Striatal Volume Increases in Active Methamphetamine-Dependent Individuals and Correlation with Cognitive Performance
Abstract
:1. Introduction
2. Results
2.1. Demographics
| Control Participants (n = 20) | MA-Dependent Participants (n = 17) | |
|---|---|---|
| Age (years) | 30.9 ± 8.2 (18–46) | 35.1 ± 6.6 (22–46) |
| Gender (males/females) | 13/7 | 12/5 |
| Social drinking (n) | 10 | 9 |
| Regular nicotine use | 0 | 14 |
| Cannabis use | 0 | 14 |
| MA use variables | ||
| Route of administration (smoking/IV/both) | - | 12/3/2 |
| Age at first use (years) | - | 23.9 ± 6.6 (12–34) |
| Duration of use (years) | - | 10.2 ± 5.8 (2–25) |
| Amount of MA used per year (g) | - | 119.7 ± 135.5 (12–520) |
| Lifetime cumulative MA use (g) | - | 1442.6 ± 1874.4 (23–5400) |
2.2. Whole Brain Volumetric Analysis
2.3. Subcortical Volumetric Analysis
2.3.1. Between-Group Analysis
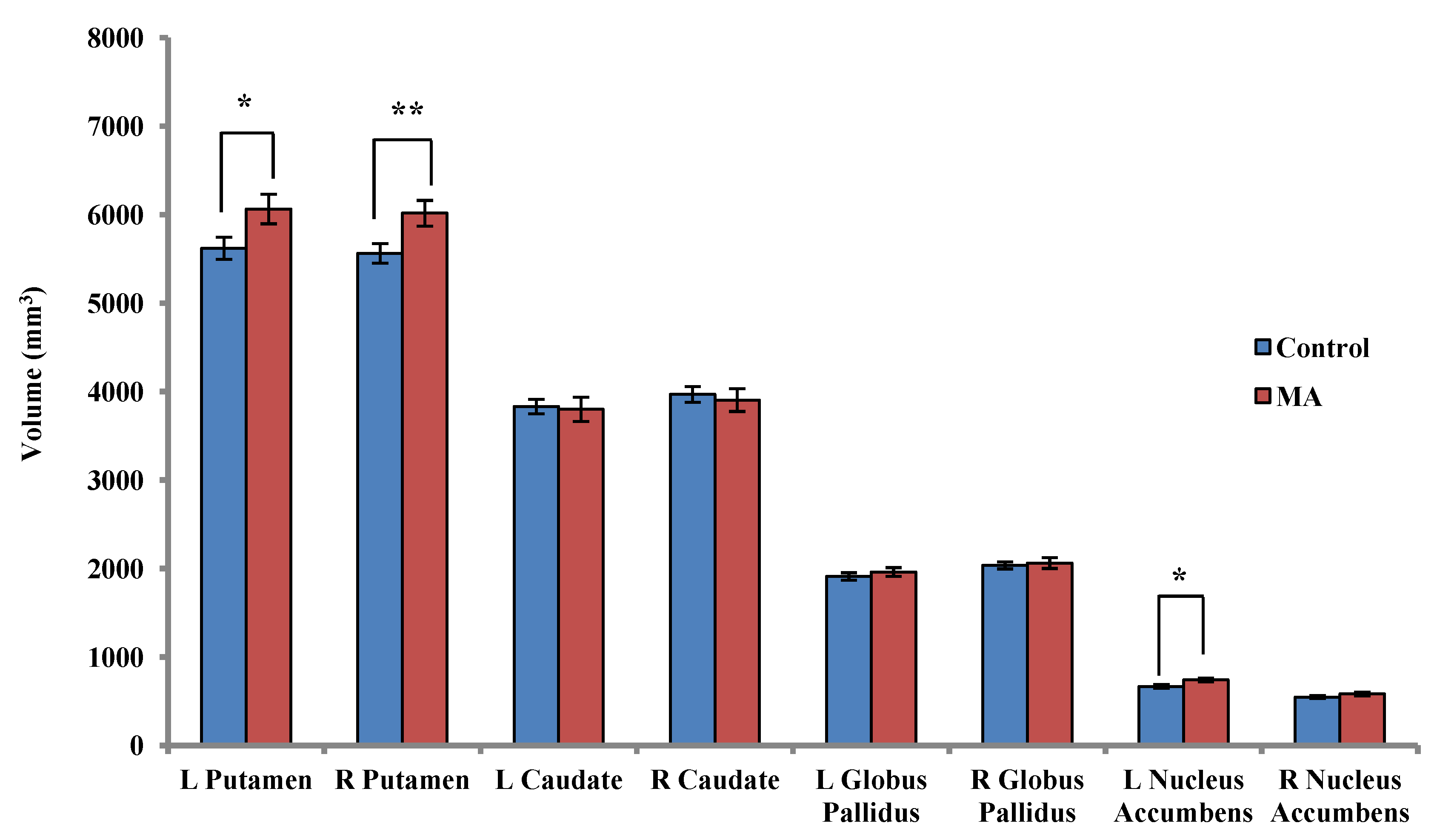
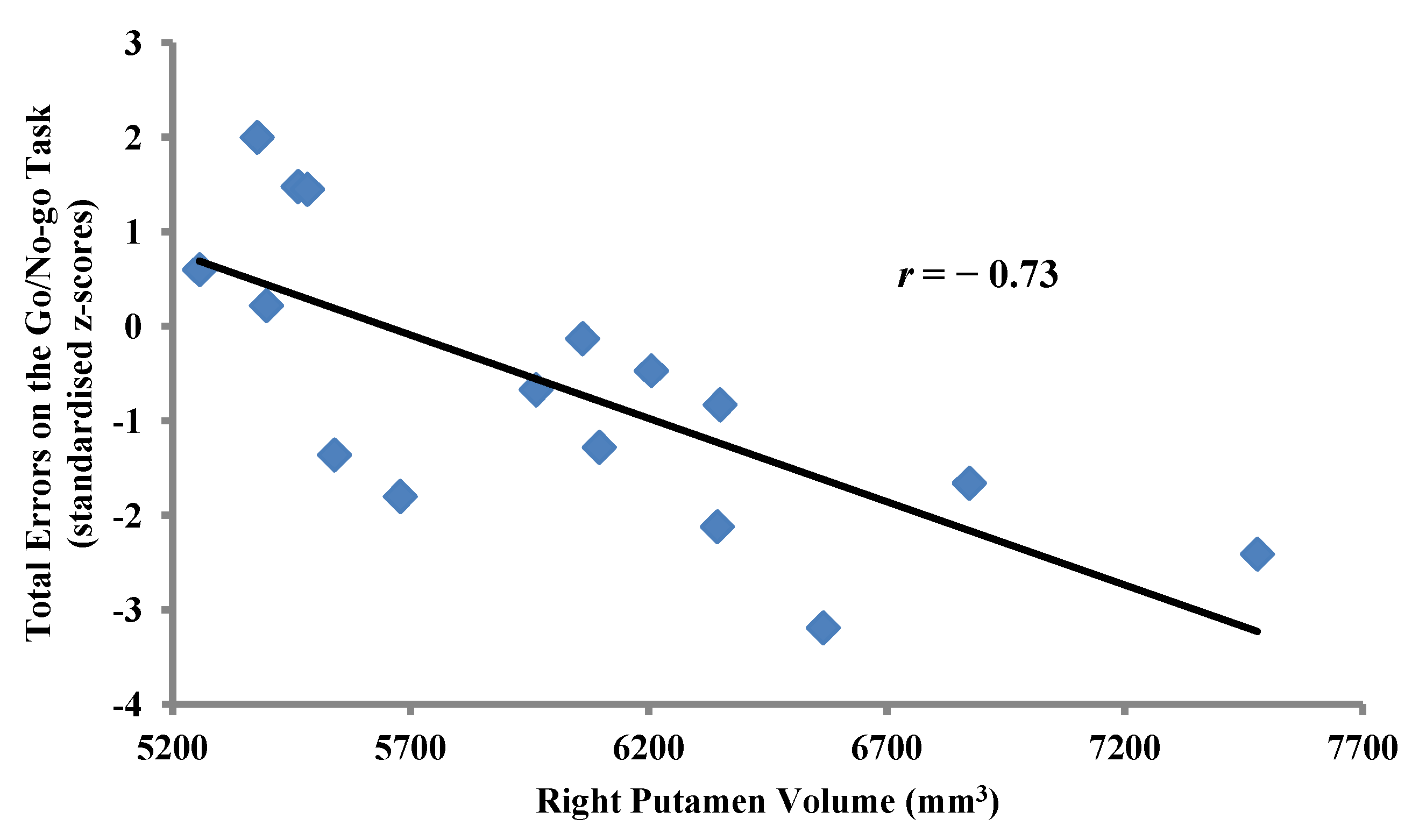
2.3.2. Within-Group Analyses: MA-Dependent Participants
2.4. Voxel-Wise Voxel-Based Morphometry
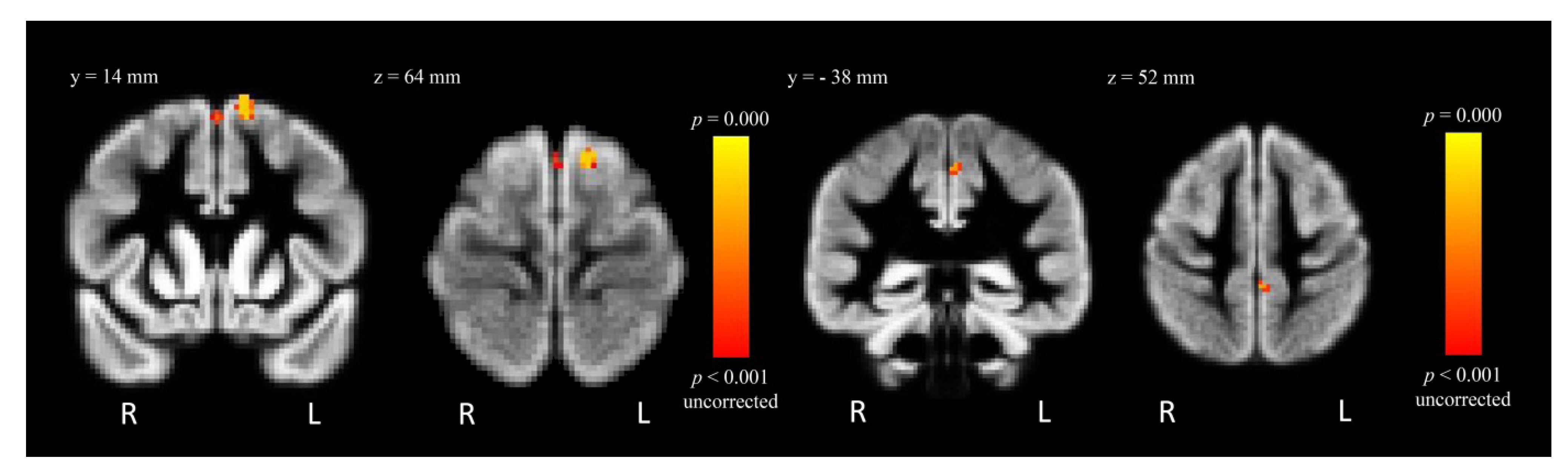
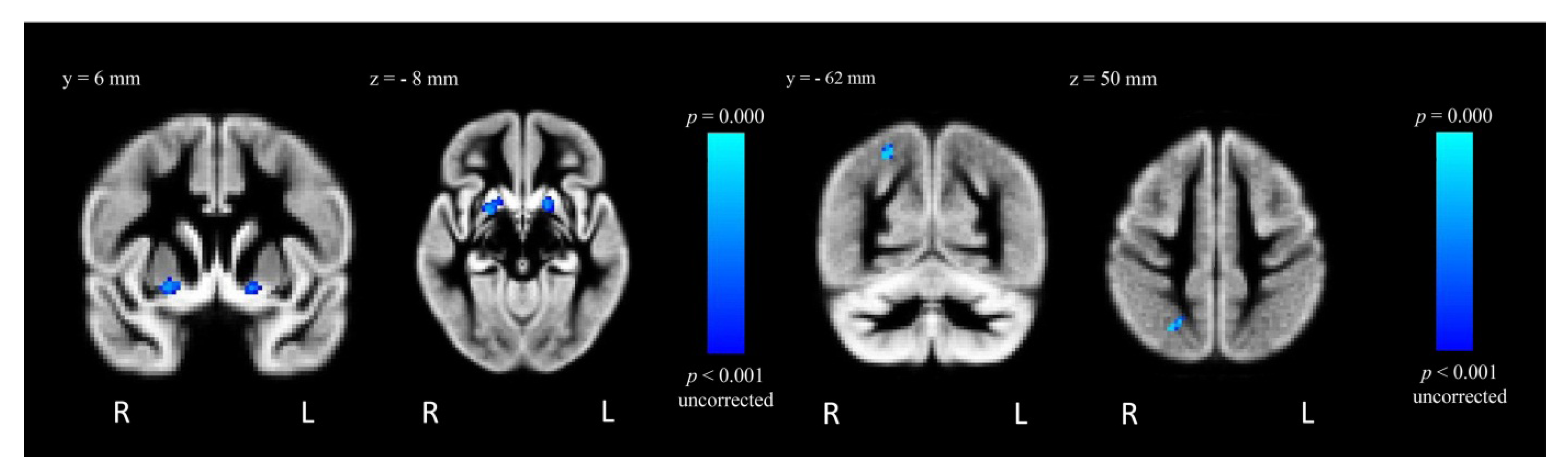
2.4.1. Between-Group Analysis
| Contrast | Regions | MNI coordinates (mm) | t-value | Cluster size (voxels) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Control > MA | L SFG | −12 | 14 | 66 | 5.25 | 48 |
| L SFG | 0 | 12 | 60 | 3.74 | 14 | |
| L precentral gyrus | −2 | −38 | 52 | 3.08 | 13 | |
| MA > Control | R putamen | 22 | 2 | −10 | 3.81 | 55 |
| R superior lateral occipital cortex | 26 | −62 | 50 | 3.37 | 42 | |
| L putamen | −16 | 6 | −8 | 3.74 | 41 | |
2.4.2. Within-Group Analysis: MA-Dependent Participants
3. Discussion
4. Experimental Procedure
4.1. Participants
4.2. Magnetic Resonance Image Acquisition
4.3. Neuropsychological Testing
4.4. Analysis
4.4.1. Whole Brain Volumetric Analysis
4.4.2. Subcortical Volumetric Analysis
4.4.3. Voxel-Wise Voxel-Based Morphometry Analysis
5. Conclusions
Acknowledgments
Conflict of Interest
References
- United Nations Office on Drugs and Crime, World Drug Report; United Nations Publications: Blue Ridge Summit, PA, USA, 2010.
- Sulzer, D.; Sonders, M.S.; Poulsen, N.W.; Galli, A. Mechanisms of neurotransmitter release by amphetamines: A review. Prog. Neurobiol. 2005, 75, 406–433. [Google Scholar] [CrossRef]
- Rothman, R.B.; Baumann, M.H.; Dersch, C.M.; Romero, D.V.; Rice, K.C.; Carroll, F.I.; Partilla, J.S. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 2001, 39, 32–41. [Google Scholar] [CrossRef]
- Rothman, R.B.; Partilla, J.S.; Baumann, M.H.; Dersch, C.M.; Carroll, F.I.; Rice, K.C. Neurochemical neutralization of methamphetamine with high-affinity nonselective inhibitors of biogenic amine transporters: A pharmacological strategy for treating stimulant abuse. Synapse 2000, 35, 222–227. [Google Scholar] [CrossRef]
- Wise, R.A. Neurobiology of addiction. Curr. Opin. Neurobiol. 1996, 6, 243–251. [Google Scholar]
- Kogan, F.J.; Nichols, W.K.; Gibb, J.W. Influence of methamphetamine on nigral and striatal tyrosine hydroxylase activity and on striatal dopamine levels. Eur. J. Pharmacol. 1976, 36, 363–371. [Google Scholar] [CrossRef]
- Melega, W.P.; Raleigh, M.J.; Stout, D.B.; Lacan, G.; Huang, S.C.; Phelps, M.E. Recovery of striatal dopamine function after acute amphetamine- and methamphetamine-induced neurotoxicity in the vervet monkey. Brain Res. 1997, 766, 113–120. [Google Scholar] [CrossRef]
- Ricaurte, G.A.; Schuster, C.R.; Seiden, L.S. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: A regional study. Brain Res. 1980, 193, 153–163. [Google Scholar] [CrossRef]
- Seiden, L.S.; Fischman, M.W.; Schuster, C.R. Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug Alcohol Depend. 1976, 1, 215–219. [Google Scholar] [CrossRef]
- Cadet, J.L.; Jayanthi, S.; Deng, X. Methamphetamine-induced neuronal apoptosis involves the activation of multiple death pathways. Review. Neurotox Res. 2005, 8, 199–206. [Google Scholar] [CrossRef]
- Jayanthi, S.; Deng, X.; Ladenheim, B.; McCoy, M.T.; Cluster, A.; Cai, N.S.; Cadet, J.L. Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc. Natl. Acad. Sci. USA 2005, 102, 868–873. [Google Scholar]
- Wilson, J.M.; Kalasinsky, K.S.; Levey, A.I.; Bergeron, C.; Reiber, G.; Anthony, R.M.; Schmunk, G.A.; Shannak, K.; Haycock, J.W.; Kish, S.J. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat. Med. 1996, 2, 699–703. [Google Scholar] [CrossRef]
- Boileau, I.; Rusjan, P.; Houle, S.; Wilkins, D.; Tong, J.; Selby, P.; Guttman, M.; Saint-Cyr, J.A.; Wilson, A.A.; Kish, S.J. Increased vesicular monoamine transporter binding during early abstinence in human methamphetamine users: Is VMAT2 a stable dopamine neuron biomarker? J. Neurosci. 2008, 28, 9850–9856. [Google Scholar] [CrossRef]
- Chang, L.; Cloak, C.; Patterson, K.; Grob, C.; Miller, E.N.; Ernst, T. Enlarged striatum in abstinent methamphetamine abusers: A possible compensatory response. Biol. Psychiatry 2005, 57, 967–974. [Google Scholar] [CrossRef]
- Schlaepfer, T.E.; Lancaster, E.; Heidbreder, R.; Strain, E.C.; Kosel, M.; Fisch, H.U.; Pearlson, G.D. Decreased frontal white-matter volume in chronic substance abuse. Int. J. Neuropsychopharmacol. 2006, 9, 147–153. [Google Scholar]
- Thompson, P.M.; Hayashi, K.M.; Simon, S.L.; Geaga, J.A.; Hong, M.S.; Sui, Y.; Lee, J.Y.; Toga, A.W.; Ling, W.; London, E.D. Structural abnormalities in the brains of human subjects who use methamphetamine. J. Neurosci. 2004, 24, 6028–6036. [Google Scholar]
- Schwartz, D.L.; Mitchell, A.D.; Lahna, D.L.; Luber, H.S.; Huckans, M.S.; Mitchell, S.H.; Hoffman, W.F. Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. Neuroimage 2010, 50, 1392–1401. [Google Scholar] [CrossRef]
- Kim, S.J.; Lyoo, I.K.; Hwang, J.; Chung, A.; Hoon Sung, Y.; Kim, J.; Kwon, D.-H.; Chang, K.H.; Renshaw, P.F. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. Int. J. Neuropsychopharmacol. 2006, 9, 221–228. [Google Scholar]
- Nakama, H.; Chang, L.; Fein, G.; Shimotsu, R.; Jiang, C.S.; Ernst, T. Methamphetamine users show greater than normal age-related cortical gray matter loss. Addiction 2011, 106, 1474–1483. [Google Scholar] [CrossRef]
- Jernigan, T.L.; Gamst, A.C.; Archibald, S.L.; Fennema-Notestine, C.; Mindt, M.R.; Marcotte, T.D.; Heaton, R.K.; Ellis, R.J.; Grant, I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am. J. Psychiatry 2005, 162, 1461–1472. [Google Scholar]
- Naqvi, N.H.; Bechara, A. The hidden island of addiction: The insula. Trends Neurosci. 2009, 32, 56–67. [Google Scholar] [CrossRef]
- Tolliver, B.K.; Price, K.L.; Baker, N.L.; LaRowe, S.D.; Simpson, A.N.; McRae-Clark, A.L.; Saladin, M.E.; DeSantis, S.M.; Chapman, E.; Garrett, M.; Brady, K.T. Impaired cognitive performance in subjects with methamphetamine dependence during exposure to neutral versus methamphetamine-related cues. Am. J. Drug Alcohol Abuse 2012, 38, 251–259. [Google Scholar] [CrossRef]
- Verdejo-Garcia, A.; Bechara, A.; Recknor, E.C.; Perez-Garcia, M. Executive dysfunction in substance dependent individuals during drug use and abstinence: An examination of the behavioral, cognitive and emotional correlates of addiction. J. Int. Neuropsychol. Soc. 2006, 12, 405–415. [Google Scholar]
- Hori, Y.; Minamimoto, T.; Kimura, M. Neuronal encoding of reward value and direction of actions in the primate putamen. J. Neurophysiol. 2009, 102, 3530–3543. [Google Scholar] [CrossRef]
- Inase, M.; Li, B.M.; Tanji, J. Dopaminergic modulation of neuronal activity in the monkey putamen through D1 and D2 receptors during a delayed Go/Nogo task. Exp. Brain Res. 1997, 117, 207–218. [Google Scholar] [CrossRef]
- Opris, I.; Hampson, R.E.; Deadwyler, S.A. The encoding of cocaine vs. natural rewards in the striatum of nonhuman primates: Categories with different activations. Neuroscience 2009, 163, 40–54. [Google Scholar] [CrossRef]
- Dibbets, P.; Evers, L.; Hurks, P.; Marchetta, N.; Jolles, J. Differences in feedback- and inhibition-related neural activity in adult ADHD. Brain Cogn. 2009, 70, 73–83. [Google Scholar] [CrossRef]
- Norman, A.L.; Pulido, C.; Squeglia, L.M.; Spadoni, A.D.; Paulus, M.P.; Tapert, S.F. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 2011, 119, 216–223. [Google Scholar] [CrossRef]
- Rubia, K.; Russell, T.; Bullmore, E.T.; Soni, W.; Brammer, M.J.; Simmons, A.; Taylor, E.; Andrew, C.; Giampietro, V.; Sharma, T. An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory function. Schizophr. Res. 2001, 52, 47–55. [Google Scholar]
- Lawyer, G.; Bjerkan, P.S.; Hammarberg, A.; Jayaram-Lindstrom, N.; Franck, J.; Agartz, I. Amphetamine dependence and co-morbid alcohol abuse: Associations to brain cortical thickness. BMC Pharmacol. 2010, 10, 5. [Google Scholar]
- Ernst, T.; Chang, L.; Leonido-Yee, M.; Speck, O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology 2000, 54, 1344–1349. [Google Scholar] [CrossRef]
- Cadet, J.L.; Ali, S.F.; Rothman, R.B.; Epstein, C.J. Neurotoxicity, drugs and abuse, and the CuZn-superoxide dismutase transgenic mice. Mol. Neurobiol. 1995, 11, 155–163. [Google Scholar] [CrossRef]
- Wagner, G.C.; Seiden, L.S.; Schuster, C.R. Methamphetamine-induced changes in brain catecholamines in rats and guinea pigs. Drug Alcohol Depend. 1979, 4, 435–438. [Google Scholar]
- Johanson, C.E.; Frey, K.A.; Lundahl, L.H.; Keenan, P.; Lockhart, N.; Roll, J.; Galloway, G.P.; Koeppe, R.A.; Kilbourn, M.R.; Robbins, T.; Schuster, C.R. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology 2006, 185, 327–338. [Google Scholar] [CrossRef]
- McCann, U.D.; Wong, D.F.; Yokoi, F.; Villemagne, V.; Dannals, R.F.; Ricaurte, G.A. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: Evidence from positron emission tomography studies with [11C]WIN-35,428. J. Neurosci. 1998, 18, 8417–8422. [Google Scholar]
- Sekine, Y.; Iyo, M.; Ouchi, Y.; Matsunaga, T.; Tsukada, H.; Okada, H.; Yoshikawa, E.; Futatsubashi, M.; Takei, N.; Mori, N. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am. J. Psychiatry 2001, 158, 1206–1214. [Google Scholar] [CrossRef]
- Volkow, N.D.; Chang, L.; Wang, G.J.; Fowler, J.S.; Ding, Y.S.; Sedler, M.; Logan, J.; Franceschi, D.; Gatley, J.; Hitzemann, R.; et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: Association with metabolism in the orbitofrontal cortex. Am. J. Psychiatry 2001, 158, 2015–2021. [Google Scholar]
- Volkow, N.D.; Chang, L.; Wang, G.J.; Fowler, J.S.; Leonido-Yee, M.; Franceschi, D.; Sedler, M.J.; Gatley, S.J.; Hitzemann, R.; Ding, Y.S.; et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am. J. Psychiatry 2001, 158, 377–382. [Google Scholar] [CrossRef]
- Ersche, K.D.; Barnes, A.; Simon Jones, P.; Morein-Zamir, S.; Robbins, T.W.; Bullmore, E.T. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain 2011, 134, 2013–2024. [Google Scholar]
- Jacobsen, L.K.; Giedd, J.N.; Gottschalk, C.; Kosten, T.R.; Krystal, J.H. Quantitative morphology of the caudate and putamen in patients with cocaine dependence. Am. J. Psychiatry 2001, 158, 486–489. [Google Scholar] [CrossRef]
- Corson, P.W.; Nopoulos, P.; Miller, D.D.; Arndt, S.; Andreasen, N.C. Change in basal ganglia volume over 2 years in patients with schizophrenia: Typical versus atypical neuroleptics. Am. J. Psychiatry 1999, 156, 1200–1204. [Google Scholar]
- Keshavan, M.S.; Bagwell, W.W.; Haas, G.L.; Sweeney, J.A.; Schooler, N.R.; Pettegrew, J.W. Changes in caudate volume with neuroleptic treatment. Lancet 1994, 344, 1434. [Google Scholar]
- Scherk, H.; Falkai, P. Effects of antipsychotics on brain structure. Curr. Opin. Psychiatry 2006, 19, 145–150. [Google Scholar] [CrossRef]
- Woodward, N.D.; Zald, D.H.; Ding, Z.; Riccardi, P.; Ansari, M.S.; Baldwin, R.M.; Cowan, R.L.; Li, R.; Kessler, R.M. Cerebral morphology and dopamine D2/D3 receptor distribution in humans: A combined [18F]fallypride and voxel-based morphometry study. Neuroimage 2009, 46, 31–38. [Google Scholar] [CrossRef]
- Martinez, D.; Greene, K.; Broft, A.; Kumar, D.; Liu, F.; Narendran, R.; Slifstein, M.; van Heertum, R.; Kleber, H.D. Lower level of endogenous dopamine in patients with cocaine dependence: Findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. Am. J. Psychiatry 2009, 166, 1170–1177. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Logan, J.; Gatley, S.J.; Hitzemann, R.; Chen, A.D.; Dewey, S.L.; Pappas, N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 1997, 386, 830–833. [Google Scholar] [CrossRef]
- Raz, N.; Torres, I.J.; Acker, J.D. Age, gender, and hemispheric differences in human striatum: A quantitative review and new data from in vivo MRI morphometry. Neurobiol. Learn. Mem. 1995, 63, 133–142. [Google Scholar] [CrossRef]
- LaVoie, M.J.; Card, J.P.; Hastings, T.G. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp. Neurol. 2004, 187, 47–57. [Google Scholar] [CrossRef]
- Sekine, Y.; Ouchi, Y.; Sugihara, G.; Takei, N.; Yoshikawa, E.; Nakamura, K.; Iwata, Y.; Tsuchiya, K.J.; Suda, S.; Suzuki, K.; et al. Methamphetamine causes microglial activation in the brains of human abusers. J. Neurosci. 2008, 28, 5756–5761. [Google Scholar]
- Seiden, L.S.; Sabol, K.E. Methamphetamine and methylenedioxymethamphetamine neurotoxicity: Possible mechanisms of cell destruction. NIDA Res. Monogr. 1996, 163, 251–276. [Google Scholar]
- Ances, B.M.; Vaida, F.; Cherner, M.; Yeh, M.J.; Liang, C.L.; Gardner, C.; Grant, I.; Ellis, R.J.; Buxton, R.B. HIV Neurobehavioral Research Center (HNRC). HIV and chronic methamphetamine dependence affect cerebral blood flow. J. Neuroimmune Pharmacol. 2011, 6, 409–419. [Google Scholar] [CrossRef]
- Hwang, J.; Lyoo, I.K.; Kim, S.J.; Sung, Y.H.; Bae, S.; Cho, S.-N.; Lee, H.Y.; Lee, D.S.; Renshaw, P.F. Decreased cerebral blood flow of the right anterior cingulate cortex in long-term and short-term abstinent methamphetamine users. Drug Alcohol Depend. 2006, 82, 177–181. [Google Scholar] [CrossRef]
- Chang, L.; Ernst, T.; Speck, O.; Patel, H.; DeSilva, M.; Leonido-Yee, M.; Miller, E.N. Perfusion MRI and computerized cognitive test abnormalities in abstinent methamphetamine users. Psychiatry Res. 2002, 114, 65–79. [Google Scholar] [CrossRef]
- Lindvall, O.; Ingvar, M.; Stenevi, U. Effects of methamphetamine on blood flow in the caudate-putamen after lesions of the nigrostriatal dopaminergic bundle in the rat. Brain Res. 1981, 211, 211–216. [Google Scholar] [CrossRef]
- Moszczynska, A.; Fitzmaurice, P.; Ang, L.; Kalasinsky, K.S.; Schmunk, G.A.; Peretti, F.J.; Aiken, S.S.; Wickham, D.J.; Kish, S.J. Why is parkinsonism not a feature of human methamphetamine users? Brain 2004, 127, 363–370. [Google Scholar] [CrossRef]
- Nestor, L.J.; Ghahremani, D.G.; Monterosso, J.; London, E.D. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. 2011, 194, 287–295. [Google Scholar] [CrossRef]
- Wilkins, C.; Sweetsur, P.; Smart, B.; Griffiths, R. Recent Trends in Illegal Drug Use in New Zealand, 2006–2010: Findings from the 2006, 2007, 2008, 2009 and 2010 Illicit Drug Monitoring System (IDMS); Massey University: Auckland, New Zealand, 2011. [Google Scholar]
- Brody, A.L.; Mandelkern, M.A.; Jarvik, M.E.; Lee, G.S.; Smith, E.C.; Huang, J.C.; Bota, R.G.; Bartzokis, G.; London, E.D. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol. Psychiatry 2004, 55, 77–84. [Google Scholar] [CrossRef]
- Tzilos, G.K.; Cintron, C.B.; Wood, J.B.R.; Simpson, N.S.; Young, A.D.; Pope, H.G., Jr.; Yurgelun-Todd, D.A. Lack of hippocampal volume change in long-term heavy cannabis users. Am. J. Addict. 2005, 14, 64–72. [Google Scholar] [CrossRef]
- Daumann, J.; Koester, P.; Becker, B.; Wagner, D.; Imperati, D.; Gouzoulis-Mayfrank, E.; Tittgemeyer, M. Medial prefrontal gray matter volume reductions in users of amphetamine-type stimulants revealed by combined tract-based spatial statistics and voxel-based morphometry. Neuroimage 2010, 54, 794–801. [Google Scholar]
- Cousijn, J.; Wiers, R.W.; Ridderinkhof, K.R.; van den Brink, W.; Veltman, D.J.; Goudriaan, A.E. Grey matter alterations associated with cannabis use: Results of a VBM study in heavy cannabis users and healthy controls. Neuroimage 2012, 59, 3845–3851. [Google Scholar] [CrossRef]
- Tanabe, J.; Tregellas, J.R.; Dalwani, M.; Thompson, L.; Owens, E.; Crowley, T.; Banich, M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol. Psychiatry 2009, 65, 160–164. [Google Scholar] [CrossRef]
- First, M.; Williams, J.; Spitzer, R.; Gibbon, M. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Clinical Trials Version (SCID-CT); New York State Psychiatric Institute: New York, NY, USA, 2007. [Google Scholar]
- Clark, C.R.; Paul, R.H.; Williams, L.M.; Arns, M.; Fallahpour, K.; Handmer, C.; Gordon, E. Standardized assessment of cognitive functioning during development and aging using an automated touchscreen battery. Arch. Clin. Neuropsychol. 2006, 21, 449–467. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; de Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004, 23, S208–S219. [Google Scholar] [CrossRef]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef]
- Jenkinson, M.; Smith, S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001, 5, 143–156. [Google Scholar] [CrossRef]
- Desikan, R.S.; Segonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Smith, S.M.; Zhang, Y.; Jenkinson, M.; Chen, J.; Matthews, P.M.; Federico, A.; de Stefano, N. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 2002, 17, 479–489. [Google Scholar] [CrossRef]
- Smith, S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002, 17, 143–155. [Google Scholar] [CrossRef]
- Zhang, Y.; Brady, M.; Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 2001, 20, 45–57. [Google Scholar] [CrossRef]
- Patenaude, B.; Smith, S.M.; Kennedy, D.N.; Jenkinson, M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 2011, 56, 907–922. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Voxel-based morphometry—The methods. Neuroimage 2000, 11, 805–821. [Google Scholar] [CrossRef]
- Good, C.D.; Johnsrude, I.S.; Ashburner, J.; Henson, R.N.; Friston, K.J.; Frackowiak, R.S. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001, 14, 21–36. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Jenkinson, M.; Smith, S. Non-Linear Optimisation; FMRIB Technical Report No. TR07JA1; FMRIB Centre: Oxford, UK, 2007 June 28. [Google Scholar]
- Andersson, J.L. R.; Jenkinson, M.; Smith, S. Non-Linear Registration, Aka Spatial Normalisation; FMRIB Technical Report No. TR07JA2; FMRIB Centre: Oxford, UK, 2007 June 28. [Google Scholar]
- Bullmore, E.T.; Suckling, J.; Overmeyer, S.; Rabe-Hesketh, S.; Taylor, E.; Brammer, M.J. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans. Med. Imaging 1999, 18, 32–42. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jan, R.K.; Lin, J.C.; Miles, S.W.; Kydd, R.R.; Russell, B.R. Striatal Volume Increases in Active Methamphetamine-Dependent Individuals and Correlation with Cognitive Performance. Brain Sci. 2012, 2, 553-572. https://doi.org/10.3390/brainsci2040553
Jan RK, Lin JC, Miles SW, Kydd RR, Russell BR. Striatal Volume Increases in Active Methamphetamine-Dependent Individuals and Correlation with Cognitive Performance. Brain Sciences. 2012; 2(4):553-572. https://doi.org/10.3390/brainsci2040553
Chicago/Turabian StyleJan, Reem K., Joanne C. Lin, Sylvester W. Miles, Rob R. Kydd, and Bruce R. Russell. 2012. "Striatal Volume Increases in Active Methamphetamine-Dependent Individuals and Correlation with Cognitive Performance" Brain Sciences 2, no. 4: 553-572. https://doi.org/10.3390/brainsci2040553




