Assessing Concentration Changes of Odorant Compounds in the Thermal-Mechanical Drying Phase of Sediment-Like Wastes from Olive Oil Extraction
Abstract
:Featured Application
Abstract
1. Introduction
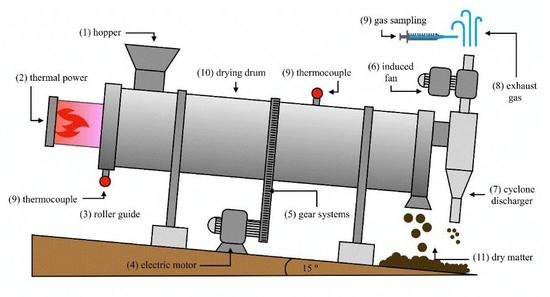
2. Materials and Methods
2.1. Identification of the Samples
2.2. Environmental Conditions
2.3. Sampling of VOCs and Conditions of Thermal Desorption-Gas Chromatography/Mass Spectrometry (TD-GC/MS)
2.4. Reagents
3. Results and Discussion
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Olive Oil. Available online: http://www.internationaloliveoil.org/store/index/48-olivae-publications (accessed on 20 March 2018).
- Chile Oliva. Available online: https://www.chileoliva.cl/es/principal/ (accessed on 25 March 2017).
- Hernández, D.; Astudillo, L.; Gutiérrez, M.; Tenreiro, C.; Retamal, C.; Rojas, C. Biodiesel production from an industrial residue: Alperujo. Ind. Crops Prod. 2014, 52, 495–498. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; González, J.; García, D.; Cegarra, J. Agrochemical characterization of “alperujo”, a solid by-product of the two-phase centrifugation method for olive oil extraction. Bioresour. Technol. 2004, 91, 195–200. [Google Scholar] [CrossRef]
- Christoforou, E.; Kylili, A.; Fokaides, P. Technical and economical evaluation of olive mills solid waste pellets. Renew. Energy 2016, 96, 33–41. [Google Scholar] [CrossRef]
- Rincón, B.; Bujalance, L.; Fermoso, F.G.; Martín, A.; Borja, R. Effect of Ultrasonic Pretreatment on Biomethane Potential of Two-Phase Olive Mill Solid Waste: Kinetic Approach and Process Performance. Sci. World J. 2014, 2014, 648624. [Google Scholar] [CrossRef] [PubMed]
- Arjona, R.; García, A.; Ollero, P. Drying of alpeorujo, a waste product of the olive oil mill industry. J. Food Energy 1999, 41, 229–234. [Google Scholar] [CrossRef]
- Gómez de la Cruza, F.; Casanova-Peláez, P.; Palomar-Carnicero, J.; Cruz-Peragóna, F. Characterization and analysis of the drying real process in an industrial olive-oil mill waste rotary dryer: A case of study in Andalusia. Appl. Therm. Eng. 2017, 116, 1–10. [Google Scholar] [CrossRef]
- Fagernäs, L.; Brammer, J.; Wilén, C.; Lauer, M.; Verhoeff, F. Drying of biomass for second generation synfuel production. Biomass Bioenergy 2010, 34, 1267–1277. [Google Scholar] [CrossRef]
- Hernández, D.; Astudillo, C.A.; Fernández-Palacios, E.; Cataldo, F.; Tenreiro, C.; Gabriel, D. Evolution of physical-chemical parameters, microbial diversity and VOC emissions of olive oil mill waste exposed to ambient conditions in open reservoirs. Waste Manag. 2018, 79, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Jauhiainen, J.; Martin-Gullon, I.; Conesa, J.; Font, R. Emissions from pyrolysis and combustion of olive oil solid waste. J. Anal. Appl. Pyrolysis 2005, 74, 512–517. [Google Scholar] [CrossRef]
- DIN. Available online: http://www.din.de/de (accessed on 25 February 2017).
- Official Methods of Analysis of AOAC INTERNATIONAL, 20th ed. 2016. Available online: http://www.aoac.org/aoac_prod_imis/AOAC/Publications/Official_Methods_of_Analysis/AOAC_Member/Publications/OMA/AOAC_Official_Methods_of_Analysis.aspx (accessed on 16 February 2017).
- NIST Chemistry Webbook. 2010. Available online: http://webbook.nist.gov/ chemistry (accessed on 10 June 2018).
- Dorado, A.D.; Husni, S.; Pascual, G.; Puigdellivol, C.; Gabriel, D. Inventory and treatment of compost maturation emissions in a municipal solid waste treatment facility. Waste Manag. 2014, 34, 344–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, W.-C. Volatile constituents detected in smoke condensates from the combination of the smoking ingredients sucrose, black tea leaves, and bread flour. J. Food Drug Anal. 2013, 21, 292–300. [Google Scholar] [CrossRef] [Green Version]
- Caprino, F.; Moretti, V.; Bellagamba, F.; Turchini, G.; Busetto, M.; Giani, I.; Paleari, M.; Pazzaglia, M. Fatty acid composition and volatile compounds of caviar from farmed white sturgeon (Acipenser transmontanus). Anal. Chim. Acta 2008, 617, 139–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buttery, B.G.; Turnbaugh, J.G.; Ling, L.C. Contribution of volatiles to rice aroma. J. Agric. Food Chem. 1988, 36, 1006–1009. [Google Scholar] [CrossRef]
- Nagata, Y. Odor Measurement Review, Measurement of Odor Threshold by Triangle Odor Bag Method; Ministery of Environmental Goverment of Japan: Tokyo, Japan, 2003; pp. 122–123.
- John, H.; Montgomery, B. Groundwater Chemicals Desk Reference, 3rd ed.; Taylor & Francis: Abingdon, UK, 1995. [Google Scholar]
- Odour Threshold Shop. Available online: http://www.odourthreshold.com/ (accessed on 3 August 2018).
- Dalai, A.; Schoenau, G.; Das, D.; Adapa, P. Volatile Organic Compounds emitted during High-temperature Alfalfa Drying. Biosyst. Eng. 2006, 94, 57–66. [Google Scholar] [CrossRef]
- Rodríguez, G.; Rodriguez, R.; Jimenez, A.; Guillén, R.; Fernández-Bolaños, J. Effect of steam treatment of alperujo on the composition, enzymatic saccharification, and in vitro digestibility of alperujo. J. Agric. Food Chem. 2007, 55, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.T.; Luna, G.; Aparicio, R. Comparative study of virgin olive oil sensory defects. Food Chem. 2005, 91, 293–301. [Google Scholar] [CrossRef]
- Castells, X. Tratamiento y valorización energética de residuos; Fundación Universitaria Iberoamericana, Ed.; Ediciones Díaz de Santos: Madrid, Spain, 2005. [Google Scholar]
- Dexter, J.; Siemers, D.; Head, J.; Miller, D. Multi-Zonemethodfor Controlling Wocand Nox Emissions in a Flatline Conveyorwafer Drying System. U.S. Patent 5,749,160, 12 May 1998. [Google Scholar]
- DIPPR: The Design Institute for Physical Properties. DIPPR 801 Thermophysical Property Database and DIADEM Predictive, Software; Brigham Young University: Provo, UT, USA, 2007.
- Vichi, S.; Pizzale, L.; Conte, L.S.; Buxaderas, S.; Lopez-Tamames, E. Solid-phase microextraction in the analysis of virgin olive oil volatile fraction: Modifications induced by oxidation and suitable markers of oxidative status. J. Agric. Food Chem. 2003, 51, 6564–6571. [Google Scholar] [CrossRef] [PubMed]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R., Jr.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 10, 273–286. [Google Scholar] [CrossRef]
- Morales, M.T.; Rios, J.J.; Aparicio, R. Changes in the Volatile Composition of Virgin Olive Oil during Oxidation: Flavors and Off-Flavors. J. Agric. Food Chem. 1997, 45, 2666–2673. [Google Scholar] [CrossRef]
- Huang, B.; Wang, G.; Chu, Z.; Qin, L. Effect of Oven Drying, Microwave Drying, and Silica Gel Drying Methods on the Volatile Components of Ginger (Zingiber officinale Roscoe) by HS-SPME-GC-MS. Dry. Technol. 2012, 30, 248–255. [Google Scholar] [CrossRef]



| Month | Moisture Content (% wb) ** Oven Method—AOAC 945.15 | Ashes (% wb) * Muffle Method—AOAC 940.26 | Measuring Heating Value (MJ kg−1) * Norm DIN Serie 51.900 | |||
|---|---|---|---|---|---|---|
| Alperujo | Orujo | Alperujo | Orujo | Alperujo | Orujo | |
| Jul | 77.7 ± 0.1 | 79.5 ± 0.2 | 2.4 ± 0.1 | 3.2 ± 0.1 | 22.3 ± 0.1 | 22.4 ± 0.2 |
| Aug | 77.9 ± 0.5 | 80.2 ± 0.3 | 2.2 ± 0.1 | 3.1 ± 0.1 | 22.1 ± 0.2 | 22.4 ± 0.1 |
| Sept | 80.0 ± 0.5 | 81.8 ± 0.4 | 1.9 ± 0.2 | 2.9 ± 0.1 | 22.4 ± 0.1 | 22.5 ± 0.1 |
| Oct | 73.1 ± 0.2 | 77.4 ± 0.2 | 1.6 ± 0.1 | 2.7 ± 0.1 | 22.2 ± 0.1 | 22.4 ± 0.2 |
| Nov | 60.5 ± 0.4 | 63.4 ± 0.2 | 1.6 ± 0.1 | 2.2 ± 0.1 | 22.1 ± 0.1 | 22.6 ± 0.2 |
| Dec | 53.0 ± 1.0 | 55.4 ± 0.4 | 1.2 ± 0.2 | 2.0 ± 0.1 | 22.3 ± 0.1 | 22.7 ± 0.2 |
| Based Compound | Sample | Standard 1 | Standard 2 | Standard 3 |
|---|---|---|---|---|
| Methanol | n-Hexane | 0.5 ppmv | 1.5 ppmv | 3.0 ppmv |
| Methanol | n-Octane | 0.5 ppmv | 1.5 ppmv | 3.0 ppmv |
| Methanol | n-Nonane | 0.5 ppmv | 2.0 ppmv | 5.0 ppmv |
| Methanol | D3710-95 | 0.5 ppmv | 1.0 ppmv | 2.5 ppmv |
| Family | Name | Months (1–6) | Odor Threshold | Reference | |
|---|---|---|---|---|---|
| Alperujo | Orujo | ppmv | |||
| Aldehydes | 2-Furancarboxaldehyde, 5-methyl- | 1,3,4 | 1 | 3.0 | Sung et al. [16] |
| 3-Cyclopentene-1-acetaldehyde, 2-oxo- | 1,3 | 1–3 | |||
| 9-Octadecenal | 3,4 | >1.0 | Caprino et al. [17] | ||
| Benzaldehyde | 1–6 | 1–6 | 0.35–3.5 | Buttery et al. [18] | |
| Furfural | 1–6 | 1–6 | 3.0–23.0 | Buttery et al. [18] | |
| Hexanal | 1–6 | 1–6 | 0.28 | Nagata [19] | |
| Methyl glyoxal | 3,4 | 3–5 | |||
| Nonanal | 1–6 | 0.34 | Nagata [19] | ||
| Octanal | 1–6 | 0.01 | Nagata [19] | ||
| Amides | Propanamide, 2-hydroxy- | 2–6 | 1–6 | ||
| Amines | Pyridine, 3-ethyl- | 2–3 | 2–3 | ||
| Phenolic alcohols | |||||
| 2-Methoxy-4-vinylphenol | 2–3 | 1–6 | 0.003 | Nagata [19] | |
| 3-tert-Butyl-4-hydroxyanisole | 2–6 | 2–3 | |||
| Phenol | 1–6 | 1–6 | 0.0056 | Nagata [19] | |
| Phenol, 2-methoxy-4-(1-propenyl)- | 2–5 | 1–6 | |||
| Phenol, 2,6-dimethoxy- | 1–2 | 1 | |||
| Phenol, 4-ethyl-2-methoxy- | 1–5 | 1–6 | |||
| 5-tert-Butylpyrogallol | 2–3 | 2-3 | |||
| Aliphatic alcohols | 1-Dodecanol, 3,7,11-trimethyl | 1–3 | |||
| 1-Dodecanol, 3,7,11-trimethyl- | 2–5 | 2–5 | |||
| 1-Hexadecanol, 2-methyl- | 1 | 1–2 | |||
| 3-Nonen-1-ol, (E)- | 2–3 | ||||
| 2-Furanmethanol | 1–6 | 1-6 | 8.0 | Montgomery [20] | |
| Aromatic HCs | Benzene, 1-azido-3-methyl- | 2–3 | 2 | ||
| Benzene, 1,3-dimethyl- | 2–5 | 2 | |||
| Toluene | 1–6 | 1–6 | 0.33 | Nagata [19] | |
| Esters | 10-Octadecenoic acid, methyl ester | 2–3 | |||
| Hexanoic acid, 2-phenylethyl ester | 2–6 | ||||
| Carboxylic acids | Acetic acid | 1–6 | 1–6 | 0.0060 | Nagata [19] |
| Propanoic acid | 4–5 | 0.0057 | Nagata [19] | ||
| 9-Hexadecenoic acid | 1–6 | ||||
| Aliphatic HCs | 1-Decene | 3–6 | 5–6 | ||
| 2-Octene | 4–5 | 99.808 | http://www.odourthreshold.com [21] | ||
| 8-Heptadecene | 5 | 3–6 | |||
| Heptane | 1–6 | ||||
| Hexane | 1–6 | 1–6 | 1.5 | Nagata [19] | |
| Nonane | 1–6 | 1–6 | 2.2 | Nagata [19] | |
| Octane | 1–6 | 1–6 | 1.7 | Nagata [19] | |
| Tetradecane, 2,6,10-trimethyl- | 3–6 | 1–6 | |||
| 1,4-Pentadiene | 2–3 | 1–2 | |||
| Ketones | 1,2-Cyclopentanedione, 3-methyl- | 5–3 | 2 | ||
| 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- | 1–6 | 4–5 | |||
| 2-Cyclopenten-1-one, 2-methyl- | 3–6 | 1–6 | |||
| 2-Propanone, 1-(acetyloxy)- | 4–5 | 1–6 | |||
| 2-Propanone, 1-hydroxy- | 2–6 | 5 | |||
| Ethers | Octane, 1-methoxy- | 1–2 | 6 | ||
| Family | Name | Boiling Temperature | Vapor Pressure | Jul | Aug | Sep | Oct | Nov | Dec | |
|---|---|---|---|---|---|---|---|---|---|---|
| ºC | bar | 1 | 2 | 3 | 4 | 5 | 6 | average | ||
| Alcohols | 2-Furanmethanol | 170.10 * | 0.45 | 3.0 ± 0.1 | 3.1 ± 0.1 | 3.5 ± 0.1 | 4.0 ± 0.1 | 4.2 ± 0.1 | 5.2 ± 0.2 | 3.8 ± 0.5 |
| Phenol | 181.93 * | 0.40 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.9 ± 0.2 | 1.0 ± 0.1 | 0.5 ± 0.4 | |
| Aldehydes | Benzaldehyde | 178.66 * | 0.46 | 3.5 ± 0.0 | 4.0 ± 0.1 | 4.2 ± 0.1 | 4.8 ± 0.2 | 4.9 ± 0.1 | 5.1 ± 0.1 | 4.4 ± 0.6 |
| Furfural | 161.55 ** | 0.73 | 6.3 ± 0.1 | 7.5 ± 0.1 | 7.8 ± 0.1 | 8.3 ± 0.1 | 8.9 ± 0.0 | 10.5 ± 0.0 | 8.2 ± 1.4 | |
| Hexanal | 128.14 * | 1.80 | 0.1 ± 0.1 | 0.5 ± 0.0 | 0.8 ±0.0 | 1.2 ± 0.0 | 1.3 ± 0.1 | 1.5 ± 0.0 | 0.9 ± 0.5 | |
| Nonanal | 194.93 * | 0.29 | 0.3 ± 0.0 | 0.6 ± 0.1 | 0.9 ± 0.1 | 1.5 ± 0.0 | 1.8 ± 0.1 | 2.1 ± 0.0 | 1.2 ± 0.7 | |
| Octanal | 174.20 * | 0.53 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.5 ± 0.1 | 0.9 ± 0.0 | 1.1 ± 0.2 | 1.2 ± 0.1 | 0.7 ± 0.5 | |
| Aromatic HCs | Toluene | 110.68 * | 2.75 | 0.5 ± 0.1 | 0.7 ± 0.2 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | 1.2 ± 0.1 | 0.8 ± 0.2 |
| Carboxylic acids | Acetic acid | 118.01 * | 2.49 | 2.8 ± 0.1 | 3.5 ± 0.1 | 2.9 ± 0.1 | 3.5 ± 0.1 | 4.2 ± 0.0 | 4.9 ± 0.1 | 3.6 ± 0.8 |
| Aliphatic HCs | Hexane | 68.73 * | 7.41 | 0.9 ± 0.0 | 1.9 ± 0.1 | 2.9 ± 0.1 | 2.8 ± 0.0 | 2.5 ± 0.1 | 1.5 ± 0.2 | 2.1 ± 0.8 |
| Octane | 125.69 * | 1.90 | 3.2 ± 0.0 | 3.2 ± 0.1 | 3.5 ± 0.2 | 2.1 ± 0.0 | 1.0 ± 0.1 | 0.5 ± 0.1 | 2.3 ± 1.3 | |
| Nonane | 150.66 * | 0.99 | 2.1 ± 0.1 | 2.9 ± 0.1 | 3.0 ± 0.1 | 1.5 ± 0.1 | 1.9 ± 0.1 | 2.0 ± 0.1 | 2.2 ± 0.6 |
| Family | Name | Boiling Temperature | Vapor Pressure | Jul | Aug | Sep | Oct | Nov | Dec | |
|---|---|---|---|---|---|---|---|---|---|---|
| °C | bar | 1 | 2 | 3 | 4 | 5 | 6 | average | ||
| Alcohols | 2-Furanmethanol | 170.10 * | 0.45 | 3.1 ± 0.1 | 3.9 ± 0.2 | 4.2 ± 0.2 | 4.2 ± 0.1 | 4.9 ± 0.0 | 5.7 ± 0.2 | 4.3 ± 0.9 |
| Phenol | 181.93 * | 0.40 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.8 ± 0.2 | 0.7 ± 0.1 | 1.2 ± 0.1 | 1.8 ± 0.2 | 0.8 ± 0.7 | |
| Aldehydes | Benzaldehyde | 178.66 * | 0.46 | 3.1 ± 0.2 | 3.2 ± 0.1 | 3.8 ± 0.1 | 4.3 ± 0.1 | 4.9 ± 0.0 | 4.9 ± 0.1 | 4.0 ± 0.8 |
| Furfural | 161.55 ** | 0.73 | 3.9 ± 0.1 | 4.3 ± 0.1 | 5.8 ± 0.1 | 5.5 ± 0.2 | 6.9 ± 0.1 | 8.6 ± 0.0 | 5.8 ± 1.7 | |
| Hexanal | 128.14 * | 1.80 | 1.7 ± 0.0 | 1.1 ± 0.1 | 1.3 ± 0.1 | 2.1 ± 0.2 | 2.2 ± 0.1 | 2.3 ± 0.0 | 1.8 ± 0.5 | |
| Nonanal | 194.93 * | 0.29 | 0.2 ± 0.0 | 0.5 ± 0.0 | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.0 | 1.5 ± 0.0 | 0.9 ± 0.5 | |
| Octanal | 174.20 * | 0.53 | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.8 ± 0.1 | 1.2 ± 0.2 | 1.8 ± 0.2 | 2.1 ± 0.1 | 1.1 ± 0.8 | |
| Aromatic HCs | Toluene | 110.68 * | 2.75 | 0.8 ± 0.1 | 0.8 ± 0.2 | 1.2 ± 0.2 | 1.3 ± 0.1 | 1.7 ± 0.2 | 2.1 ± 0.1 | 1.3 ± 0.5 |
| Carboxylic acids | Acetic acid | 118.01 * | 2.49 | 2.4 ± 0.1 | 3.2 ± 0.1 | 3.8 ± 0.1 | 4.1 ± 0.0 | 4.5 ± 0.1 | 5.2 ± 0.1 | 3.9 ± 1.0 |
| Aliphatic HCs | Hexane | 68.73 * | 7.41 | 2.1 ± 0.1 | 2.0 ± 0.1 | 2.1 ± 0.2 | 1.9 ± 0.1 | 1.2 ± 0.0 | 1.0 ± 0.2 | 1.7 ± 0.5 |
| Octane | 125.69 * | 1.90 | 1.2 ± 0.1 | 2.1 ± 0.1 | 2.2 ± 0.3 | 1.9 ± 0.1 | 0.5 ± 0.2 | 0.2 ± 0.0 | 1.4 ± 0.9 | |
| Nonane | 150.66 * | 0.99 | 2.4 ± 0.1 | 3.1 ± 0.1 | 3.4 ± 0.1 | 3.8 ± 0.0 | 4.2 ± 0.1 | 4.1 ± 0.1 | 3.5 ± 0.7 |
| VOC | Compound | Odor Impact Value | |
|---|---|---|---|
| Family | Alperujo | Orujo | |
| Phenolic alcohols | Phenol | 89.29 | 142.86 |
| Aliphatic alcohols | 2-Furanmethanol | 0.48 | 0.54 |
| Aldehyde | Benzaldehyde | 1.26 | 1.14 |
| Furfural | 0.36 | 0.25 | |
| Hexanal | 3.21 | 6.43 | |
| Nonanal | 3.53 | 2.65 | |
| Octanal | 70.00 | 110.00 | |
| Aromatic | Toluene | 2.42 | 3.94 |
| Acetic acid | 3.60 | 3.90 | |
| Aliphatic HC | Hexane | 1.40 | 1.13 |
| Nonane | 1.00 | 1.59 | |
| Octane | 1.35 | 0.82 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, D.; Quinteros-Lama, H.; Tenreiro, C.; Gabriel, D. Assessing Concentration Changes of Odorant Compounds in the Thermal-Mechanical Drying Phase of Sediment-Like Wastes from Olive Oil Extraction. Appl. Sci. 2019, 9, 519. https://doi.org/10.3390/app9030519
Hernández D, Quinteros-Lama H, Tenreiro C, Gabriel D. Assessing Concentration Changes of Odorant Compounds in the Thermal-Mechanical Drying Phase of Sediment-Like Wastes from Olive Oil Extraction. Applied Sciences. 2019; 9(3):519. https://doi.org/10.3390/app9030519
Chicago/Turabian StyleHernández, Diógenes, Héctor Quinteros-Lama, Claudio Tenreiro, and David Gabriel. 2019. "Assessing Concentration Changes of Odorant Compounds in the Thermal-Mechanical Drying Phase of Sediment-Like Wastes from Olive Oil Extraction" Applied Sciences 9, no. 3: 519. https://doi.org/10.3390/app9030519