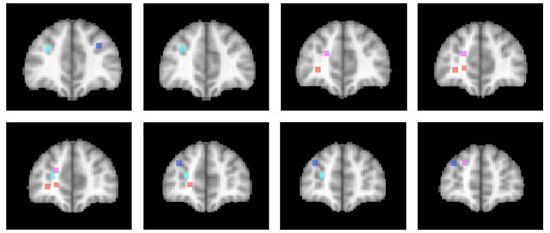
Anterior Prefrontal Contributions to Implicit Attention Control
Abstract
:1. Introduction
2. Prefrontal Involvement in High-Level Cognition in the Absence of Awareness
3. Frontopolar Involvement in the Implicit Control of Attention
3.1. Cross-Dimensional Visual Singleton Search
3.2. Contextual Cueing

3.3. Relation to Theoretical Accounts of Anterior Prefrontal Cortex Function
3.4. Differentiation within Anterior Prefrontal Cortex?

3.5. How Implicit Is Implicit Attention Control?
4. Conclusions
Acknowledgments
References
- Gilbert, S.J.; Spengler, S.; Simons, J.S.; Steele, J.D.; Lawrie, S.M.; Frith, C.D.; Burgess, P.W. Functional specialization within rostral prefrontal cortex (area 10): A meta-analysis. J. Cogn. Neurosci. 2006, 18, 932–948. [Google Scholar]
- Koechlin, E.; Hyafil, A. Anterior prefrontal function and the limits of human decision-making. Science 2007, 318, 594–598. [Google Scholar] [CrossRef]
- Pollmann, S. Switching between dimensions, locations, and responses: The role of the left frontopolar cortex. NeuroImage 2001, 14, S118–S124. [Google Scholar] [CrossRef]
- Pollmann, S. Anterior prefrontal cortex contributions to attention control. Exp. Psychol. 2004, 51, 270–278. [Google Scholar]
- van Gaal, S.; Lamme, V.A.F. Unconscious high-level information processing: Implication for neurobiological theories of consciousness. Neuroscientist 2011, 18, 287–301. [Google Scholar] [CrossRef]
- Del Cul, A.D.; Dehaene, S.; Reyes, P.; Bravo, E.; Slachevsky, A. Causal role of prefrontal cortex in the threshold for access to consciousness. Brain 2009, 132, 2531–2540. [Google Scholar]
- Lumer, E.D.; Friston, K.J.; Rees, G. Neural correlates of perceptual rivalry in the human brain. Science 1998, 280, 1930–1934. [Google Scholar] [CrossRef]
- Beck, D.M.; Rees, G.; Frith, C.D.; Lavie, N. Neural correlates of change detection and change blindness. Nat. Neurosci. 2001, 4, 645–650. [Google Scholar]
- Dehaene, S.; Naccache, L. Towards a cognitive neuroscience of consciousness: Basic evidence and a workspace framework. Cognition 2001, 79, 1–37. [Google Scholar]
- Dehaene, S.; Changeux, J.-P.; Naccache, L.; Sackur, J.; Sergent, C. Conscious, preconscious, and subliminal processing: A testable taxonomy. Trends Cogn. Sci. 2006, 10, 204–211. [Google Scholar]
- Marois, R.; Yi, D.-J.; Chun, M.M. The neural fate of consciously perceived and missed events in the attentional blink. Neuron 2004, 41, 465–472. [Google Scholar] [CrossRef]
- Pollen, D.A. On the neural correlates of visual perception. Cereb. Cortex 1999, 9, 4–19. [Google Scholar] [CrossRef]
- Lau, H.C.; Passingham, R.E. Unconscious activation of the cognitive control system in the human prefrontal cortex. J. Neurosci. 2007, 27, 5805–5811. [Google Scholar]
- Vorberg, D.; Mattler, U.; Heinecke, A.; Schmidt, T.; Schwarzbach, J. Different time courses for visual perception and action priming. Proc. Natl. Acad. Sci. USA 2003, 100, 6275–6280. [Google Scholar]
- van Gaal, S.; Ridderinkhof, K.R.; Fahrenfort, J.J.; Scholte, H.S.; Lamme, V.A.F. Frontal cortex mediates unconsciously triggered inhibitory control. J. Neurosci. 2008, 28, 8053–8062. [Google Scholar] [CrossRef]
- van Gaal, S.; Ridderinkhof, K.R.; Scholte, H.S.; Lamme, V.A.F. Unconscious activation of the prefrontal no-go network. J. Neurosci. 2010, 30, 4143–4150. [Google Scholar] [CrossRef]
- Tsushima, Y.; Sasaki, Y.; Watanabe, T. Greater disruption due to failure of inhibitory control on an ambiguous distractor. Science 2006, 314, 1786–1788. [Google Scholar] [CrossRef]
- Seitz, A.; Lefebvre, C.; Watanabe, T.; Jolicoeur, P. Requirement for high-level processing in subliminal learning. Curr. Biol. 2005, 15, R753–R755. [Google Scholar] [CrossRef]
- Yotsumoto, Y.; Watanabe, T. Defining a link between perceptual learning and attention. PLoS Biol. 2008, 6. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Kuai, S.-G.; Xiao, L.-Q.; Klein, S.A.; Levi, D.M.; Yu, C. Stimulus coding rules for perceptual learning. PLoS Biol. 2008, 6. [Google Scholar] [CrossRef]
- Stroop, J. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Eriksen, B.A.; Eriksen, C.W. Effects of noise letters upon the identification of a target letter in a non-search task. Percept. Psychophys. 1974, 16, 143–149. [Google Scholar] [CrossRef]
- Cohen, J.D.; Dunbar, K.; McClelland, J.L. On the control of automatic processes: A parallel distributed processing account of the Stroop effect. Psychol. Rev. 1990, 97, 332–361. [Google Scholar]
- Ridderinkhof, K.R.; Ullsperger, M.; Crone, E.A.; Nieuwenhuis, S. The role of the medial frontal cortex in cognitive control. Science 2004, 306, 443–447. [Google Scholar]
- Blais, C.; Harris, M.B.; Guerrero, J.V.; Bunge, S.A. Rethinking the role of automaticity in cognitive control. Q. J. Exp. Psychol. 2012, 65, 268–276. [Google Scholar]
- Jacoby, L.L.; Lindsay, D.S.; Hessels, S. Item-specific control of automatic processes: Stroop process dissociations. Psychon. Bull. Rev. 2003, 10, 638–644. [Google Scholar] [CrossRef]
- Risko, E.F.; Stolz, J.A. The proportion valid effect in covert orienting: Strategic control or implicit learning? Conscious Cogn. 2010, 19, 432–442. [Google Scholar] [CrossRef]
- Blais, C.; Bunge, S. Behavioral and neural evidence for item-specific performance monitoring. J. Cogn. Neurosci. 2010, 22, 2758–2767. [Google Scholar] [CrossRef]
- Zurawska Vel Grajewska, B.; Sim, E.-J.; Hoenig, K.; Herrnberger, B.; Kiefer, M. Mechanisms underlying flexible adaptation of cognitive control: Behavioral and neuroimaging evidence in a flanker task. Brain Res. 2011, 1421, 52–65. [Google Scholar] [CrossRef]
- Christoff, K.; Prabhakaran, V.; Dorfman, J.; Zhao, Z.; Kroger, J.K.; Holyoak, K.J.; Gabrieli, J.D. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. NeuroImage 2001, 14, 1136–1149. [Google Scholar]
- Braver, T.S.; Bongiolatti, S.R. The role of frontopolar cortex in subgoal processing during working memory. NeuroImage 2002, 15, 523–536. [Google Scholar] [CrossRef]
- Koechlin, E.; Basso, G.; Pietrini, P.; Panzer, S.; Grafman, J. The role of the anterior prefrontal cortex in human cognition. Nature 1999, 399, 148–151. [Google Scholar]
- Christoff, G.; Gabrieli, J.D.E. The frontopolar cortex and human cognition: Evidence for a rostro-caudal hierarchical organization within the human prefrontal cortex. Psychobiology 2000, 28, 168–186. [Google Scholar]
- Burgess, P.W.; Simons, J.S.; Dumontheil, I.; Gilbert, J.S. The gateway hypothesis of rostral prefrontal cortex (area 10) function. In Measuring the Mind: Speed, Control, and Age; Duncan, J., Phillips, L., McLoad, P., Eds.; Oxford University Press: Oxford, UK, 2005; pp. 217–248. [Google Scholar]
- Burgess, P.W.; Scott, S.K.; Frith, C.D. The role of the rostral frontal cortex (area 10) in prospective memory: A lateral versus medial dissociation. Neuropsychologia 2003, 41, 906–918. [Google Scholar] [CrossRef]
- Pollmann, S.; Weidner, R.; Müller, H.J.; von Cramon, D.Y. A fronto-posterior network involved in visual dimension changes. J. Cogn. Neurosci. 2000, 12, 480–494. [Google Scholar] [CrossRef]
- Weidner, R.; Pollmann, S.; Müller, H.J.; von Cramon, D.Y. Top-down controlled visual dimension weighting: An event-related fMRI study. Cereb. Cortex 2002, 12, 318–328. [Google Scholar] [CrossRef]
- Müller, H.J.; Heller, D.; Ziegler, J. Visual search for singleton feature targets within and across feature dimensions. Percept. Psychophys. 1995, 57, 1–17. [Google Scholar] [CrossRef]
- Pollmann, S.; Weidner, R.; Müller, H.; von Cramon, D. Neural Correlates of visual dimension weighting. Vis. Cogn. 2006, 14, 877–897. [Google Scholar] [CrossRef]
- Pollmann, S.; Mahn, K.; Reimann, B.; Weidner, R.; Tittgemeyer, M.; Preul, C.; Müller, H.J.; von Cramon, D.Y. Selective visual dimension weighting deficit after left lateral frontopolar lesions. J. Cogn. Neurosci. 2007, 19, 365–375. [Google Scholar] [CrossRef]
- Chun, M.M.; Jiang, Y. Contextual cueing: Implicit learning and memory of visual context guides spatial attention. Cogn. Psychol. 1998, 36, 28–71. [Google Scholar] [CrossRef]
- Geyer, T.; Shi, Z.; Müller, H.J. Contextual cueing in multiconjunction visual search is dependent on color- and configuration-based intertrial contingencies. J. Exp. Psychol. Hum. Percept. Perform. 2010, 36, 515–532. [Google Scholar] [CrossRef]
- Preston, A.R.; Gabrieli, J.D.E. Dissociation between explicit memory and configural memory in the human medial temporal lobe. Cereb. Cortex 2008, 18, 2192–2207. [Google Scholar] [CrossRef]
- Smyth, A.C.; Shanks, D.R. Awareness in contextual cuing with extended and concurrent explicit tests. Mem. Cogn. 2008, 36, 403–415. [Google Scholar] [CrossRef]
- Manginelli, A.A.; Pollmann, S. Misleading contextual cues: How do they affect visual search? Psychol. Res. 2009, 73, 212–221. [Google Scholar] [CrossRef]
- Pollmann, S.; Manginelli, A.A. Anterior prefrontal involvement in implicit contextual change detection. Front Hum. Neurosci. 2009, 3. [Google Scholar] [CrossRef]
- Friedrich, F.J.; Egly, R.; Rafal, R.D.; Beck, D. Spatial attention deficits in humans: A comparison of superior parietal and temporal-parietal junction lesions. Neuropsychology 1998, 12, 193–207. [Google Scholar] [CrossRef]
- Corbetta, M.; Patel, G.; Shulman, G.L. The reorienting system of the human brain: From environment to theory of mind. Neuron 2008, 58, 306–324. [Google Scholar] [CrossRef]
- Pollmann, S.; Manginelli, A.A. Repeated contextual search cues lead to reduced BOLD-onset times in early visual and left inferior frontal cortex. Open Neuroimag. J. 2010, 4, 9–15. [Google Scholar] [CrossRef]
- Henson, R.N.A. Neuroimaging studies of priming. Prog. Neurobiol. 2003, 70, 53–81. [Google Scholar] [CrossRef]
- Schacter, D.L.; Buckner, R.L. Priming and the brain. Neuron 1998, 20, 185–195. [Google Scholar] [CrossRef]
- Pollmann, S.; Manginelli, A.A. Early implicit contextual change detection in anterior prefrontal cortex. Brain Res. 2009, 1263, 87–92. [Google Scholar] [CrossRef]
- Soto, D.; Humphreys, G.W.; Rotshtein, P. Dissociating the neural mechanisms of memory-based guidance of visual selection. Proc. Natl. Acad. Sci. USA 2007, 104, 17186–17191. [Google Scholar]
- Reynolds, J.R.; McDermott, K.B.; Braver, T.S. A direct comparison of anterior prefrontal cortex involvement in episodic retrieval and integration. Cereb. Cortex 2006, 16, 519–528. [Google Scholar]
- Ranganath, C.; Johnson, M.K.; DEsposito, M. Left anterior prefrontal activation increases with demands to recall specific perceptual information. J. Neurosci. 2000, 20, RC108:1–RC108:5. [Google Scholar]
- King, J.A.; Hartley, T.; Spiers, H.J.; Maguire, E.A.; Burgess, N. Anterior prefrontal involvement in episodic retrieval reflects contextual interference. NeuroImage 2005, 28, 256–267. [Google Scholar] [CrossRef]
- Ramnani, N.; Owen, A.M. Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nat. Rev. Neurosci. 2004, 5, 184–194. [Google Scholar] [CrossRef]
- Talairach, J.; Tournoux, P. Co-Planar Stereotactic Atlas of the Human Brain; Thieme: Stuttgart, Germany, 1998. [Google Scholar]
- Goel, V.; Grafman, J. Role of the right prefrontal cortex in ill-structured planning. Cogn. Neuropsychol. 2000, 17, 415–436. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pollmann, S. Anterior Prefrontal Contributions to Implicit Attention Control. Brain Sci. 2012, 2, 254-266. https://doi.org/10.3390/brainsci2020254
Pollmann S. Anterior Prefrontal Contributions to Implicit Attention Control. Brain Sciences. 2012; 2(2):254-266. https://doi.org/10.3390/brainsci2020254
Chicago/Turabian StylePollmann, Stefan. 2012. "Anterior Prefrontal Contributions to Implicit Attention Control" Brain Sciences 2, no. 2: 254-266. https://doi.org/10.3390/brainsci2020254