Mg-Based Hydrogen Absorbing Materials for Thermal Energy Storage—A Review
Abstract
:1. Introduction
2. Chemical Fundamentals
3. Different Types of Mg-Based Materials for Thermal Energy Storage
3.1. Different Heat Storage Systems of Mg-Based Materials
3.1.1. Mg/MgH2 System
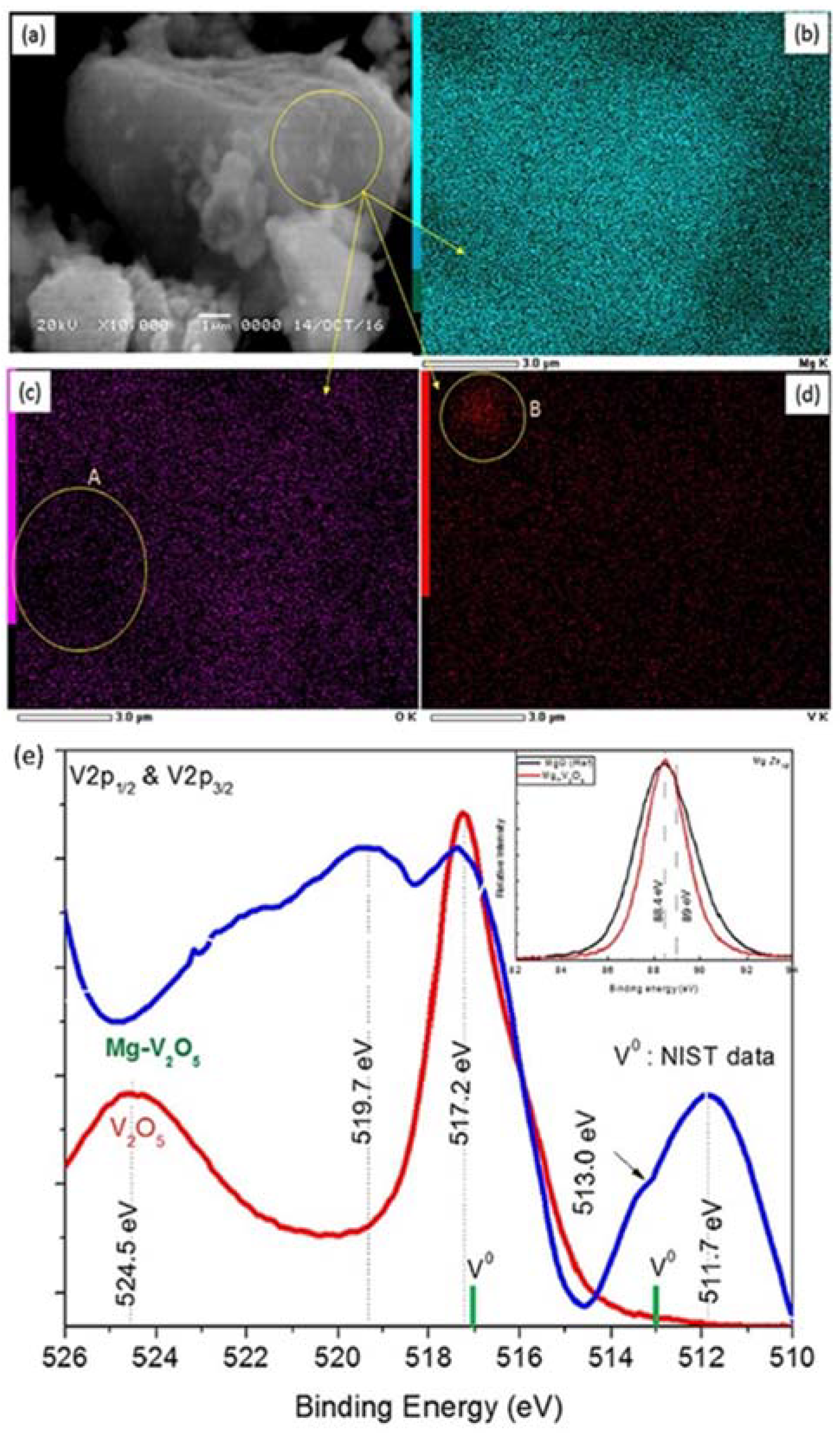
3.1.2. Doped Mg/MgH2 System
3.1.3. The Mg-Fe/Mg2FeH6 System
3.1.4. The Mg-Co-H System
3.1.5. The NaMgH3 System
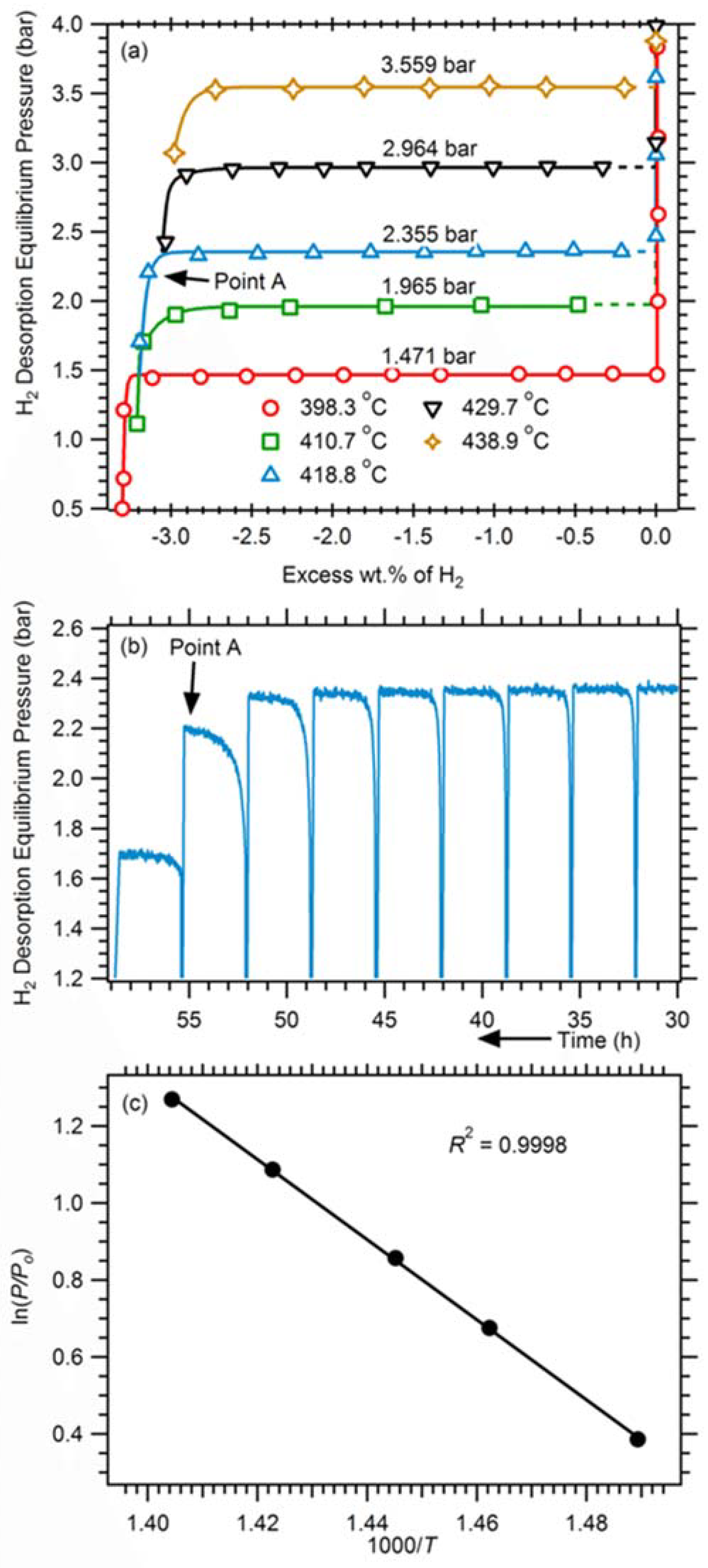
3.2. Kinetics and Thermal Conductivity Properties
4. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Gil, A.; Medrano, M.; Martorell, I.; Lázaro, A.; Dolado, P.; Zalba, B.; Cabeza, L.F. State of the art on high temperature thermal energy storage for power generation. Part 1—Concepts, materials and modellization. Renew. Sustain. Energy Rev. 2010, 14, 31–55. [Google Scholar] [CrossRef]
- Aydin, D.; Casey, S.P.; Riffat, S. The latest advancements on thermochemical heat storage systems. Renew. Sustain. Energy Rev. 2015, 41, 356–367. [Google Scholar] [CrossRef]
- Yan, T.; Wang, R.Z.; Li, T.X.; Wang, L.W.; Fred, I.T. A review of promising candidate reactions for chemical heat storage. Renew. Sustain. Energy Rev. 2015, 43, 13–31. [Google Scholar] [CrossRef]
- Ward, P.A.; Corgnale, C.; Teprovich, J.A.; Motyka, T.; Hardy, B.; Peters, B.; Zidan, R. High performance metal hydride based thermal energy storage systems for concentrating solar power applications. J. Alloys Compd. 2015, 645, S374–S378. [Google Scholar] [CrossRef] [Green Version]
- Qu, X.; Li, Y.; Li, P.; Wan, Q.; Zhai, F. The development of metal hydrides using as concentrating solar thermal storage materials. Front. Mater. Sci. 2015, 9, 317–331. [Google Scholar] [CrossRef]
- Abbott, D. Keeping the energy debate clean: How do we supply the world’s energy needs? Proc. IEEE 2010, 98, 42–66. [Google Scholar] [CrossRef]
- Mendoza, B. Total solar irradiance and climate. Adv. Space Res. 2005, 35, 882–890. [Google Scholar] [CrossRef]
- Hermann, W.A. Quantifying global exergy resources. Energy 2006, 31, 1685–1702. [Google Scholar] [CrossRef]
- Archer, C.L.; Jacobson, M.Z. Evaluation of global wind power. J. Geophys. Res. Atmos. 2005, 110. [Google Scholar] [CrossRef] [Green Version]
- Pollack, H.N.; Hurter, S.J.; Johnson, J.R. Heat flow from the earth’s interior: Analysis of the global data set. Rev. Geophys. 1993, 31, 267–280. [Google Scholar] [CrossRef]
- Milliman, J.; Farnsworth, K. Fluvial Discharge of Silicate to the Oceans: A Global Perspective; School of Marine Science, College of William and Mary: Gloucester Point, VA, USA, 1999. [Google Scholar]
- Panicker, N.N. Power resource estimate of ocean surface waves. Ocean Eng. 1975, 3, 429–439. [Google Scholar] [CrossRef]
- Ray, R.; Eanes, R.; Chao, B. Detection of tidal dissipation in the solid earth by satellite tracking and altimetry. Nature 1996, 381, 595–597. [Google Scholar] [CrossRef]
- Quayle, R.G.; Changery, M.J. Estimates of coastal deepwater wave energy potential for the world. In OCEANS 81; IEEE: Piscataway, NJ, USA, 1982; pp. 903–907. [Google Scholar]
- N’Tsoukpoe, K.E.; Liu, H.; Le Pierrès, N.; Luo, L. A review on long-term sorption solar energy storage. Renew. Sustain. Energy Rev. 2009, 13, 2385–2396. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Møller, K.T.; Sheppard, D.; Ravnsbæk, D.B.; Buckley, C.E.; Akiba, E.; Li, H.W.; Jensen, T.R. Complex metal hydrides for hydrogen, thermal and electrochemical energy storage. Energies 2017, 10, 1645. [Google Scholar] [CrossRef]
- Felderhoff, M.; Urbanczyk, R.; Peil, S. Thermochemical heat storage for high temperature applications—A review. Green 2013, 3, 113–123. [Google Scholar] [CrossRef]
- Felderhoff, M.; Bogdanovic, B. High temperature metal hydrides as heat storage materials for solar and related applications. Int. J. Mol. Sci. 2009, 10, 325–344. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, C.Y. A review of solar collectors and thermal energy storage in solar thermal applications. Appl. Energy 2013, 104, 538–553. [Google Scholar] [CrossRef] [Green Version]
- Bogdanović, B.; Ritter, A.; Spliethoff, B. Active MgH2-Mg systems for reversible chemical energy storage. Angew. Chem. Int. Ed. 1990, 29, 223–234. [Google Scholar] [CrossRef]
- Aochi, A.; Tadokoro, T.; Yoshida, K.; Kameyama, H.; Nobue, M.; Yamaguchi, T. Economical and technical evaluation of ut-3 thermochemical hydrogen production process for an industrial scale plant. Int. J. Hydrogen Energy 1989, 14, 421–429. [Google Scholar] [CrossRef]
- Alefeld, G. Energiespeicherung durch heterogenverdampfung (I). Wärme 1975, 81, 89. [Google Scholar]
- Herrmann, U.; Kearney, D.W. Survey of thermal energy storage for parabolic trough power plants. J. Sol. Energy Eng. 2002, 124, 145–152. [Google Scholar] [CrossRef]
- Reilly, H.; Kolb, G. Evaluation of Molten Salt Power Tower Technology Based on Experience at Solar Two; Report No. SAND2001-3674; Sandia National Laboratories: Albuquerque, New Mexico, 2001. [Google Scholar]
- Kubota, M.; Yokoyama, K.; Watanabe, F.; Hasatani, M. Heat releasing characteristics of CaO/CaCO3 reaction in a packed bed for high temperature heat storage and temperature up-grading. In Proceedings of the 8th International Conference on Thermal Energy Storage (Terrastock 2000), Stuttgart, Germany, 28 August–1 September 2000. [Google Scholar]
- Visscher, K. Solarcombi systems, heading for 100% solar fraction. Presented at the VSK Exhibition, Utrecht, The Netherlands, 2004. [Google Scholar]
- Hahne, E. Thermal energy storage: Some views on some problems. In Proceedings of the 8th International Heat Transfer Conference, San Francisco, CA, USA, 17–22 August 1986; pp. 279–292. [Google Scholar]
- Lovegrove, K.; Luzzi, A.; Kreetz, H. A solar-driven ammonia-based thermochemical energy storage system. Sol. Energy 1999, 67, 309–316. [Google Scholar] [CrossRef]
- Steinfeld, A.; Sanders, S.; Palumbo, R. Design aspects of solar thermochemical engineering—A case study: Two-step water-splitting cycle using the Fe3O4/FeO redox system. Sol. Energy 1999, 65, 43–53. [Google Scholar] [CrossRef]
- Shiizaki, S.; Nagashimga, I.; Iwata, K.; Hosoda, T.; Kameyama, H. Development of plate fin reactor for heat recovery system using methanol decomposition. In Proceedings of the 8th International Conference on Thermal Energy Storage (Terrastock 2000), Stuttgart, Germany, 28 August–1 September 2000. [Google Scholar]
- Rönnebro, E.; Whyatt, G.; Powell, M.; Simmons, K.; Fang, Z.; White, R. Reversible metal hydride thermal energy storage for high temperature power generation systems. In Proceedings of the Concentrating Solar Power Program Review, Phoenix, AZ, USA, 23–25 April 2013; pp. 29–30. [Google Scholar]
- Shao, H.; Xin, G.; Zheng, J.; Li, X.; Akiba, E. Nanotechnology in Mg-based materials for hydrogen storage. Nano Energy 2012, 1, 590–601. [Google Scholar] [CrossRef]
- Shao, H.; He, L.; Lin, H.; Li, H.-W. Progress and trends in magnesium-based materials for energy-storage research: A review. Energy Technol. 2018, 6, 445–458. [Google Scholar] [CrossRef]
- Li, J.; Xu, J.; Li, B.; He, L.; Lin, H.; Li, H.W.; Shao, H. Advanced sem and tem techniques applied in Mg-based hydrogen storage research. Scanning 2018, 2018. [Google Scholar] [CrossRef]
- Shao, H. Heat modeling and material development of Mg-based nanomaterials combined with solid oxide fuel cell for stationary energy storage. Energies 2017, 10, 1767. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Shao, H.; Li, W.; Lin, H. Catalysis and downsizing in Mg-based hydrogen storage materials. Catalysts 2018, 8, 89. [Google Scholar] [CrossRef]
- Bogdanović, B.; Ritter, A.; Spliethoff, B.; Straβburger, K. A process steam generator based on the high temperature magnesium hydride/magnesium heat storage system. Int. J. Hydrogen Energy 1995, 20, 811–822. [Google Scholar] [CrossRef]
- Bogdanović, B.; Spliethoff, B.; Ritter, A. The magnesium hydride system for heat storage and cooling. Z. Phys. Chem. 1989, 164, 1497–1508. [Google Scholar] [CrossRef]
- Reiser, A.; Bogdanović, B.; Schlichte, K. The application of Mg-based metal-hydrides as heat energy storage systems. Int. J. Hydrogen Energy 2000, 25, 425–430. [Google Scholar] [CrossRef]
- Friedlmeier, G.; Groll, M. Experimental analysis and modelling of the hydriding kinetics of Ni-doped and pure mg. J. Alloys Compd. 1997, 253, 550–555. [Google Scholar] [CrossRef]
- Steiner, D.; Groll, M.; Wierse, M. Development and Investigation of Thermal Energy Storage Systems for the Medium Temperature Range; American Society of Mechanical Engineers: New York, NY, USA, 1995. [Google Scholar]
- Buchner, H.; Povel, R. The daimler-benz hydride vehicle project. Int. J. Hydrogen Energy 1982, 7, 259–266. [Google Scholar] [CrossRef]
- Marty, P.; Fourmigue, J.-F.; De Rango, P.; Fruchart, D.; Charbonnier, J. Numerical simulation of heat and mass transfer during the absorption of hydrogen in a magnesium hydride. Energy Convers. Manag. 2006, 47, 3632–3643. [Google Scholar] [CrossRef]
- Askri, F.; Jemni, A.; Nasrallah, S.B. Study of two-dimensional and dynamic heat and mass transfer in a metal–hydrogen reactor. Int. J. Hydrogen Energy 2003, 28, 537–557. [Google Scholar] [CrossRef]
- Sheppard, D.; Humphries, T.; Buckley, C. What is old is new again. Mater. Today 2015, 18, 415. [Google Scholar] [CrossRef]
- Humphries, T.D.; Sheppard, D.A.; Rowles, M.R.; Sofianos, M.V.; Buckley, C. Fluoride substitution in sodium hydride for thermal energy storage applications. J. Mater. Chem. A 2016, 4, 12170–12178. [Google Scholar] [CrossRef] [Green Version]
- Sheppard, D.; Corgnale, C.; Hardy, B.; Motyka, T.; Zidan, R.; Paskevicius, M.; Buckley, C. Hydriding characteristics of NaMgH2F with preliminary technical and cost evaluation of magnesium-based metal hydride materials for concentrating solar power thermal storage. RSC Adv. 2014, 4, 26552–26562. [Google Scholar] [CrossRef]
- Tortoza, M.S.; Humphries, T.D.; Sheppard, D.A.; Paskevicius, M.; Rowles, M.R.; Sofianos, M.V.; Aguey-Zinsou, K.-F.; Buckley, C.E. Thermodynamics and performance of the Mg-H-F system for thermochemical energy storage applications. Phys. Chem. Chem. Phys. 2018, 20, 2274–2283. [Google Scholar] [CrossRef] [PubMed]
- Bogdanović, B.; Liao, S.T.; Schwickardi, M.; Sikorsky, P.; Spliethoff, B. Katalytische synthese von magnesiumhydrid unter milden bedingungen. Angew. Chem. 1980, 92, 845–846. [Google Scholar] [CrossRef]
- Bogdanović, B.; Hartwig, T.; Spliethoff, B. The development, testing and optimization of energy storage materials based on the MgH2-Mg system. Int. J. Hydrogen Energy 1993, 18, 575–589. [Google Scholar] [CrossRef]
- Chen, J.; Xia, G.; Guo, Z.; Huang, Z.; Liu, H.; Yu, X. Porous Ni nanofibers with enhanced catalytic effect on the hydrogen storage performance of MgH2. J. Mater. Chem. A 2015, 3, 15843–15848. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Fast hydrogen sorption kinetics of nanocrystalline mg using Nb2O5 as catalyst. Scr. Mater. 2003, 49, 213–217. [Google Scholar] [CrossRef]
- Kumar, S.; Kojima, Y.; Dey, G.K. Morphological effects of Nb2O5 on MgH2-Mg system for thermal energy storage application. Int. J. Hydrogen Energy 2018, 43, 809–816. [Google Scholar] [CrossRef]
- Kumar, S.; Kojima, Y.; Kain, V. Nano-engineered MgH2-Mg system for solar thermal energy storage. Sol. Energy 2017, 150, 532–537. [Google Scholar] [CrossRef]
- Mistry, P.C.; Grant, D.M.; Stuart, A.D.; Manickam, K.; Walker, G.S. Evolution of catalyst coated atomised magnesium spheres—An alternative thermal storage medium for concentrated solar power applications. Int. J. Hydrogen Energy 2017, 42, 28453–28463. [Google Scholar] [CrossRef]
- Didisheim, J.; Zolliker, P.; Yvon, K.; Fischer, P.; Schefer, J.; Gubelmann, M.; Williams, A. Dimagnesium iron (II) hydride, MgH2FeH6, containing octahedral FeH64− anions. Inorg. Chem. 1984, 23, 1953–1957. [Google Scholar] [CrossRef]
- Huot, J.; Boily, S.; Akiba, E.; Schulz, R. Direct synthesis of Mg 2 FeH 6 by mechanical alloying. J. Alloys Compd. 1998, 280, 306–309. [Google Scholar] [CrossRef]
- Gennari, F.; Castro, F.; Gamboa, J.A. Synthesis of MgH2FeH6 by reactive mechanical alloying: Formation and decomposition properties. J. Alloys Compd. 2002, 339, 261–267. [Google Scholar] [CrossRef]
- Varin, R.; Li, S.; Calka, A.; Wexler, D. Formation and environmental stability of nanocrystalline and amorphous hydrides in the 2Mg–Fe mixture processed by controlled reactive mechanical alloying (CRMA). J. Alloys Compd. 2004, 373, 270–286. [Google Scholar] [CrossRef]
- Bogdanović, B.; Reiser, A.; Schlichte, K.; Spliethoff, B.; Tesche, B. Thermodynamics and dynamics of the Mg–Fe–H system and its potential for thermochemical thermal energy storage. J. Alloy. Compd. 2002, 345, 77–89. [Google Scholar] [CrossRef]
- Urbanczyk, R.; Peinecke, K.; Peil, S.; Felderhoff, M. Development of a heat storage demonstration unit on the basis of MgH2FeH6 as heat storage material and molten salt as heat transfer media. Int. J. Hydrogen Energy 2017, 42, 13818–13826. [Google Scholar] [CrossRef]
- Zolliker, P.; Yvon, K.; Fischer, P.; Schefer, J. Dimagnesium cobalt (I) pentahydride, Mg2CoH5, containing square-pyramidal pentahydrocobaltate (4−)(CoH54−) anions. Inorg. Chem. 1985, 24, 4177–4180. [Google Scholar] [CrossRef]
- Černý, R.; Bonhomme, F.; Yvon, K.; Fischer, P.; Zolliker, P.; Cox, D.; Hewat, A. Hexamagnesium dicobalt undecadeuteride Mg6Co2D11: Containing [CoD4]5− and [CoD5]4− complex anions conforming to the 18-electron rule. J. Alloys Compd. 1992, 187, 233–241. [Google Scholar] [CrossRef]
- Ivanov, E.Y.; Konstanchuk, I.; Stepanov, A.; Jie, Y.; Pezat, M.; Darriet, B. The ternary system magnesium-cobalt-hydrogen. Inorg. Chem. 1989, 28, 613–615. [Google Scholar] [CrossRef]
- Shao, H.; Xu, H.; Wang, Y.; Li, X. Synthesis and hydrogen storage behavior of Mg–Co–H system at nanometer scale. J. Solid State Chem. 2004, 177, 3626–3632. [Google Scholar] [CrossRef]
- Yoshida, M.; Bonhomme, F.; Yvon, K.; Fischer, P. On the composition and structure of the cubic δ-phase in the Mg–Co–H system. J. Alloys Compd. 1993, 190, 45–46. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, W.; Udovic, T.J.; Rush, J.J.; Yildirim, T. Crystal chemistry of perovskite-type hydride NaMgH3: Implications for hydrogen storage. Chem. Mater. 2008, 20, 2335–2342. [Google Scholar] [CrossRef]
- Sheppard, D.A.; Paskevicius, M.; Buckley, C.E. Thermodynamics of hydrogen desorption from NaMgH3 and its application as a solar heat storage medium. Chem. Mater. 2011, 23, 4298–4300. [Google Scholar] [CrossRef]
- Ikeda, K.; Kato, S.; Shinzato, Y.; Okuda, N.; Nakamori, Y.; Kitano, A.; Yukawa, H.; Morinaga, M.; Orimo, S. Thermodynamical stability and electronic structure of a perovskite-type hydride, NaMgH3. J. Alloys Compd. 2007, 446, 162–165. [Google Scholar] [CrossRef]
- Komiya, K.; Morisaku, N.; Rong, R.; Takahashi, Y.; Shinzato, Y.; Yukawa, H.; Morinaga, M. Synthesis and decomposition of perovskite-type hydrides, MMgH3 (M = Na, K, Rb). J. Alloys Compd. 2008, 453, 157–160. [Google Scholar] [CrossRef]
- Ikeda, K.; Kogure, Y.; Nakamori, Y.; Orimo, S. Reversible hydriding and dehydriding reactions of perovskite-type hydride NaMgH3. Scr. Mater. 2005, 53, 319–322. [Google Scholar] [CrossRef]
- Pottmaier, D.; Pinatel, E.R.; Vitillo, J.G.; Garroni, S.; Orlova, M.; Baró, M.D.; Vaughan, G.B.; Fichtner, M.; Lohstroh, W.; Baricco, M. Structure and thermodynamic properties of the NaMgH3 perovskite: A comprehensive study. Chem. Mater. 2011, 23, 2317–2326. [Google Scholar] [CrossRef]
- Nomura, K.; Akiba, E.; Ono, S. Kinetics of the reaction between Mg2Ni and hydrogen. Int. J. Hydrogen Energy 1981, 6, 295–303. [Google Scholar] [CrossRef]
- Satya Sekhar, B.; Muthukumar, P.; Saikia, R. Tests on a metal hydride based thermal energy storage system. Int. J. Hydrogen Energy 2012, 37, 3818–3824. [Google Scholar] [CrossRef]
- Hardian, R.; Pistidda, C.; Chaudhary, A.L.; Capurso, G.; Gizer, G.; Cao, H.; Milanese, C.; Girella, A.; Santoru, A.; Yigit, D.; et al. Waste Mg-Al based alloys for hydrogen storage. Int. J. Hydrogen Energy 2017. [Google Scholar] [CrossRef]
- Shao, H.; Ma, W.; Kohno, M.; Takata, Y.; Xin, G.; Fujikawa, S.; Fujino, S.; Bishop, S.; Li, X. Hydrogen storage and thermal conductivity properties of Mg-based materials with different structures. Int. J. Hydrogen Energy 2014, 39, 9893–9898. [Google Scholar] [CrossRef]
- Wilhelm, J. Reaktionen im festen zustande bei höheren temperaturen. Reaktionsgeschwindigkeiten endotherm verlaufender umsetzungen. Z. Anorg. Allg. Chem. 1927, 163, 1–30. [Google Scholar]
- Toberer, E.S.; Baranowski, L.L.; Dames, C. Advances in thermal conductivity. Annu. Rev. Mater. Res. 2012, 42, 179–209. [Google Scholar] [CrossRef]
- Popilevsky, L.; Skripnyuk, V.M.; Amouyal, Y.; Rabkin, E. Tuning the thermal conductivity of hydrogenated porous magnesium hydride composites with the aid of carbonaceous additives. Int. J. Hydrogen Energy 2017, 42, 22395–22405. [Google Scholar] [CrossRef]
- Xia, G.; Tan, Y.; Chen, X.; Sun, D.; Guo, Z.; Liu, H.; Ouyang, L.; Zhu, M.; Yu, X. Monodisperse magnesium hydride nanoparticles uniformly self-assembled on graphene. Adv. Mater. 2015, 27, 5981–5988. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-H.; Park, M.; Lee, Y.H.; Kim, S.; Im, Y.H.; Suh, J.-Y.; Cho, Y.W. Effective thermal conductivity of MgH2 compacts containing expanded natural graphite under a hydrogen atmosphere. Int. J. Hydrogen Energy 2014, 39, 349–355. [Google Scholar] [CrossRef]
- Crivello, J.C.; Denys, R.V.; Dornheim, M.; Felderhoff, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Walker, G.S.; et al. Mg-based compounds for hydrogen and energy storage. Appl. Phys. A 2016, 122, 85. [Google Scholar] [CrossRef]
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef] [Green Version]








| Renewable Source | Max. Power (TW) | Percentage of Total Solar Energy |
|---|---|---|
| Total surface solar [7] | 85,000 | 100% |
| Desert solar [6] | 7,650 | 9% |
| Ocean thermal [8] | 100 | 0.12% |
| Wind [9] | 72 | 0.08% |
| Geothermal [10] | 44 | 0.05% |
| River hydroelectric [11] | 7 | 0.008% |
| Biomass [6] | 7 | 0.008% |
| Open ocean wave [12] | 7 | 0.008% |
| Tidal wave [13] | 4 | 0.003% |
| Coastal wave [14] | 3 | 0.003% |
| Heat Storage Method | Materials | Specific Heat (kJ/kg/K) | Energy Density (GJ/m3) | Working Temperature (°C) | Reference |
|---|---|---|---|---|---|
| Sensible heat | Rock | 1.30 | n.a. | 200−300 | [25] |
| Concrete | 0.85 | n.a. | 200−400 | [25] | |
| Mineral oil | 2.60 | n.a. | 200−300 | [1] | |
| Carbonate salts | 1.80 | n.a | 450−850 | [26] | |
| Latent heat | KNO3/KCl | 1.21 | n.a. | 320 | [1] |
| E117 (commercial) | 2.61 | 0.25 | 117 | [1] | |
| A164 (commercial) | n.a. | 0.46 | 164 | [1] | |
| AlSi12 | 1.04 | 1.51 | 576 | [1] | |
| Na2CO3 | n.a. | 0.70 | 854 | [25] | |
| Chemical heat | Calcium carbonate | n.a | 4.40 | 800−900 | [27] |
| Iron carbonate | n.a. | 2.60 | 180 | [28] | |
| Metal hydrides | n.a. | 4.00 | 200−500 | [29] | |
| Magnesium oxide | n.a. | 3.30 | 250−400 | [1] | |
| Hydroxides | n.a. | 3.00 | 500 | [29] |
| Compound | Reaction | Material Energy Density | Reaction Temperature (°C) |
|---|---|---|---|
| Ammonia [30] | NH3 + ΔH ↔ 1/2N2 + 3/2H2 | 67 kJ/mol | 400–500 |
| Methane/water [29] | CH4 + H2O ↔ CO + 3H2 | n.a. | 500–1000 |
| Hydroxides [29] | Ca(OH)2 ↔ CaO + H2O | 3 GJ/m3 | 500 |
| Calcium carbonate [27] | CaCO3 ↔ CaO + CO2 | 4.4 GJ/m3 | 800–900 |
| Iron carbonate [28] | FeCO3 ↔ FeO + CO2 | 2.6 GJ/m3 | 180 |
| Metal hydrides [29] | Metal xH2 ↔ metal yH2 + (x − y) H2 | 4 GJ/m3 | 200–500 |
| Metal oxides (Zn and Fe) [31] | e.g., 2-step water splitting using Fe3O4/FeO redox system | n.a. | 2000–2500 |
| Aluminium ore alumina [1] | n.a. | n.a. | 2100–2300 |
| Methanolation–demethanolation [32] | CH3OH ↔ CO + 2H2 | n.a. | 200–250 |
| Magnesium oxide [1] | MgO + H2O ↔ Mg (OH)2 | 3.3 GJ/m3 | 250–400 |
| Sample | Heat Capacity (kJ/kg/K) | Thermal Conductivity (W/m/K) | Hydrogen Absorption Kinetics |
|---|---|---|---|
| 325 mesh Mg | 1.046 | 10.42 | poor |
| Mg single crystal (0001) | 1.082 | 168.0 | poor |
| Mg nanoparticles | 0.954 | 4.985 | good |
| Mg50Co50 bcc alloy | 0.594 | 0.432 | superior |
| Pd capped Mg thin film | 1.020 | 82.0 | superior |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Li, J.; Shao, H.; He, L. Mg-Based Hydrogen Absorbing Materials for Thermal Energy Storage—A Review. Appl. Sci. 2018, 8, 1375. https://doi.org/10.3390/app8081375
Li B, Li J, Shao H, He L. Mg-Based Hydrogen Absorbing Materials for Thermal Energy Storage—A Review. Applied Sciences. 2018; 8(8):1375. https://doi.org/10.3390/app8081375
Chicago/Turabian StyleLi, Bo, Jianding Li, Huaiyu Shao, and Liqing He. 2018. "Mg-Based Hydrogen Absorbing Materials for Thermal Energy Storage—A Review" Applied Sciences 8, no. 8: 1375. https://doi.org/10.3390/app8081375





