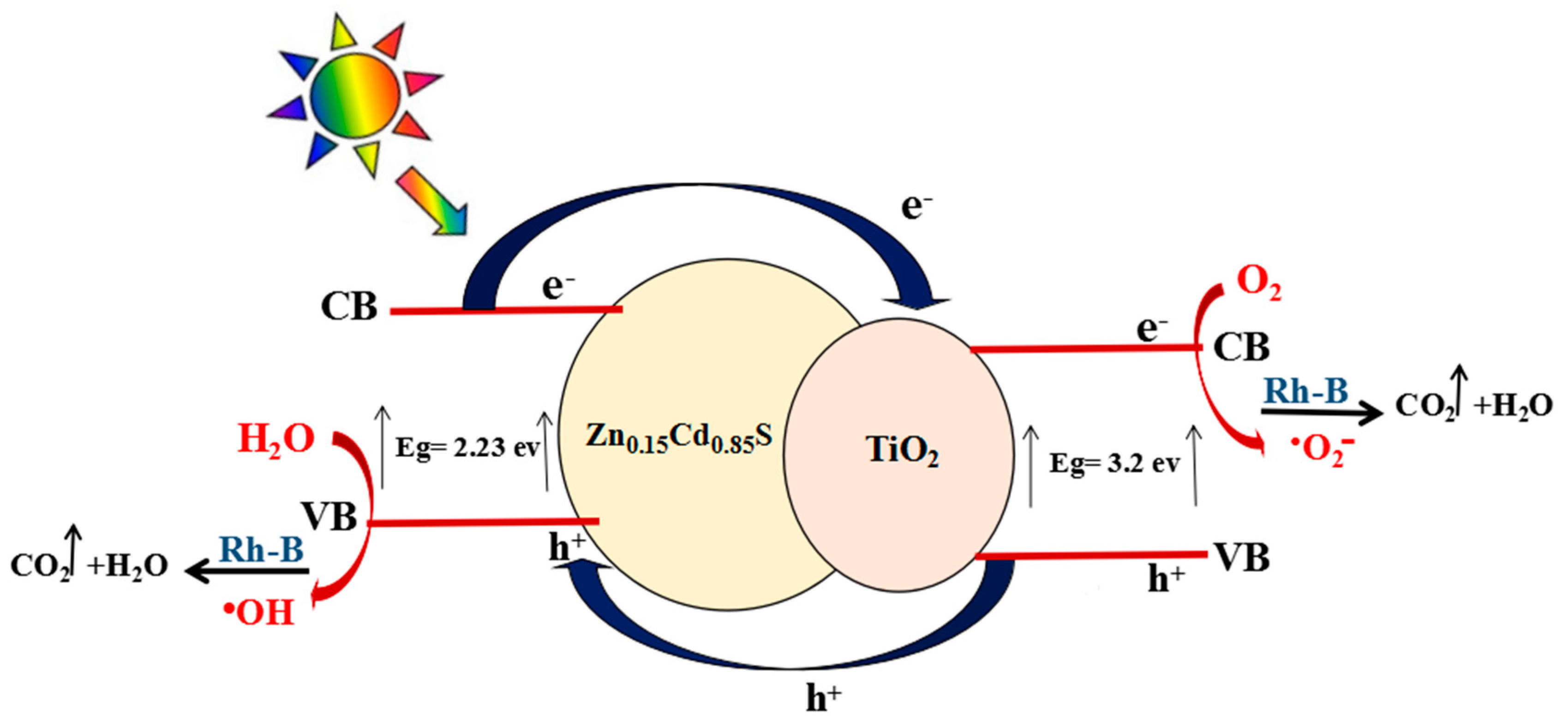
3.5. Mechanism of Photocatalytic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lin, Y.; Li, D.; Hu, J.; Xiao, G.; Wang, J.; Li, W.; Fu, X. Highly efficient photocatalytic degradation of organic pollutants by PANI-modified TiO2 composite. J. Phys. Chem. C 2012, 116, 5764–5772. [Google Scholar] [CrossRef]
- Chen, F.; Jia, D.; Cao, Y.; Jin, X.; Liu, A. Facile synthesis of CdS nanorods with enhanced photocatalytic activity. Ceram. Int. 2015, 41, 14604–14609. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Z.; Chen, Z.; Lv, C.; Humphrey, M.G.; Zhang, C. Cobalt phosphide nanorods as an efficient electrocatalyst for the hydrogen evolution reaction. Nano Energy 2014, 9, 373–382. [Google Scholar] [CrossRef]
- Xiong, S.; Xi, B.; Qian, Y. CdS hierarchical nanostructures with tunable morphologies: Preparation and photocatalytic properties. J. Phys. Chem. C 2010, 114, 14029–14035. [Google Scholar] [CrossRef]
- Wu, S.-D.; Zhu, Z.; Zhang, Z.; Zhang, L. Preparation of the CdS semiconductor nanofibril by UV irradiation. Mater. Sci. Eng. B 2002, 90, 206–208. [Google Scholar] [CrossRef]
- Chang, W.-S.; Wu, C.-C.; Jeng, M.-S.; Cheng, K.-W.; Huang, C.-M.; Lee, T.-C. Ternary Ag–In–S polycrystalline films deposited using chemical bath deposition for photoelectrochemical applications. Mater. Chem. Phys. 2010, 120, 307–312. [Google Scholar] [CrossRef]
- Roy, A.; De, G.; Sasmal, N.; Bhattacharyya, S. Determination of the flatband potential of semiconductor particles in suspension by photovoltage measurement. Int. J. Hydrogen Energy 1995, 20, 627–630. [Google Scholar] [CrossRef]
- Ren, L.; Yang, F.; Deng, Y.-R.; Yan, N.-N.; Huang, S.; Lei, D.; Sun, Q.; Yu, Y. Synthesis of (CuIn)xCd2(1 − x)S2 photocatalysts for H2 evolution under visible light by using a low-temperature hydrothermal method. Int. J. Hydrogen Energy 2010, 35, 3297–3305. [Google Scholar] [CrossRef]
- Chauhan, R.; Kumar, A.; Chaudhary, R.P. Visible-light photocatalytic degradation of methylene blue with Fe doped CdS nanoparticles. Appl. Surf. Sci. 2013, 270, 655–660. [Google Scholar] [CrossRef]
- Wang, X.W.; Lui, G.; Chen, Z.-G.; Li, F.; Wang, L.Z.; Lu, G.Q.; Cheng, H.-M. Enhanced photocatalytic hydrogen evolution by prolonging the lifetime of carriers in ZnO/CdS heterostructures. Chem. Commun. 2009, 23, 3452–3454. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, R.; Shirole, A.R.; Sudarsan, V.; Girija, K.G.; Rao, R.; Sudakar, C.; Bharadwaj, S.R. Improved photocatalytic activity of indium doped cadmium sulfide dispersed on zirconia. J. Mater. Chem. 2011, 21, 16566–16573. [Google Scholar] [CrossRef]
- Li, W.; Li, D.; Chen, Z.; Huang, H.; Sun, M.; He, Y.; Fu, X. High-efficient degradation of dyes by ZnxCd1 − xS solid solutions under visible light irradiation. J. Phys. Chem. C 2008, 112, 14943–14947. [Google Scholar] [CrossRef]
- Li, W.; Li, D.; Zhang, W.; Hu, Y.; He, Y.; Fu, X. Microwave synthesis of ZnxCd1 − xS nanorods and their photocatalytic activity under visible light. J. Phys. Chem. C 2010, 114, 2154–2159. [Google Scholar] [CrossRef]
- Yang, F.; Yan, N.-N.; Huang, S.; Sun, Q.; Zhang, L.-Z.; Yu, Y. Zn-doped CdS nanoarchitectures prepared by hydrothermal synthesis: Mechanism for enhanced photocatalytic activity and stability under visible light. J. Phys. Chem. C 2012, 116, 9078–9084. [Google Scholar] [CrossRef]
- Yang, F.; Messing, M.E.; Mergenthaler, K.; Ghasemi, M.; Johansson, J.; Wallenberg, L.R.; Pistol, M.-E.; Deppert, K.; Samuelson, L.; Magnusson, M.H. Zn-doping of GaAs nanowires grown by Aerotaxy. J. Cryst. Growth 2015, 414, 181–186. [Google Scholar] [CrossRef]
- Baghchesara, M.A.; Yousefi, R.; Cheraghizade, M.; Jamali-Sheini, F.; Saáedi, A. Photocurrent application of Zn-doped CdS nanostructures grown by thermal evaporation method. Ceram. Int. 2016, 42, 1891–1896. [Google Scholar] [CrossRef]
- Chowdhury, C.; Karmakar, S.; Datta, A. Monolayer group IV–VI monochalcogenides: Low-dimensional materials for photocatalytic water splitting. J. Phys. Chem. C 2017, 121, 7615–7624. [Google Scholar] [CrossRef]
- Liu, Y.; Kanhere, P.D.; Wong, C.L.; Tian, Y.; Feng, Y.; Boey, F.; Wu, T.; Chen, H.; White, T.J.; Chen, Z.; et al. Hydrazine-hydrothermal method to synthesize three-dimensional chalcogenide framework for photocatalytic hydrogen generation. J. Solid State Chem. 2010, 183, 2644–2649. [Google Scholar] [CrossRef]
- Nie, L.; Zhang, Q. Recent progress in crystalline metal chalcogenides as efficient photocatalysts for organic pollutant degradation. Inorg. Chem. Front. 2017, 4, 1953–1962. [Google Scholar] [CrossRef]
- Reshak, A.H. Active photocatalytic water splitting solar-to-hydrogen energy conversion: Chalcogenide photocatalyst Ba2ZnSe3 under visible irradiation. Appl. Catal. B Environ. 2018, 221, 17–26. [Google Scholar] [CrossRef]
- Wang, J.; Meng, J.; Li, Q.; Yang, J. Single-layer cadmium chalcogenides: Promising visible-light driven photocatalysts for water splitting. Phys. Chem. Chem. Phys. 2016, 18, 17029–17036. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Y.; Mu, Z.; Li, J.J.; Jin, Y.G.; Cheng, J.; Lu, G.Q.; Hao, Z.P.; Qiao, S.Z. Mesoporous Co3O4 and Au/Co3O4 catalysts for low-temperature oxidation of trace ethylene. J. Am. Chem. Soc. 2010, 132, 2608–2613. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Leung, M.K.; Leung, D.Y.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Yan, W.; Wu, Y.P.; Wang, Z.H. Synthesis of TiO2 nanotubes coupled with CdS nanoparticles and production of hydrogen by photocatalytic water decomposition. Mater. Lett. 2008, 62, 3846–3848. [Google Scholar] [CrossRef]
- Basahel, S.N.; Lee, K.; Hahn, R.; Schmuki, P.; Bawaked, S.M.; Al-Thabaiti, S.A. Self-decoration of Pt metal particles on TiO2 nanotubes used for highly efficient photocatalytic H2 production. Chem. Commun. 2014, 50, 6123–6125. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.; Lee, J.; Lee, D.C.; Moon, J.H. Uniform decoration of CdS nanoparticles on TiO2 inverse opals for visible light photoelectrochemical cell. Electrochim. Acta 2015, 166, 350–355. [Google Scholar] [CrossRef]
- Tsuji, I.; Kato, H.; Kobayashi, H.; Kudo, A. Photocatalytic H2 evolution reaction from aqueous solutions over band structure-controlled (AgIn)xZn2(1− x)S2 solid solution photocatalysts with visible-light response and their surface nanostructures. J. Am. Chem. Soc. 2004, 126, 13406–13413. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Chen, Y.; Zhou, W.; Pan, K.; Tian, C.; Huang, X.-R.; Fu, H. 3D hierarchical flower-like TiO2 nanostructure: Morphology control and its photocatalytic property. CrystEngComm 2011, 13, 2994–3000. [Google Scholar] [CrossRef]
- Hu, Z.; Quan, H.; Chen, Z.; Shao, Y.; Li, D. New insight into an efficient visible light-driven photocatalytic organic transformation over CdS/TiO2 photocatalysts. Photochem. Photobiol. Sci. 2018, 17, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, C.; Liu, S. Effect of PSS on morphology and optical properties of ZnO. J. Colloid Interface Sci. 2008, 326, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Weller, H. Colloidal semiconductor Q-particles: Chemistry in the transition region between solid state and molecules. Angew. Chem. Int. Ed. 1993, 32, 41–53. [Google Scholar] [CrossRef]
- Su, X.; Zhao, J.; Li, Y.; Zhu, Y.; Ma, X.; Sun, F.; Wang, Z. Solution synthesis of Cu2O/TiO2 core-shell nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2009, 349, 151–155. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, H.; Quan, X.; Chen, S. Green synthesis of feather-shaped MoS2/CdS photocatalyst for effective hydrogen production. Int. J. Photoenergy 2013, 2013, 247516. [Google Scholar] [CrossRef]
- Setty, M. Burstein effect in thick films of cadmium orthostannate (Cd2SnO4). J. Mater. Sci. Lett. 1987, 6, 909–911. [Google Scholar] [CrossRef]
- Houng, M.; Fu, S.; Wu, T. Effect of surface chemisorption on the photoconductivity enhancement of CdS thick films. J. Mater. Sci. Lett. 1986, 5, 96–98. [Google Scholar] [CrossRef]
- Gonbeau, D.; Guimon, C.; Pfister-Guillouzo, G.; Levasseur, A.; Meunier, G.; Dormoy, R. XPS study of thin films of titanium oxysulfides. Surf. Sci. 1991, 254, 81–89. [Google Scholar] [CrossRef]
- Slink, W.E.; De Groot, P.B. Vanadium-titanium oxide catalysts for oxidation of butene to acetic acid. J. Catal. 1981, 68, 423–432. [Google Scholar] [CrossRef]
- Sanjines, R.; Tang, H.; Berger, H.; Gozzo, F.; Margaritondo, G.; Levy, F. Electronic structure of anatase TiO2 oxide. J. Appl. Phys. 1994, 75, 2945–2951. [Google Scholar] [CrossRef]
- Leinen, D.; Fernandez, A.; Espinos, J.; Belderrain, T.; González-Elipe, A. Ion beam induced chemical vapor deposition for the preparation of thin film oxides. Thin Solid Films 1994, 241, 198–201. [Google Scholar] [CrossRef]
- Chadwick, D.; Hashemi, T. Adsorbed corrosion inhibitors studied by electron spectroscopy: Benzotriazole on copper and copper alloys. Corros. Sci. 1978, 18, 39–51. [Google Scholar] [CrossRef]
- Kowalczyk, S.; Ley, L.; McFeely, F.; Pollak, R.; Shirley, D. Relative effect of extra-atomic relaxation on Auger and binding-energy shifts in transition metals and salts. Phys. Rev. B 1974, 9, 381. [Google Scholar] [CrossRef]
- Wagner, C.D. Chemical shifts of Auger lines, and the Auger parameter. Faraday Discuss. Chem. Soc. 1975, 60, 291–300. [Google Scholar] [CrossRef]
- Xu, X.; Lu, R.; Zhao, X.; Xu, S.; Lei, X.; Zhang, F.; Evans, D.G. Fabrication and photocatalytic performance of a ZnxCd1 − xS solid solution prepared by sulfuration of a single layered double hydroxide precursor. Appl. Catal. B Environ. 2011, 102, 147–156. [Google Scholar] [CrossRef]
- Zhang, L.-S.; Wong, K.-H.; Zhang, D.-Q.; Hu, C.; Jimmy, C.Y.; Chan, C.-Y.; Wong, P.-K. Zn:In(OH)ySz solid solution nanoplates: Synthesis, characterization, and photocatalytic mechanism. Environ. Sci. Technol. 2009, 43, 7883–7888. [Google Scholar] [CrossRef] [PubMed]




| Sample Label | Molar Ratio P25:Cd | BET (m2·g−1) | Band Gap (eV) |
|---|---|---|---|
| CdS | - | 0.26 | 2.20 |
| Zn0.15Cd0.85S | - | 0.63 | 2.23 |
| P25/Zn0.15Cd0.85S 1-10 | 1:10 | 7.50 | 2.24 |
| P25/Zn0.15Cd0.85S 1-5 | 1:5 | 21.4 | 2.28 |
| P25/Zn0.15Cd0.85S 1-3 | 1:3 | 37.8 | 2.30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alomar, M.; Liu, Y.; Chen, W. Surface Decorated Zn0.15Cd0.85S Nanoflowers with P25 for Enhanced Visible Light Driven Photocatalytic Degradation of Rh-B and Stability. Appl. Sci. 2018, 8, 327. https://doi.org/10.3390/app8030327
Alomar M, Liu Y, Chen W. Surface Decorated Zn0.15Cd0.85S Nanoflowers with P25 for Enhanced Visible Light Driven Photocatalytic Degradation of Rh-B and Stability. Applied Sciences. 2018; 8(3):327. https://doi.org/10.3390/app8030327
Chicago/Turabian StyleAlomar, Muneerah, Yueli Liu, and Wen Chen. 2018. "Surface Decorated Zn0.15Cd0.85S Nanoflowers with P25 for Enhanced Visible Light Driven Photocatalytic Degradation of Rh-B and Stability" Applied Sciences 8, no. 3: 327. https://doi.org/10.3390/app8030327