Effects of Chronic Central Arginine Vasopressin (AVP) on Maternal Behavior in Chronically Stressed Rat Dams
Abstract
:1. Introduction
2. Results and Discussion
2.1. Maternal Care Testing
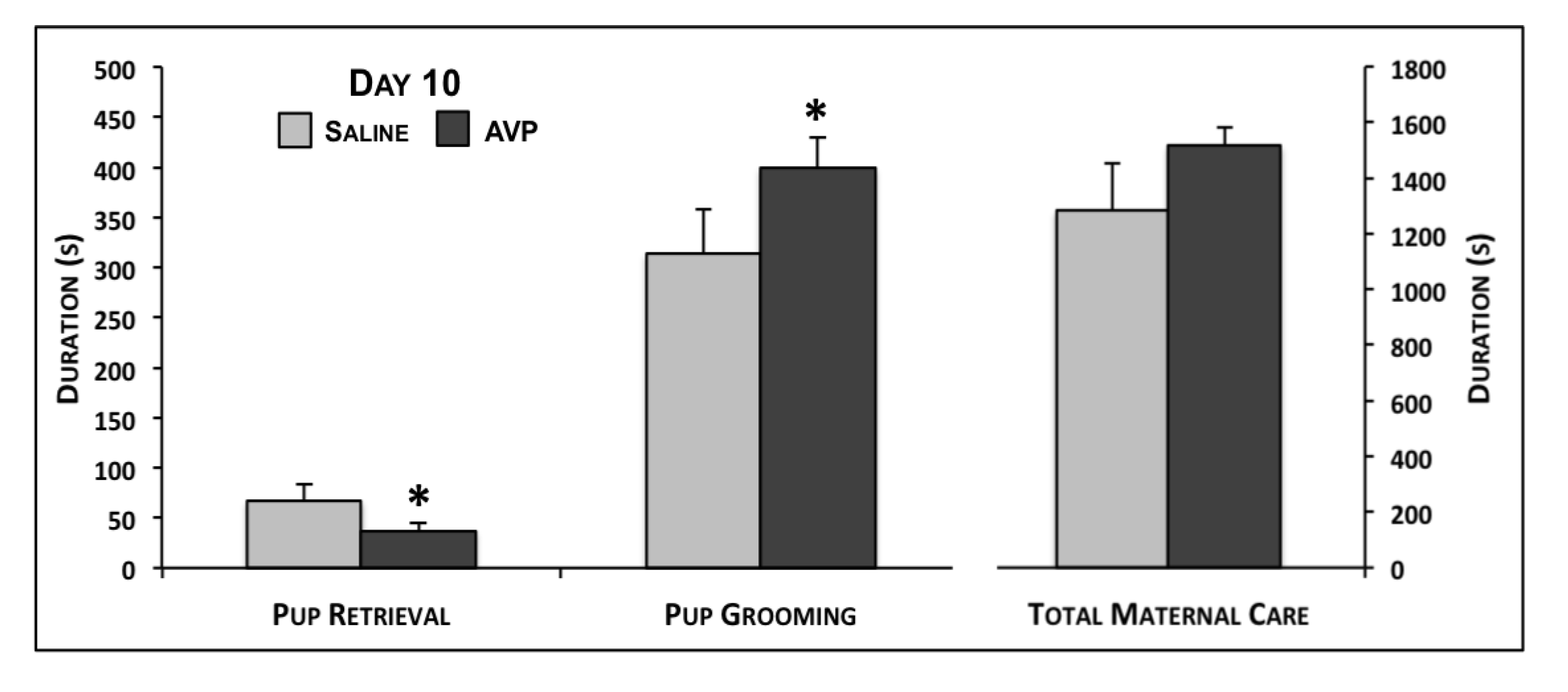
| Behavior Variable a | Day 3 | Day 10 | Day 17 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Saline (n = 16) | AVP (n = 26) | p | Saline (n = 14) | AVP (n = 25) | p | Saline (n = 13) | AVP (n = 25) | p | |
| Pup Grooming | 139.9 ± 33.5 | 162.7 ± 17.4 | 0.23 | 313.8 ± 44.7 | 399.3 ± 29.8 | 0.05 | 362.6 ± 43.7 | 328.5 ± 29.3 | 0.26 |
| Nursing | 744.2 ± 118.5 | 781.8 ± 74.4 | 0.39 | 969.2 ± 135.2 | 1116.9 ± 62.2 | 0.13 | 884.5 ± 140.1 | 926.1 ± 80.0 | 0.39 |
| Nursing Latency | 684.7 ± 123.7 | 649.8 ± 73.7 | 0.40 | 544.3 ± 121.1 | 411.0 ± 44.1 | 0.11 | 640.4 ± 135.5 | 580.0 ± 55.4 | 0.31 |
| Total Maternal Care | 884.1 ± 137.5 | 944.5 ± 81.1 | 0.34 | 1283.1 ± 169.1 | 1516.3 ± 65.1 | 0.07 | 1247.0 ± 171.5 | 1254.6 ± 90.9 | 0.48 |
| Nesting | 124.7 ± 17.1 | 144.0 ± 19.4 | 0.25 | 75.4 ± 14.0 | 69.2 ± 8.5 | 0.34 | 30.7 ± 12.3 | 41.6 ± 8.0 | 0.22 |
| Self Grooming | 160.0 ± 34.5 | 169.6 ± 23.9 | 0.41 | 65.6 ± 13.0 | 81.2 ± 17.4 | 0.27 | 79.9 ± 19.1 | 65.9 ± 13.6 | 0.28 |
| Retrieval | 55.8 ± 11.1 | 51.0 ± 7.0 | 0.35 | 67.1 ± 16.6 | 36.8 ± 8.2 | 0.04 | -- | -- | -- |
| Full Retrieval | 415.7 ± 121.5 | 357.7 ± 49.6 | 0.31 | 397.1 ± 111.9 | 352.8 ± 76.5 | 0.37 | -- | -- | -- |
| Activity | 100.1 ± 13.6 | 102.4 ± 12.7 | 0.45 | 88.1 ± 12.5 | 82.3 ± 7.4 | 0.34 | 108.6 ± 14.1 | 103.3 ± 8.0 | 0.36 |
2.2. Maternal Aggression Testing
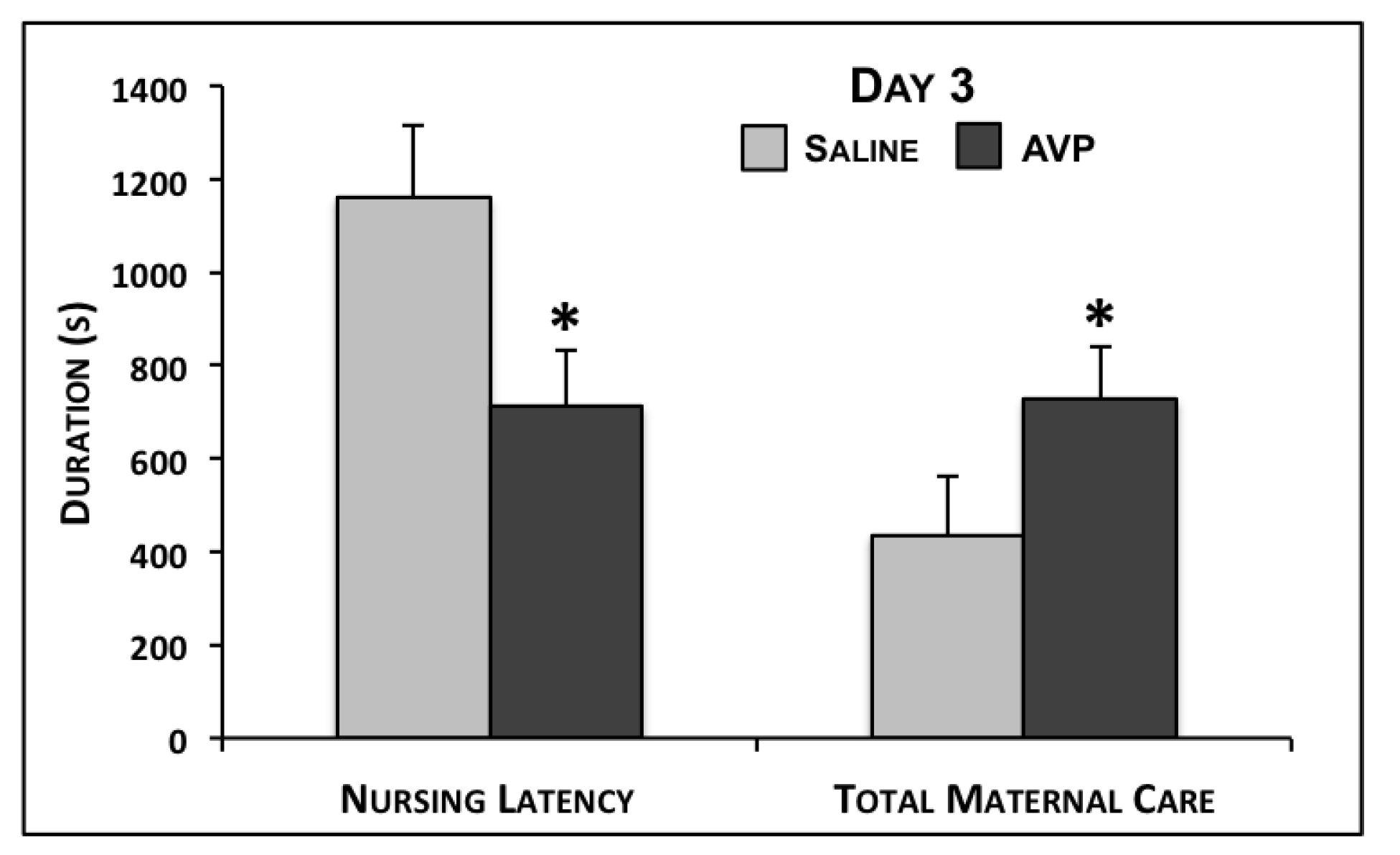
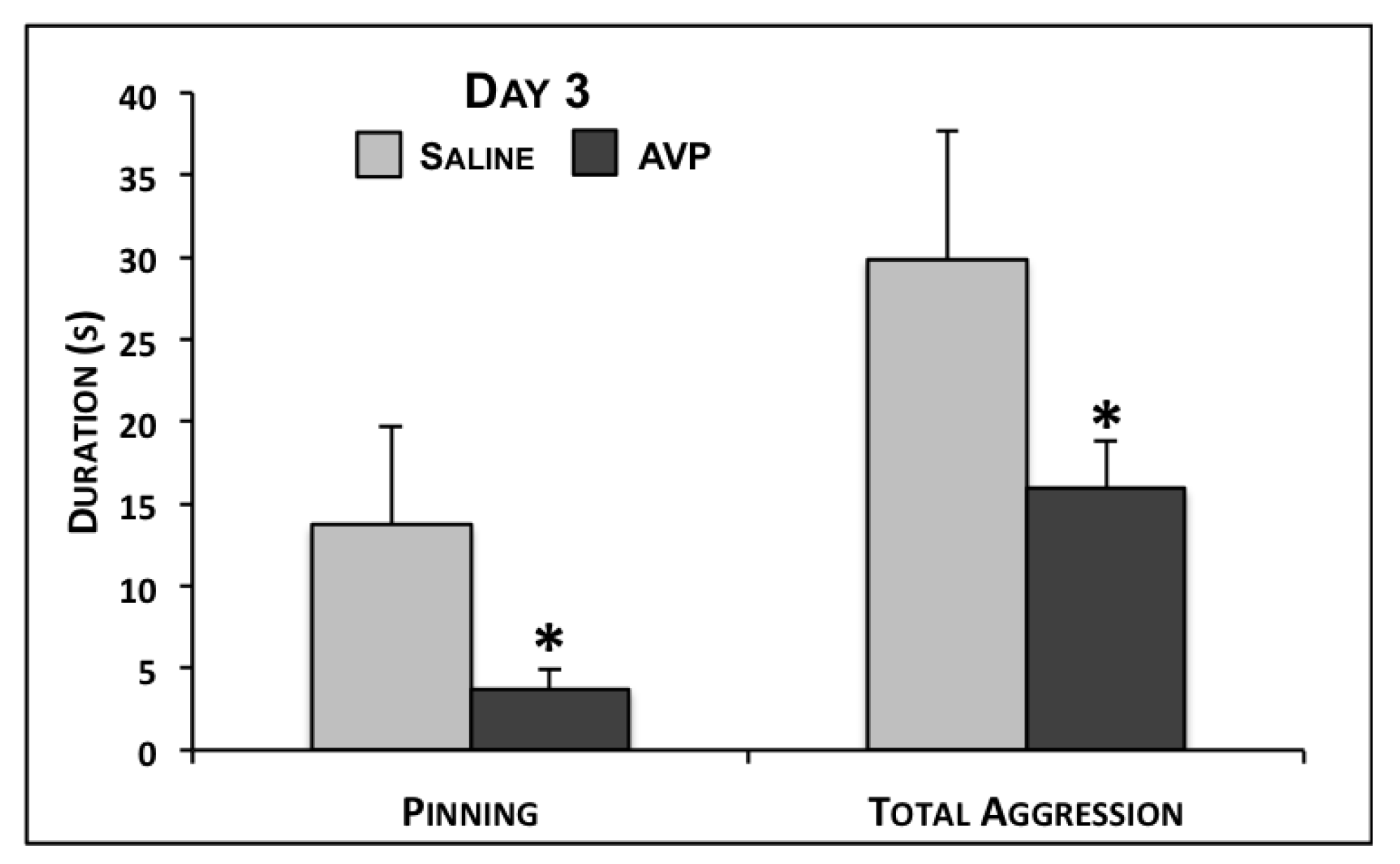
| Behavior Variable a | Day 3 | Day 10 | Day 17 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Saline (n = 16) | AVP (n = 26) | p | Saline (n = 14) | AVP (n = 25) | p | Saline (n = 13) | AVP (n = 25) | p | |
| Pup Grooming | 14.8 ± 5.2 | 27.1 ± 6.1 | 0.08 | 188.7 ± 43.4 | 169.1 ± 26.1 | 0.34 | 138.0 ± 45.2 | 117.7 ± 24.5 | 0.33 |
| Nursing | 420.3 ± 127.4 | 700.6 ± 110.5 | 0.06 | 880.7 ± 176.3 | 741.7 ± 108.6 | 0.24 | 572.4 ± 110.4 | 559.5 ± 90.0 | 0.46 |
| Nursing Latency | 1160.9 ± 153.5 | 714.2 ± 117.2 | 0.01 | 680.4 ± 158.5 | 681.2 ± 126.8 | 0.50 | 887.3 ± 115.7 | 814.6 ± 135.3 | 0.36 |
| Total Maternal Care | 435.1 ± 128.0 | 727.8 ± 113.2 | 0.05 | 1069.4 ± 206.7 | 910.9 ± 126.7 | 0.25 | 710.3 ± 143.0 | 677.2 ± 106.1 | 0.43 |
| Nesting | 23.7 ± 9.3 | 67.3 ± 22.8 | 0.08 | 65.3 ± 29.5 | 46.4 ± 12.7 | 0.25 | 28.5 ± 12.9 | 30.5 ± 8.1 | 0.44 |
| Self Grooming | 165.5 ± 33.1 | 165.0 ± 18.7 | 0.50 | 135.5 ± 22.2 | 146.5 ± 24.2 | 0.38 | 109.7 ± 20.6 | 105.3 ± 9.8 | 0.41 |
| Activity | 95.9 ± 11.6 | 63.4 ± 6.0 | <0.01 | 69.2 ± 12.6 | 76.8 ± 8.3 | 0.30 | 98.3 ± 12.4 | 92.8 ± 11.2 | 0.38 |
| Attacking | 12.3 ± 2.2 | 8.9 ± 1.6 | 0.10 | 7.6 ± 1.7 | 10.8 ± 1.3 | 0.08 | 6.4 ± 1.0 | 7.4 ± 1.2 | 0.29 |
| Biting | 1.5 ± 0.5 | 1.9 ± 0.8 | 0.36 | 0.9 ± 0.5 | 1.3 ± 0.6 | 0.30 | 1.4 ± 0.5 | 1.2 ± 0.6 | 0.40 |
| Kicking | 2.3 ± 0.5 | 1.4 ± 0.4 | 0.08 | 1.2 ± 0.4 | 2.2 ± 0.8 | 0.19 | 0.3 ± 0.1 | 1.1 ± 0.4 | 0.06 |
| Pinning | 13.7 ± 6.0 | 3.7 ± 1.1 | 0.02 | 1.3 ± 0.6 | 15.9 ± 10.6 | 0.16 | 1.0 ± 0.7 | 9.5 ± 6.0 | 0.16 |
| Total Aggression | 29.9 ± 7.8 | 15.9 ± 2.9 | 0.03 | 11.0 ± 2.2 | 30.3 ± 12.0 | 0.12 | 9.2 ± 1.4 | 19.2 ± 6.9 | 0.15 |
| Aggression Bout | 0.8 ± 0.1 | 0.5 ± 0.1 | 0.01 | 0.6 ± 0.1 | 0.8 ± 0.1 | 0.23 | 0.5 ± 0.0 | 0.8 ± 0.2 | 0.17 |
| Attack Latency | 62.5 ± 21.5 | 44.8 ± 12.3 | 0.22 | 54.6 ± 17.0 | 76.0 ± 29.8 | 0.31 | 209.6 ± 99.6 | 226.3 ± 78.0 | 0.44 |
2.3. Growth
| Age | Day 10 | Day 17 | ||||
|---|---|---|---|---|---|---|
| Saline (n = 14) | AVP (n = 25) | p | Saline (n = 13) | AVP (n = 25) | p | |
| Dams (g) | 22.5±3.6 | 22.8±2.8 | 0.47 | 38.5±5.1 | 34.5±3.4 | 0.26 |
| Dams (%) | 7.0 ± 1.1 | 7.3 ± 1.0 | 0.42 | 12.0 ± 1.6 | 11.2 ± 1.2 | 0.36 |
| Pups a (g) | 16.2 ± 0.4 | 16.6 ± 0.3 | 0.17 | 37.0 ± 0.7 | 36.8 ± 0.6 | 0.42 |
| Pups a (%) | 211.4 ± 9.3 | 200.0 ± 5.2 | 0.13 | 483.0 ± 18.9 | 443.2 ± 11.8 | 0.04 |
2.4. Discussion
3. Experimental Section
3.1. Animals
3.2. Chronic Social Stress Paradigm
3.3. Behavior Testing
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Patel, V.N.; DeSouza, N.; Rodrigues, M. Postnatal depression and infant growth and development in low income countries: A cohort study from Goa, India. Arch. Dis. Child. 2003, 88, 34–37. [Google Scholar] [CrossRef]
- Surkan, P.J.; Kawachi, I.; Ryan, L.M.; Berkman, L.F.; Carvalho Vieira, L.M.; Peterson, K.E. Maternal depressive symptoms, parenting self-efficacy, and child growth. Am. J. Public Health 2008, 98, 125–132. [Google Scholar] [CrossRef]
- Goodman, S.H. Depression in mothers. Annu. Rev. Clin. Psychol. 2007, 3, 107–135. [Google Scholar]
- Paykel, E.; Emms, E.; Fletcher, J.; Rassaby, E. Life events and social support in puerperal depression. Br. J. Psychiatry 1980, 136, 339–346. [Google Scholar] [CrossRef]
- O’Hara, M.W.; Rehm, L.P.; Campbell, S.B. Postpartum depression: A role for social network and life stress variables. J. Nerv. Ment. Dis. 1983, 171, 336–341. [Google Scholar] [CrossRef]
- O’Hara, M.W. Social support, life events, and depression during pregnancy and the puerperium. Arch. Gen. Psychiatry 1986, 43, 569–573. [Google Scholar] [CrossRef]
- Seguin, L.; Potvin, L.; St-Denis, M.L.; Loiselle, J. Chronic stressors, social support, and depression during pregnancy. Obstet. Gynecol. 1995, 85, 583–589. [Google Scholar] [CrossRef]
- Beck, C.T. A meta-analysis of predictors of postpartum depression. Nurs. Res. 1996, 45, 297–303. [Google Scholar]
- O’Hara, M.W.; Swain, A.M. Rates and risk of postpartum depression: A meta-analysis. Int. Rev. Psychiatry 1996, 8, 37–54. [Google Scholar] [CrossRef]
- Beck, C.T. Predictors of postpartum depression: An update. Nurs. Res. 2001, 50, 275–285. [Google Scholar] [CrossRef]
- Robertson, E.; Grace, S.; Wallington, T.; Stewart, D.E. Antenatal risk factors for postpartum depression: A synthesis of recent literature. Gen. Hosp. Psychiatry 2004, 26, 289–295. [Google Scholar] [CrossRef]
- Klier, C.M.; Rosenblum, K.L.; Zeller, M.; Steinhardt, K.; Bergemann, N.; Muzik, M. A multirisk approach to predicting chronicity of postpartum depression symptoms. Depress. Anxiety 2008, 25, 718–724. [Google Scholar] [CrossRef]
- Hillerer, K.M.; Reber, S.O.; Neumann, I.D.; Slattery, D.A. Exposure to chronic pregnancy stress reverses peripartum-associated adaptations: Implications for postpartum anxiety and mood disorders. Endocrinology 2011, 152, 3930–3940. [Google Scholar] [CrossRef]
- Westdahl, C.; Milan, S.; Magriples, U.; Kershaw, T.S.; Rising, S.S.; Ickovics, J.R. Social support and social conflict as predictors of prenatal depression. Obstet. Gynecol. 2007, 110, 134–140. [Google Scholar] [CrossRef]
- Maestripieri, D.; Badiani, A.; Puglisi-Allegra, S. Prepartal chronic stress increases anxiety and decreases aggression in lactating female mice. Behav. Neurosci. 1991, 105, 663–668. [Google Scholar]
- Léonhardt, M.; Matthews, S.G.; Meaney, M.J.; Walker, C.D. Psychological stressors as a model of maternal adversity: Diurnal modulation of corticosterone responses and changes in maternal behavior. Horm. Behav. 2007, 51, 77–88. [Google Scholar] [CrossRef]
- Neumann, I.; Toschi, N.; Ohl, F.; Torner, L.; Kromer, S.A. Maternal defense as an emotional stressor in female rats: Correlation of neuroendocrine and behavioral parameters and involvement of brain oxytocin. Eur. J. Neurosci. 2001, 13, 1016–1024. [Google Scholar] [CrossRef]
- Douglas, A.J.; Meddle, S.L.; Kroemer, S.; Muesch, W.; Bosch, O.J.; Neumann, I.D. Social stress induces hypothalamo-pituitary-adrenal axis responses in lactating rats bred for high trait anxiety. Eur. J. Neurosci. 2007, 25, 1599–1603. [Google Scholar] [CrossRef]
- Nephew, B.C.; Bridges, R.S. Central actions of arginine vasopressin and a V1a receptor antagonist on maternal aggression, maternal behavior, and grooming in lactating rats. Pharmacol. Biochem. Behav. 2008, 91, 77–83. [Google Scholar] [CrossRef]
- Nephew, B.C.; Bridges, R.S.; Lovelock, D.F.; Byrnes, E.M. Enhanced maternal aggression and associated changes in neuropeptide gene expression in reproductively experienced rats. Behav. Neurosci. 2009, 123, 949–957. [Google Scholar] [CrossRef]
- Nephew, B.C.; Bridges, R.S. Effects of chronic social stress during lactation on maternal behavior and growth in rats. Stress 2011, 14, 677–684. [Google Scholar]
- Murgatroyd, C.; Carini, L.; Nephew, B.C. Effects of chronic social stress on maternal behavior, anhedonia, milk intake, pup growth, and gene expression. Eur. J. Psychotraumtol. 2012, 3 Suppl. 1. [Google Scholar]
- Murgatroyd, C.; Nephew, B.C. Effects of early life stress on maternal behavior and neuroendocrinology. Psychoneuroendocrinology 2012, in press.. [Google Scholar]
- Insel, T.R.; Young, L.J. Neuropeptides and the evolution of social behavior. Curr. Opin. Neurobiol. 2000, 103, 1035–1046. [Google Scholar]
- Rotzinger, S.; Lovejoy, D.A.; Tan, L.A. Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides 2010, 31, 736–756. [Google Scholar]
- Bosch, O.J. Maternal nurturing is dependent on her innate anxiety: The behavioral roles of brain oxytocin and vasopressin. Horm. Behav. 2011, 59, 202–212. [Google Scholar] [CrossRef]
- Bosch, O.J.; Neumann, I.D. Both oxytocin and vasopressin are mediators of maternal are and aggression in rodents: From central release to sites of action. Horm. Behav. 2011, 61, 293–303. [Google Scholar]
- Fleming, A.S. Maternal nest defense in the desert woodrat Neotoma lepida. Behav. Neural. Biol. 1979, 26, 41–63. [Google Scholar] [CrossRef]
- Wolff, J.O. Maternal aggression as a deterrent to infanticide in Peromyscus leucopus and P. maniculatus. Anim. Behav. 1985, 33, 117–123. [Google Scholar] [CrossRef]
- Maestripieri, D.; Alleva, E. Maternal aggression and litter size in the female house mouse. Ethology 1990, 84, 27–34. [Google Scholar] [CrossRef]
- Wolff, J.O. Why are female small mammals territorial? Oikos 1993, 68, 364–370. [Google Scholar] [CrossRef]
- Vom Saal, F.S.; Franks, P.; Boechler, M.; Palanza, P.; Parmigiani, S. Nest defense and survival of offspring in highly aggressive wild Canadian female house mice. Physiol. Behav. 1995, 58, 669–678. [Google Scholar] [CrossRef]
- Wolff, J.O.; Peterson, J.A. An offspring-defense hypothesis for territoriality in female mammals. Ethol. Ecol. Evol. 1998, 10, 227–239. [Google Scholar] [CrossRef]
- Numan, M.; Insel, T.R. The Neurobiology of Parental Behavior; Springer-Verlag: New York, NY, USA, 2003. [Google Scholar]
- Ferris, C.F.; Meenan, D.M.; Axelson, J.F.; Albers, H.E. A vasopressin antagonist can reverse dominant/subordinate behavior in hamsters. Physiol. Behav. 1986, 38, 135–138. [Google Scholar] [CrossRef]
- Ferris, C.F.; Potegal, M. Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol. Behav. 1988, 44, 235–239. [Google Scholar] [CrossRef]
- Elkabir, D.R.; Wyatt, M.E.; Vellucci, S.V.; Herbert, J. The effects of separate or combined infusions of corticotropin releasing factor and vasopressin either intraventricularly or into the amygdala on aggressive and investigative behavior inthe rat. Regul. Pept. 1990, 28, 199–214. [Google Scholar] [CrossRef]
- Compaan, J.C.; Buijs, R.M.; Pool, C.W.; de Ruiter, J.H.; Koolhaas, J.M. Differential lateral septal vasopressin innervation in aggressive and nonaggressive male mice. Brain Res. Bull. 1993, 30, 1–6. [Google Scholar] [CrossRef]
- Delville, Y.; Mansour, K.M.; Ferris, C.F. Serotonin blocks vasopressin-facilitated offensive aggression: Interactions within the ventrolateral hypothalamus of golden hamsters. Physiol. Behav. 1996, 59, 813–816. [Google Scholar] [CrossRef]
- Delville, Y.; Mansour, K.M.; Ferris, C.F. Testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus. Physiol. Behav. 1996, 60, 25–29. [Google Scholar] [CrossRef]
- Ferris, C.F.; Melloni, R.H.; Koppel, G.; Perry, K.W.; Fuller, R.W.; Delville, Y. Vasopressin/serotonin interaction in the anterior hypothalamus control aggressive behavior in golden hamsters. Neuroscience 1997, 17, 4331–4340. [Google Scholar]
- Stribley, J.M.; Carter, C.S. Developmental exposure to vasopressin increases aggression in adult prairie voles. Proc. Natl. Acad. Sci. USA 1996, 96, 12601–12604. [Google Scholar] [CrossRef]
- Caldwell, H.K.; Lee, H.J.; Macbeth, A.H.; Young, W.S., III. Vasopressin: Behavioral roles of an “original” neuropeptide. Prog. Neurobiol. 2008, 84, 1–24. [Google Scholar] [CrossRef]
- Gutzler, S.J.; Karom, M.; Albers, H.E. A Vasopressin (V1a) Receptor Antagonist Stimulates Aggression in Female Syrian Hamsters, but not during Behavioral Estrus. In Presented at the 37th annual meeting of the Society for Neuroscience, San Diego, CA, USA, 3–7 November 2007.
- Erskine, M.S.; Barfield, R.J.; Goldman, B.D. Intraspecific fighting during late pregnancy and lactation in rats and effects of litter removal. Behav. Biol. 1978, 23, 206–218. [Google Scholar] [CrossRef]
- Mayer, A.D.; Rosenblatt, J.S. Hormonal factors influence the onset of maternal aggression in laboratory rats. Horm. Behav. 1987, 21, 253–267. [Google Scholar] [CrossRef]
- Caughey, S.D.; Klampfl, S.M.; Bishop, V.R.; Pfortsch, J.; Neumann, I.D.; Bosch, O.J.; Meddle, S.L. Changes in the intensity of maternal aggression and central oxytocin and vasopressin V1a receptors across the peripartum period in the rat. J. Neuroendocrinol. 2011, 23, 1113–1124. [Google Scholar] [CrossRef]
- Bosch, O.J.; Neumann, I.D. Vassopressin released through the central amygdala promotes maternal aggression. J. Eur. Neurosci. 2010, 31, 883–891. [Google Scholar] [CrossRef]
- Nephew, B.C.; Byrnes, E.M.; Bridges, R.S. Vasopressin mediates enhanced offspring protection in multiparous rats. Neuropharmacology 2010, 58, 102–106. [Google Scholar]
- Caffrey, M.K.; Nephew, B.C.; Febo, M. Central vasopressin V1a receptors modulate neural processing in mothers facing intruder threat to pups. Neuropharmacology 2010, 58, 107–116. [Google Scholar] [CrossRef]
- Bosch, O.J.; Neumann, I.D. Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proc. Natl. Acad. Sci. USA 2008, 105, 17139–17144. [Google Scholar]
- Nephew, B.C.; Bridges, R.S. Arginine vasopressin V1a receptor antagonist impairs maternal memory in rats. Physiol. Behav. 2008, 95, 182–186. [Google Scholar] [CrossRef]
- Pedersen, C.A.; Caldwell, J.D.; Walker, C.; Ayers, G.; Mason, G.A. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and preoptic areas. Behav. Neurosci. 1994, 108, 1163–1171. [Google Scholar]
- Heinrichs, M.; Domes, G. Neuropeptides and social behaviour: Effects of oxytocin and vasopressin in humans. Prog. Brain Res. 2008, 170, 337–350. [Google Scholar] [CrossRef]
- Scott, L.V.; Dinan, T.G. Vasopressin and the regulation of hypothalamic-pituitary-adrenal axis function: implications for the pathophysiology of depression. Life Sci. 1998, 62, 1985–1998. [Google Scholar]
- Nakase, S.; Kitayama, I.; Soya, H.; Hamanaka, K.; Nomura, J. Increased expression of magnocellular arginine vasopressin mRNA in paraventricular nucleus of stress induced depression-model rats. Life Sci. 1998, 63, 23–31. [Google Scholar] [CrossRef]
- Muller, M.B.; Landgraf, R.; Keck, M.E. Vasopressin, major depression, and hypothalamic-pituitary-adrenocortical desensitization. Biol. Psychiatry 2000, 48, 330–333. [Google Scholar] [CrossRef]
- Goekoop, J.G.; de Winter, R.P.F.; de Rijk, R.; Zwinderman, K.H.; Frankhuijzen-Sierevogel, A.; Wiegant, V.M. Depression with above-normal plasma vasopressin: Validation by relations with family history of depression and mixed anxiety and retardation. Psychiatry Res. 2006, 141, 201–211. [Google Scholar] [CrossRef]
- Veenema, A.H.; Blume, A.; Niederle, D.; Buwalda, B.; Neumann, I.D. Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. Eur. J. Neurosci. 2006, 24, 1711–1720. [Google Scholar] [CrossRef]
- Surget, A.; Belzung, C. Involvement of vasopressin in affective disorders. Eur. J. Pharmacol. 2008, 583, 340–349. [Google Scholar]
- Scott, L.V.; Dinan, T.G. Vasopressin as a target for antidepressant development: an assessment of the available evidence. J. Affect. Disord. 2002, 72, 113–124. [Google Scholar] [CrossRef]
- Litvin, Y.; Murakami, G.; Pfaff, D.W. Effects of chronic social defeat on behavioral and neural correlates of sociality: Vasopressin, oxytocin and the vasopressinergic V1b receptor. Physiol. Behav. 2011, 103, 393–403. [Google Scholar] [CrossRef]
- Thompson, R.R.; George, K.; Walton, J.C.; Orr, S.P.; Benson, J. Sex-specific influences of vasopressin on human social communication. Proc. Natl. Acad. Sci. USA 2006, 103, 7889–7894. [Google Scholar]
- Neumann, I.; Johnston, H.; Hatzinger, M.; Liebsch, G.; Shipston, M.; Russell, J.A.; Landgraf, R.; Douglas, A.J. Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. J. Physiol. 1998, 508, 289–300. [Google Scholar]
- Douglas, A.J.; Brunton, P.J.; Bosch, O.J.; Russell, J.A.; Neumann, I.D. Neuroendocrine responses to stress in mice: Hyporesponsiveness in pregnancy and partuition. Endocrinology 2003, 144, 5268–5276. [Google Scholar] [CrossRef]
- Landgraf, R.; Wigger, A.; Holsboer, F.; Neumann, I.D. Hyperreactive hypothalamo-pituitary-adrenocortical (HPA) axis in rats bred for high anxiety-related behavior (rapid communication). J. Neuroendocrinol. 1999, 11, 405–407. [Google Scholar]
- Keck, M.E.; Wigger, A.; Welt, T.; Müller, M.B.; Gesing, A.; Reul, J.M.; Holsboer, F.; Landgraf, R.; Neumann, I.D. Vasopressin mediates the response of the combined dexamethasone/CRH test in hyper-anxious rats: implications for pathogenesis of affective disorders. Neuropsychoparmacology 2002, 26, 94–105. [Google Scholar] [CrossRef]
- Keck, M.E.; Welt, T.; Müller, M.B.; Uhr, M.; Ohl, F.; Wigger, A.; Toschi, N.; Holsboer, F.; Landgraf, R. Reduction of hypothalamic vasopressinergic hyperdrive contributes to clinically relevant behavioral and neuroendocrine effects of chronic paroxetine treatment in a psychopathological rat model. Neuropsychoparmacology 2003, 28, 235–243. [Google Scholar] [CrossRef]
- Murgatroyd, C.; Wigger, A.; Frank, E.; Singewald, N.; Bunck, M.; Holsboer, F.; Landgraf, R.; Spengler, D. Impaired repression at a vasopressin promoter polymorphism underlies overexpression of vasopressin in a rat model of trait anxiety. J. Neurosci. 2004, 24, 7762–7770. [Google Scholar]
- Wigger, A.; Sanchez, M.M.; Mathys, K.C.; Ebner, K.; Frank, E.; Liu, D. Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: Critical role of vasopressin. Neuropsychopharmacology 2004, 29, 1–14. [Google Scholar] [CrossRef]
- Neumann, I.D.; Krömer, S.A.; Bosch, O.J. Effects of psycho-social stress during pregnancy on neuroendocrine and behavioral parameters in lactation depend on the genetically determined stress vulnerability. Psychoneuroendocrinology 2005, 30, 791–806. [Google Scholar] [CrossRef]
- Crowley, R.S.; Insel, T.R.; O’Keefe, J.A.; Amico, J.A. Cytoplasmic oxytocin and vasopressin gene transcripts decline postpartum in the hypothalamus of the lactating rat. Endocrinology 1993, 133, 2704–2710. [Google Scholar]
- Champagne, F.A.; Francis, D.D.; Mar, A.; Meaney, M.J. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol. Behav. 2003, 79, 359–371. [Google Scholar] [CrossRef]
- Adewuya, A.O.; Ola, B.O.; Aloba, O.O.; Mapayi, B.M.; Okeniyi, J.A.O. Impact of postnatal depression on infants’ growth in Nigeria. J. Affect. Disord. 2008, 108, 191–193. [Google Scholar]
- Lau, C. Effects of stress on lactation. Pediatr. Clin. North Am. 2001, 48, 221–234. [Google Scholar] [CrossRef]
- De Goeij, D.C.; Dijkstra, H.; Tilders, F.J. Chronic psychosocial stress enhances vasopressin, but not corticotropic-releasing factor, in the external zone of the median eminence of male rats: Relationship to subordinate status. Endocrinology 1992, 131, 847–853. [Google Scholar] [CrossRef]
- Meyer-Lindenberg, A.; Domes, G.; Kirsch, P.; Heinrichs, M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat. Rev. Neurosci. 2011, 12, 524–538. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Coverdill, A.J.; McCarthy, M.; Bridges, R.S.; Nephew, B.C. Effects of Chronic Central Arginine Vasopressin (AVP) on Maternal Behavior in Chronically Stressed Rat Dams. Brain Sci. 2012, 2, 589-604. https://doi.org/10.3390/brainsci2040589
Coverdill AJ, McCarthy M, Bridges RS, Nephew BC. Effects of Chronic Central Arginine Vasopressin (AVP) on Maternal Behavior in Chronically Stressed Rat Dams. Brain Sciences. 2012; 2(4):589-604. https://doi.org/10.3390/brainsci2040589
Chicago/Turabian StyleCoverdill, Alexander J., Megan McCarthy, Robert S. Bridges, and Benjamin C. Nephew. 2012. "Effects of Chronic Central Arginine Vasopressin (AVP) on Maternal Behavior in Chronically Stressed Rat Dams" Brain Sciences 2, no. 4: 589-604. https://doi.org/10.3390/brainsci2040589




