Cobalt (II) Complexes with Schiff Base Ligands Derived from Terephthalaldehyde and ortho-Substituted Anilines: Synthesis, Characterization and Antibacterial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
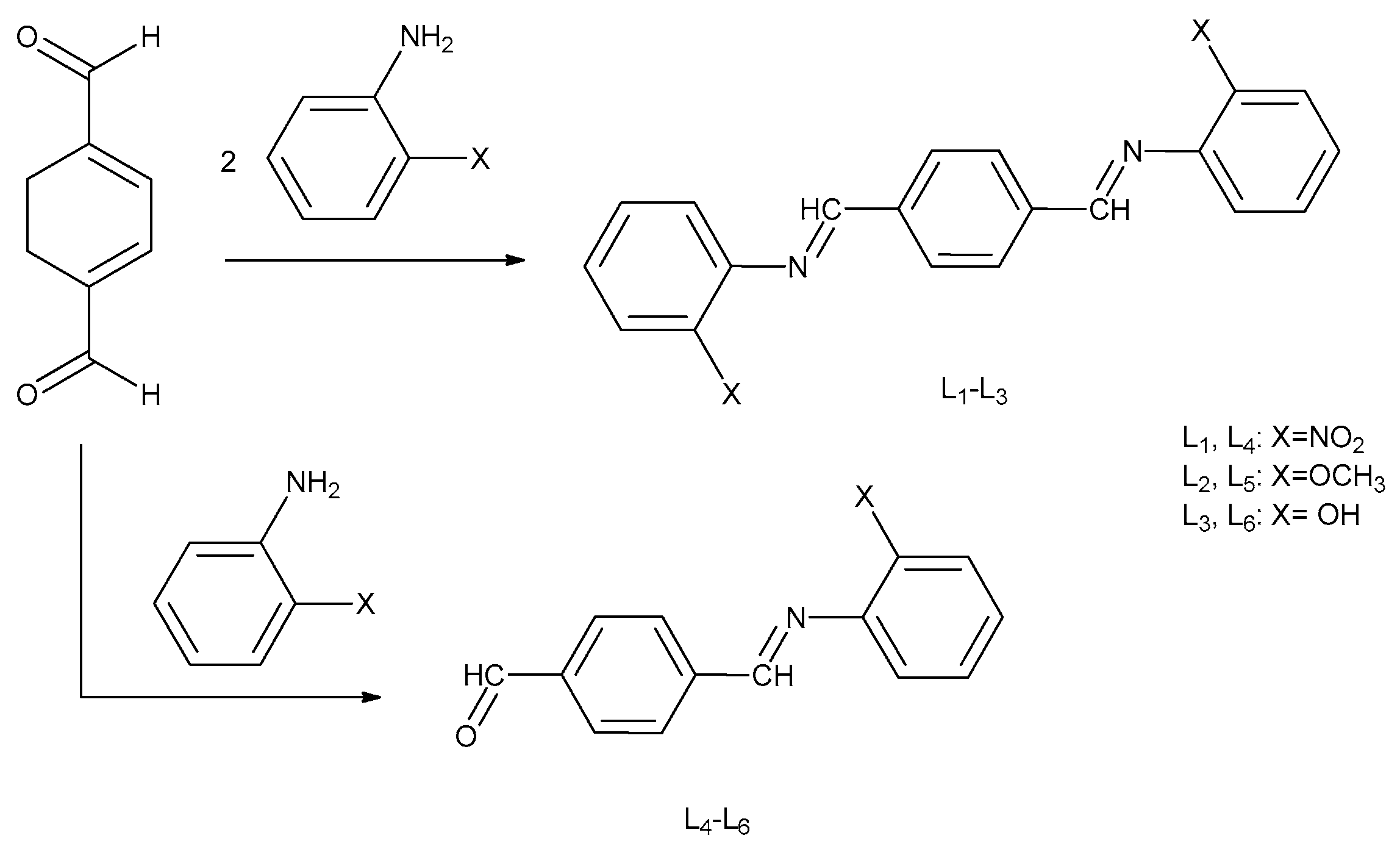
2.2. Preparation of Schiff Base Ligands
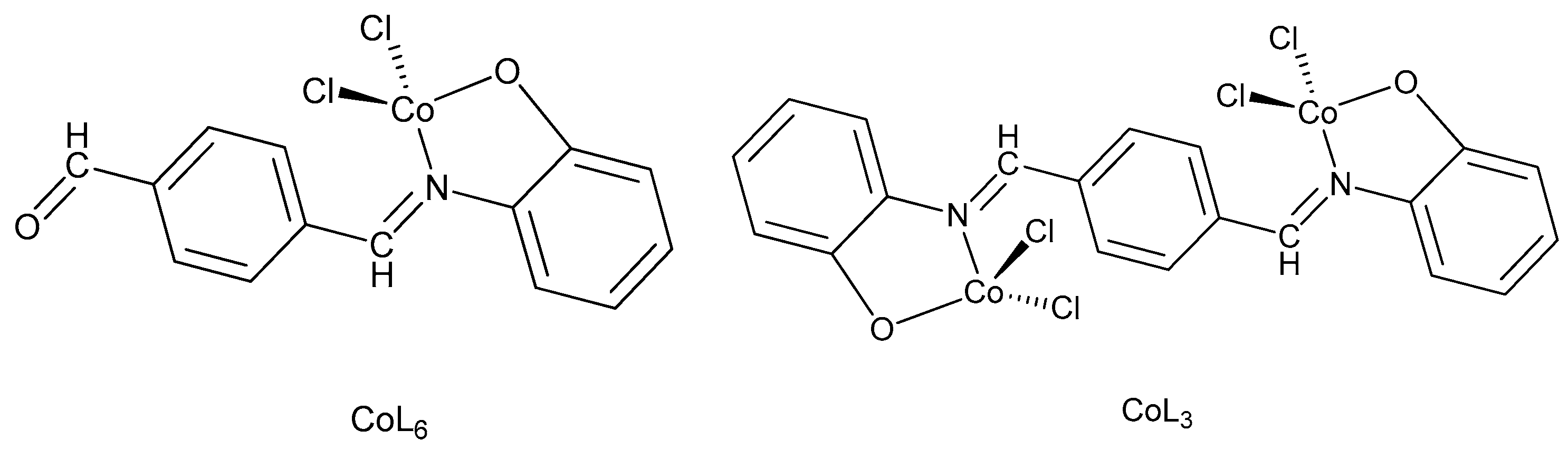
2.3. Preparation of Co (II) Complexes
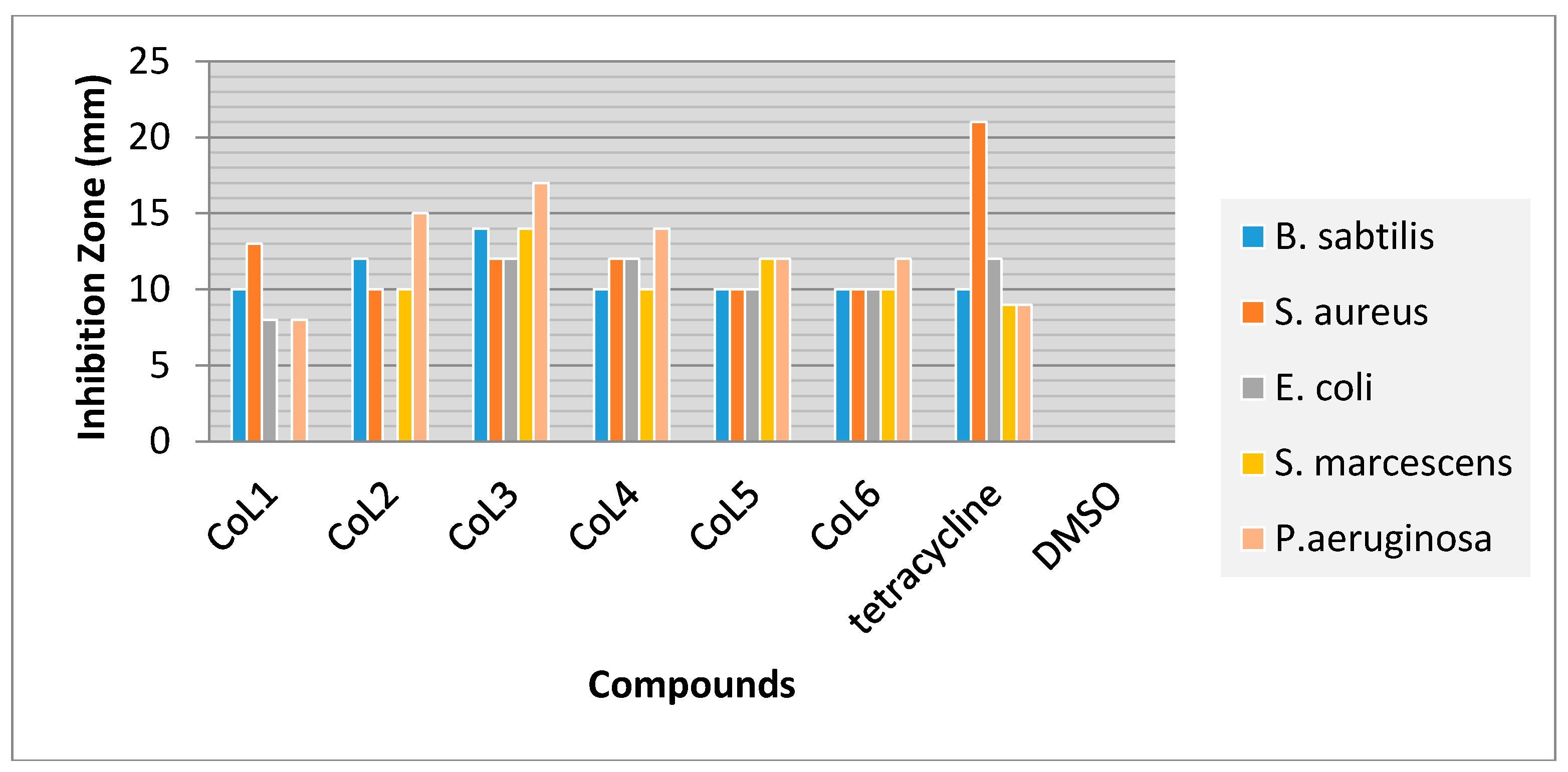
2.4. Antibacterial Study
2.4.1. Disc Diffusion Method
2.4.2. Broth Dilution Method
3. Results and Discussion
3.1. Characterization of Ligands
3.2. Characterization of Metal Complexes
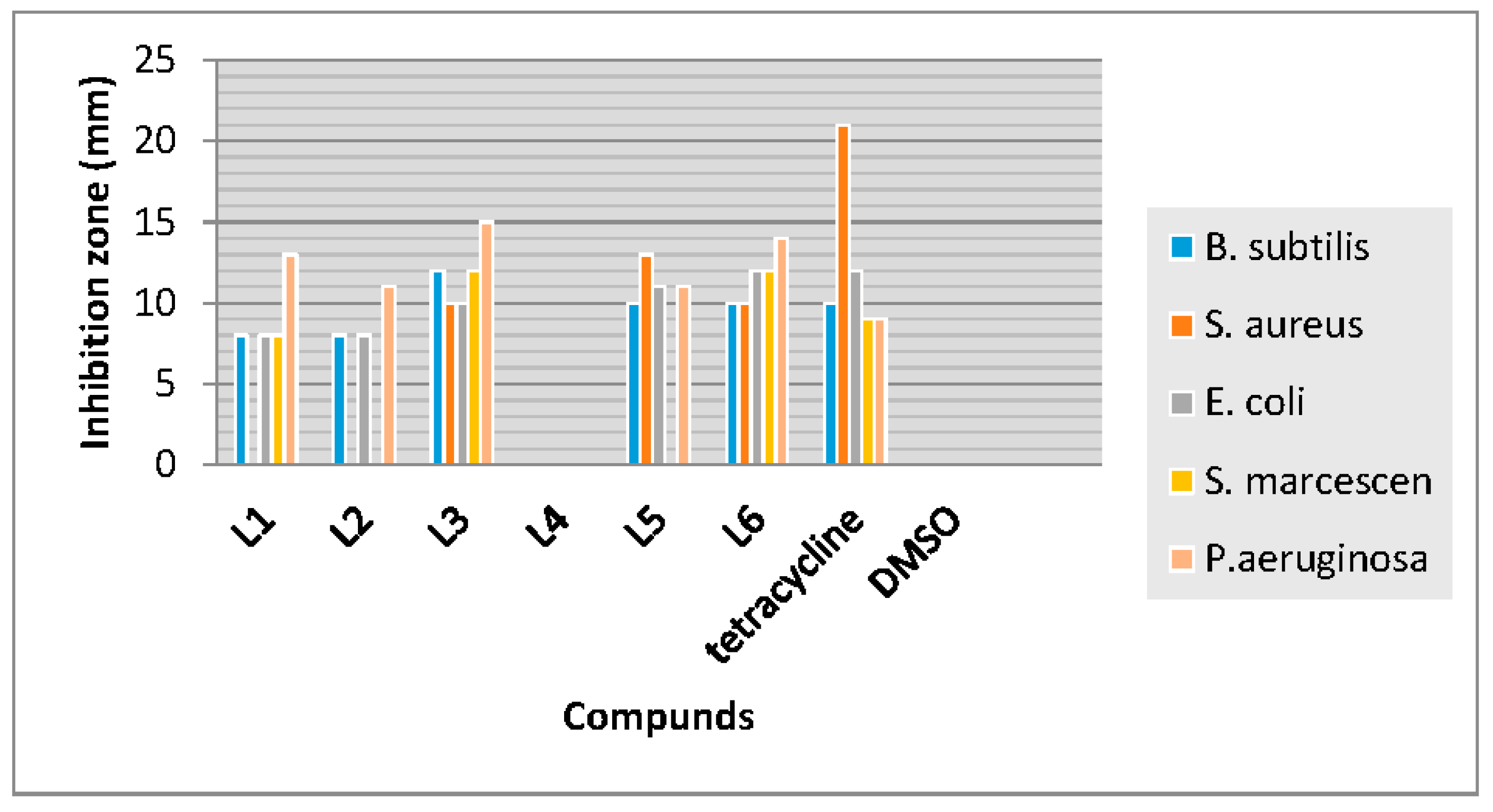
3.3. Antibacterial Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, Y.-T.; Sheng, J.; Yin, D.-W.; Xin, H.; Yang, X.-M.; Qiao, Q.-Y.; Yang, Z.-J. Ferrocenyl chaconne-based Schiff bases and their metal complexes: Highly efficient, solvent-free synthesis, characterization, biological research. J. Organomet. Chem. 2018, 856, 27–33. [Google Scholar] [CrossRef]
- Lashanizadegan, M.; Shayegan, S.; Sarkheil, M. Copper(II) complex of (±)trans-1,2-cyclohexanediamine azo-linked Schiff base ligand encapsulated in nanocavity of zeolite-Y for the catalytic oxidation of olefins. J. Serbian Chem. Soc. 2016, 81, 153–162. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, W.; Yu, Q.; Huang, F.-P.; Bian, H.-D.; Liang, H. Ni(II) Complexes with Schiff Base Ligands: Preparation, Characterization, DNA/Protein Interaction and Cytotoxicity Studies. Molecules 2017, 22, 1772. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Sreenivasulu, B.; Vital, J. Amino acid—Containing reduced Schiff bases as the building blocks for metallasupramolecular structures. Coord. Chem. Rev. 2008, 252, 1027–1050. [Google Scholar] [CrossRef]
- Golcu, A.; Tumer, M.; Demirelli, H.; Wheatley, R.A. Cd and Cu complexes of polydentate Schiff base ligands: Synthesis, characterization, properties and biological activity. Inorg. Chim. Acta 2005, 153, 1785–1797. [Google Scholar] [CrossRef]
- Sarkar, S.; Jana, M.; Mondal, T.; Sinha, C. Ru-halide-carbonyl complexes of naphthylazoimidazoles; synthesis, spectra, electrochemistry, catalytic and electronic structure. J. Organomet. Chem. 2012, 716, 129–137. [Google Scholar] [CrossRef]
- Khandar, A.; Nejati, K.; Rezvani, Z. Syntesis, characterization, and study of the use of cobalt Schiff base complexes as catalysts for the oxidation of styrene by molecular oxygen. Molecules 2005, 10, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Raman, N.; Ravichandran, S.; Thangaraja, C. Copper, Cobalt, Nickel and zink complexes of Schiff base derived from benzil-2,4-dinitrophenylhydrazone wiyh aniline. J. Chem. Sci. 2004, 116, 215–219. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Ko, Y.G. Schiff base complexes and their versatile applications as catalysts in oxidation of organic compounds: Part I. Appl. Organomet. Chem. 2016, 31. [Google Scholar] [CrossRef]
- Siva, C.; Silva, D.; Modolo, L.; Alves, R.; Rwsende, M.; Martins, C.; Fatima, A. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Afradi, M.; Foroughifar, N.; Psdar, H.; Moghhanian, H. Facile green one-pot synthesis of novel thiazolo[3,2-a]pyrimidine derivatives using Fe3O4 L-argainine and their biological investigation as potent antimicrobial agents. Appl. Organomet. Chem. 2016, 1–16. [Google Scholar] [CrossRef]
- Anitha, C.; Sheela, C.; Tharmaraj, P.; Sumathi, S. Spectroscopic studies and biological evaluation of some transition metal complexses of azo schiff base ligand derived from (1-phenyl-2,3-dimethyl-4-aminopyrazol-5-one) and 5-((4-chlorophenyl)diazenyl)-2-hydroxybenzaldehyde. Spectrochim. Acta Part A 2002, 96, 493–900. [Google Scholar] [CrossRef] [PubMed]
- Raman, N.; Raja, Y.P.; Kulandaisamy, A. Synthesis and characterisation of Cu(II), Ni(II), Mn(II), Zn(II) and VO(II) Schiff base complexes derived fromo-phenylenediamine and acetoacetanilide. J. Chem. Sci. 2001, 113, 183–189. [Google Scholar] [CrossRef]
- Raman, N.; Thangaraja, C.; Johnsonraja, S. Synthesis, spectral characterization, redox and antimicrobial activity of Schiff base transition metal(II) complexes derived from 4-aminoantipyrine and 3-salicylideneacetylacetone. Cent. Eur. J. Chem. 2005, 3, 537–555. [Google Scholar] [CrossRef]
- Streater, M.; Taylor, P.D.; Hider, R.C.; Porter, J. Novel 3-hydroxyl-2(1H)-pyridinones. Synthesis, iron(III)-chelating properties and biological activity. J. Med. Chem. 1990, 33, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, S.A.; Hassib, H.B.; Issa, Y.M. Studies on some salycylaldehyde schiff base derivatives and their complexes with Cr, Mn, Ni, Cu. Spectrochim. Acta Part A 2007, 67, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.; Cornell, H.; Nicoletti, G.; Jackson, N.; Hügel, H. Study of Fluorinated β-Nitrostyrenes as Antimicrobial Agents. Appl. Sci. 2012, 2, 114–128. [Google Scholar] [CrossRef]
- Tolazzal, M.; Tarafder, H.; Ali, M.; Juan, D. Complexes of a tridentate ONS Schiff base. Synthesis and biological properties. Transit. Metal Chem. 2000, 25, 456–460. [Google Scholar] [CrossRef]
- Khojasteh, R.; Matin, S.J. Synthesis, characterization and antimicrobial activity of some metal complexes of heptadentate schiif base ligand derived from acetylacetone. Russ. J. Appl. Chem. 2015, 88, 921–925. [Google Scholar] [CrossRef]
- Hou, S.H.; Jihui, J.; Huang, X.; Wang, X.; Ma, L.; Shen, W.; Kang, F.; Hiang, Z.-H. Silver Nanoparticles-Loaded Exfoliated Graphite and Its Anti-Bacterial Performance. Appl. Sci. 2017, 7, 852. [Google Scholar] [CrossRef]
- Lee, J.; Purushothaman, B.; Li, Z.; Kulsi, G.; Song, J.M. Synthesis, Characterization, and Antibacterial Activities of High-Valence Silver Propamidine Nanoparticles. Appl. Sci. 2017, 7, 736. [Google Scholar] [CrossRef]
- Wang, S.X.; Zhang, F.J.; Feng, Q.P.; Li, Y.L. Synthesis, characterization, and antibacterial activity of transition metal complexes with 5-hydroxy-7,4′-dimethoxyflavone. J. Inorg. Biochem. 1992, 46, 251–257. [Google Scholar] [CrossRef]
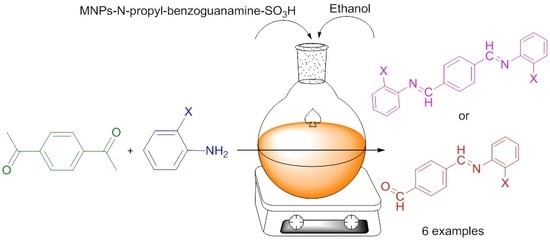
- Gholami Dehbalaei, M.; Foroughifar, N.; Pasdar, H.; Khajeh-Amiri, A. N-Propyl benzoguanamine sulfonic acid supported on magnetic Fe3O4 nanoparticles: A novel and efficient magnetically heterogeneous catalyst for the synthesis of 1,8-dioxo-decahydroacridine derivatives. New J. Chem. 2018, 42, 327–335. [Google Scholar] [CrossRef]
- Camus, A.; Marsich, N.; Lanfredi, A.M.M.; Ugozzoli, F.; Massera, C. Copper(II) nitrito complexes with 2,20-dipyridylamine. Crystal structures of the [(acetato)(2,20-dipyridylamine)(nitrito-O,O0)copper(II)] and [(2,20-dipyridylamine) (nitrito-O,O0)(_-nitrito-O)copper(II)]2_2(acetonitrile). Inorg. Chim. Acta 2000, 309, 1–9. [Google Scholar] [CrossRef]
- Mautner, F.A.; Vicente, R.; Massoud, S.S. Structure determination of nitrito- and thiocyanato-copper(II) complexes: X-ray structures of [Cu(Medpt)(ONO)(H2O)]ClO4(1), [Cu(dien)(ONO)]ClO4 (2) and [Cu2(Medpt)2(_N,S-NCS)2](ClO4)2 (3) (Medpt = 3,30-diamino-N-methyldipropylamine and dien = diethylenetriamine). Polyhedron 2006, 25, 1673–1680. [Google Scholar] [CrossRef]
- Eslami, A. Thermoanalytical study of linkage isomerism in coordination compounds: Part I. Reinvestigation of thermodynamic and thermokinetic of solid state interconversion of nitrito (ONO) and nitro (NO2) isomers of pentaaminecobalt(III) chloride by means of DSC. Thermochim. Acta 2004, 409, 189–193. [Google Scholar] [CrossRef]
- Pasdar, H.; Hedayati Saghavaz, B.; Foroughifar, N.; Davallo, M. Synthesis, Characterization and Antibacterial Activity of Novel 1,3-Diethyl-1,3-bis(4-nitrophenyl)urea and Its Metal(II) Complexes. Molecules 2017, 22, 2125. [Google Scholar] [CrossRef] [PubMed]
- El-Megharbel, S.M.; Adam, A.M.; Meghdad, A.S.; Refat, M.S. Synthesis and molecular structure of moxifloxacin drug with metal ions as amodel drug against some kinds of bacteria and fungi. Russ. J. Gen. Chem. 2015, 85, 2366–2371. [Google Scholar] [CrossRef]
- Patel, M.N.; Pansuriya, P.B.; Parmar, P.A.; Gandhi, D.S. Synthesis, characterization and thermal and biocidal aspects of drug-based metal complexes. Pharm. Chem. J. 2008, 42, 687–692. [Google Scholar] [CrossRef]
- Hedayati Saghavaz, B.; Pasdar, H.; Foroughifar, N. Novel dinuclear metal complexes of guanidine-pyridine hybrid ligand: Synthesis, structural characterization and biological activity. Biointerface Res. Appl. Chem. 2016, 6, 1842–1846. [Google Scholar]
- Khadivi, R.; Pasdar, H.; Foroughifar, N.; Davallo, M. Synthesis and structural characterization of metal complexes derived from substituted guanidine-pyridine as potential antibacterial agents. Biointerface Res. Appl. Chem. 2017, 7, 2238–2242. [Google Scholar]


| Entry | Catalyst (mg) | Solvent | Time (min) | Yield (%) |
|---|---|---|---|---|
| 1 | MNPs-N-propyl-benzoguanamine-SO3H (3) | H2O | 300 | 45 |
| 2 | MNPs-N-propyl-benzoguanamine-SO3H (3) | EtOH:H2O | 210 | 53 |
| 3 | MNPs-N-propyl-benzoguanamine-SO3H (3) | EtOH | 180 | 68 |
| 4 | MNPs-N-propyl-benzoguanamine-SO3H (6) | EtOH | 120 | 84 |
| 5 | MNPs-N-propyl-benzoguanamine-SO3H (9) | EtOH | 210 | 75 |
| 6 | MNPs-N-propyl-benzoguanamine-SO3H (11) | EtOH | 300 | 63 |
| 7 | PTSA (6) | EtOH | 480 | 35 |
| Compounds | HCN (ppm) | CHO (ppm) | Ar-H (ppm) | CH3 (ppm) | OH (ppm) |
|---|---|---|---|---|---|
| L1 | 10.04 | - | 8.02–7.50 | - | - |
| L2 | 10.08 | - | 8.66–6.95 | 3.80 | - |
| L3 | 9.72 | - | 8.34–6.82 | - | 1.16 |
| L4 | 10.06 | 10.99 | 8.16–7.69 | - | - |
| L5 | 8.64 | 10.07 | 8.03–6.95 | 3.79 | - |
| L6 | 9.73 | 10.18 | 8.41–7.45 | - | 1.21 |
| Compounds | Band Position (nm) | Assignment |
|---|---|---|
| L1 | 260 | π→π* |
| 350 | n→p* | |
| L2 | 300 | π→π* |
| 390 | n→p* | |
| L3 | 310 | π→π* |
| L4 | 290 | π→π* |
| 320 | n→p* | |
| L5 | 290 | π→π* |
| 370 | n→p* | |
| L6 | 340 | π→π* |
| CoL1 | 310 | π→π* |
| 350 | n→p* | |
| 600 | 4A1→4B1 | |
| 690 | 4A1→4B2 | |
| CoL2 | 280 | π→π* |
| 410 | n→p* | |
| 610 | 4A1→4B1 | |
| 680 | 4A1→4B2 | |
| CoL3 | 280 | π→π* |
| 370 | n→p* | |
| 600 | 4A1→4B1 | |
| 680 | 4A1→4B2 | |
| CoL4 | 290 | π→π* |
| 350 | n→p* | |
| 610 | 4A1→4B1 | |
| 690 | 4A1→4B2 | |
| CoL5 | 260 | π→π* |
| 310 | n→p* | |
| 590 | 4A1→4B1 | |
| 660 | 4A1→4B2 | |
| CoL6 | 270 | π→π* |
| 390 | n→p* | |
| 605 | 4A1→4B1 | |
| 680 | 4A1→4B2 |
| Compounds | M. W. (g/mol) | Yield (%) | Color | Molar Conductivity (Ω−1 mol−1 cm2) | M. P. (°C) |
|---|---|---|---|---|---|
| L1 | 374 | 84 | Dark yellow | - | 208–210 |
| L2 | 344 | 86 | Orange | - | 190–192 |
| L3 | 316 | 64 | Brown | - | 295–297 |
| L4 | 254 | 68 | Yellow | - | 203–205 |
| L5 | 239 | 73 | Pale Yellow | - | 181–183 |
| L6 | 225 | 65 | Light green | - | 282–284 |
| CoL1 | 633 | 65 | Pale green | 22 | 284–250 |
| CoL2 | 603 | 68 | Dark green | 12 | 313–315 |
| CoL3 | 575 | 75 | Dark brown | 14 | 230–232 |
| CoL4 | 503 | 80 | Green | 18 | 280–28 |
| CoL5 | 473 | 82 | Orange-red | 16 | 305–307 |
| CoL6 | 445 | 87 | Dark-pink | 10 | 236–238 |
| Compounds | B. subtils | S. aureus | E. coli | S. marcescen | P. aeruginosa |
|---|---|---|---|---|---|
| L1 | 2.5 | 10 | 5 | 5 | 2.5 |
| L2 | 2.5 | 10 | 2.5 | 5 | 2.5 |
| L3 | 1.25 | 5 | 5 | 2.5 | 0.31 |
| L4 | - | - | - | - | - |
| L5 | 2.5 | 1.25 | 2.5 | 5 | 2.5 |
| L6 | 2.5 | 2.5 | 2.5 | 2.5 | 1.25 |
| CoL1 | 1.25 | 2.5 | 2.5 | 2.5 | 2.5 |
| CoL2 | 1.25 | 5 | 10 | 2.5 | 1.25 |
| CoL3 | 0.15 | 2.5 | 1.25 | 0.31 | 0.15 |
| CoL4 | 2.5 | 1.25 | 1.25 | 1.25 | 0.62 |
| CoL5 | 1.25 | 1.25 | 1.25 | 0.62 | 0.62 |
| CoL6 | 1.25 | 1.25 | 2.5 | 1.25 | 0.62 |
| Tetracycline | 5 | 2.5 | 5 | 5 | 5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaygan, S.; Pasdar, H.; Foroughifar, N.; Davallo, M.; Motiee, F. Cobalt (II) Complexes with Schiff Base Ligands Derived from Terephthalaldehyde and ortho-Substituted Anilines: Synthesis, Characterization and Antibacterial Activity. Appl. Sci. 2018, 8, 385. https://doi.org/10.3390/app8030385
Shaygan S, Pasdar H, Foroughifar N, Davallo M, Motiee F. Cobalt (II) Complexes with Schiff Base Ligands Derived from Terephthalaldehyde and ortho-Substituted Anilines: Synthesis, Characterization and Antibacterial Activity. Applied Sciences. 2018; 8(3):385. https://doi.org/10.3390/app8030385
Chicago/Turabian StyleShaygan, Sahar, Hoda Pasdar, Naser Foroughifar, Mehran Davallo, and Fereshteh Motiee. 2018. "Cobalt (II) Complexes with Schiff Base Ligands Derived from Terephthalaldehyde and ortho-Substituted Anilines: Synthesis, Characterization and Antibacterial Activity" Applied Sciences 8, no. 3: 385. https://doi.org/10.3390/app8030385