Neuroadaptive Changes Associated with Smoking: Structural and Functional Neural Changes in Nicotine Dependence
Abstract
:1. Introduction
2. Current Neurobiological Models of Substance Dependence
2.1. Vulnerability Factors for Substance Dependence
2.2. Development and Acquisition of Dependence: The Role of Positive Reinforcement and of the Brain Reward System
2.2.1. Mechanisms of Positive Reinforcement in the Acquisition of Substance Dependence
2.2.2. The Hedonic Homeostasis and Mechanisms of Negative Reinforcement in the Acquisition of Substance Dependence
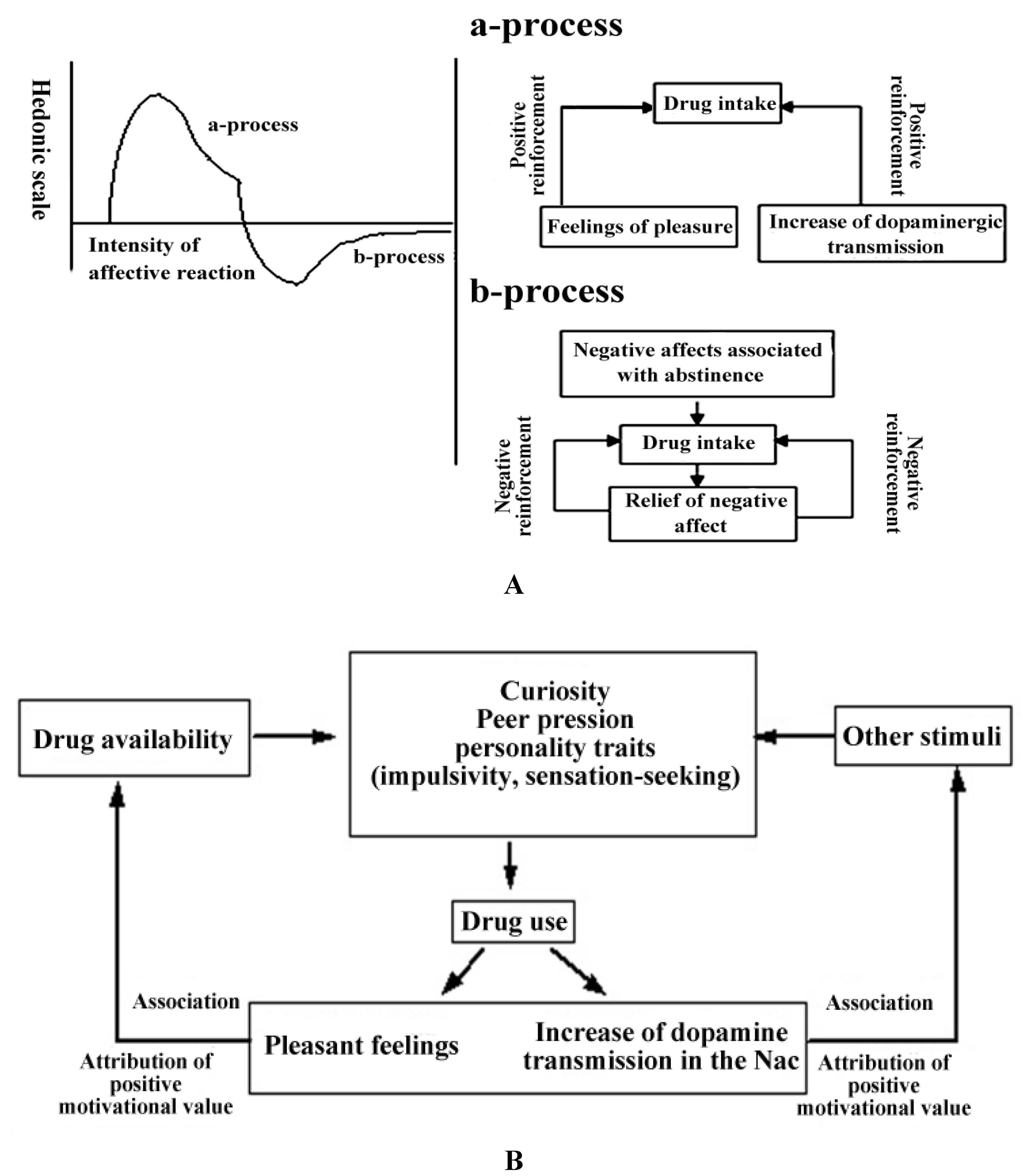
2.2.3. The Conditioning of Drug-Related Cues
2.2.4. The Role of the Dorsal Striatum in the Development of Addiction Habits
2.3. Maintenance of Dependence and Relapse: The Role of Drug Cues, Addictive Memory and of Neuroadaptation
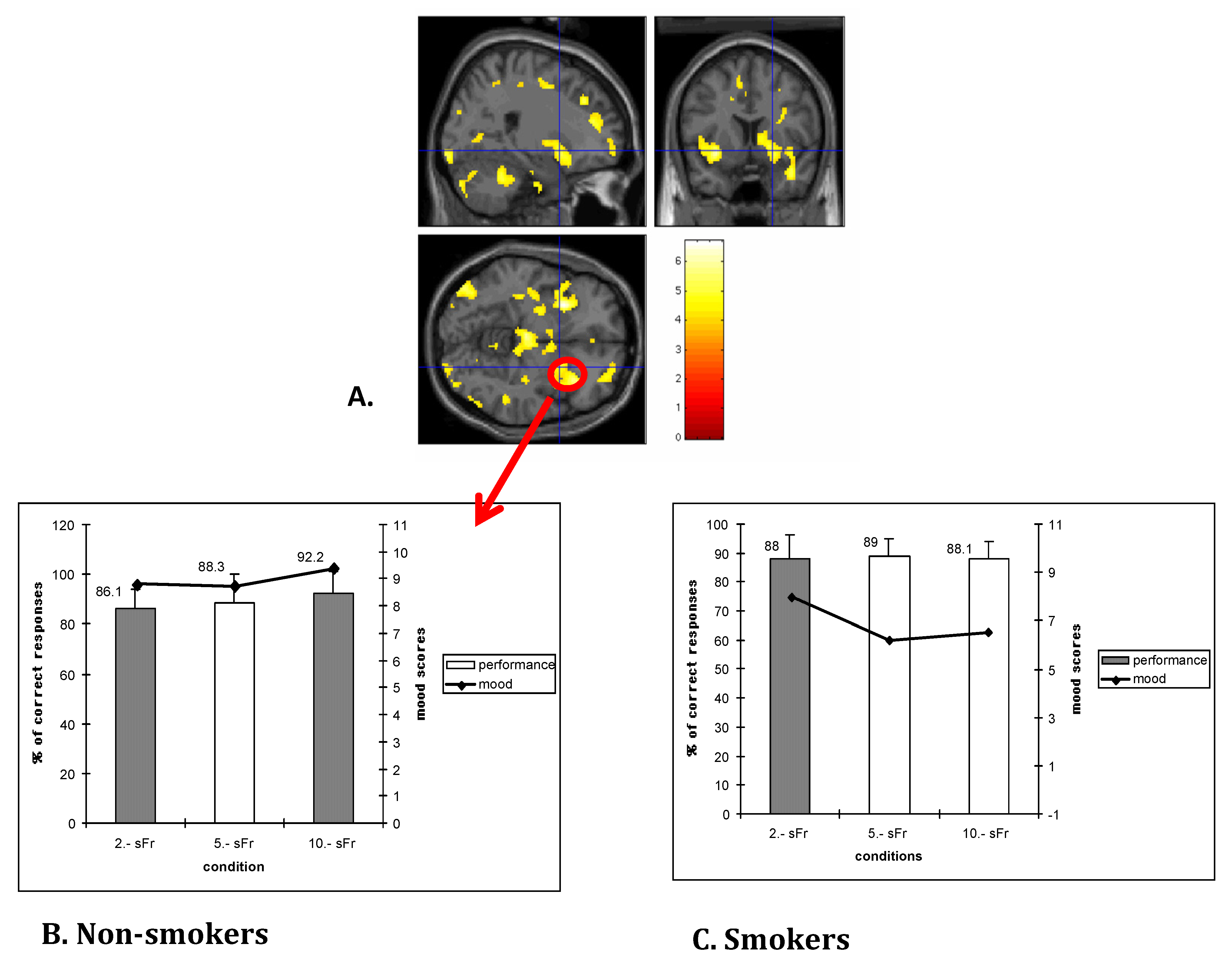
2.4. Cognitive Control Dysregulation and Deficits in Executive Functions
2.4.1. Decision-Making Deficits and the Ventromedial Prefrontal Cortex
2.4.2. Impaired Insights and the Insula
2.4.3. Dysfunctional Inhibitory Systems
3. Findings in Human Smokers
3.1. Changes in Brain Regions and Neurotransmitter Systems Associated with the Brain Reward Circuitry
3.1.1. Neurochemical Changes in Smokers
3.1.2. Structural Changes and Functional Connectivity
3.2. Changes in Brain Regions and Neurotransmitter Systems Associated with Smoking-Associated Cues and the “Anti-Reward” System
3.3. Changes Associated with Cognitive Control Systems
4. Conclusion
Acknowledgments
Conflict of Interest
References
- Ray, R.; Ruparel, K.; Newberg, A.; Wileyto, E.P.; Loughead, J.W.; Divgi, C.; Blendy, J.A.; Logan, J.; Zubieta, J.K.; Lerman, C. Human mu opioid receptor (OPRM1 A118G) polymorphism is associated with brain mu-opioid receptor binding potential in smokers. Proc. Natl. Acad. Sci. USA 2011, 108, 9268–9273. [Google Scholar]
- The Health Consequences of Smoking: A Report of the Surgeon General; U.S. Department of Health & Human Services: Atlanta, GA, USA, 2004.
- Quaak, M.; van Schayck, C.P.; Knaapen, A.M.; van Schooten, F.J. Genetic variation as a predictor of smoking cessation success. A promising preventive and intervention tool for chronic respiratory diseases? Eur. Respir. J. 2009, 33, 468–480. [Google Scholar] [CrossRef]
- Benowitz, N.L. Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addition. N. Engl. J. Med. 1988, 319, 1318–1330. [Google Scholar] [CrossRef]
- Whiting, P.J.; Lindstrom, J.M. Characterization of bovine and human neuronal nicotinic acetylcholine receptors using monoclonal antibodies. J. Neurosci. 1988, 8, 3395–3404. [Google Scholar]
- Corrigall, W.; Franklin, K.; Coen, K.; Clarke, P. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology 1992, 107, 285–289. [Google Scholar] [CrossRef]
- Sannerud, C.A.; Prada, J.; Goldberg, D.M.; Goldberg, S.R. The effects of sertraline on nicotine self-administration and food-maintained responding in squirrel monkeys. Eur. J. Pharmacol. 1994, 271, 461–469. [Google Scholar] [CrossRef]
- Henningfield, J.E.; Miyasato, K.; Jasinski, D.R. Cigarette smokers self-administer intravenous nicotine. Pharmacol. Biochem. Behav. 1983, 19, 887–890. [Google Scholar] [CrossRef]
- Clarke, P.; Fu, D.; Jakubovic, A.; Fibiger, H. Evidence that mesolimbic dopaminergic activation underlies the locomotor stimulant action of nicotine in rats. J. Pharmacol. Exp. Ther. 1988, 246, 701–708. [Google Scholar]
- Cumming, P.; Rosa-Neto, P.; Watanabe, H.; Smith, D.; Bender, D.; Clarke, P.B.; Gjedde, A. Effects of acute nicotine on hemodynamics and binding of [11C]raclopride to dopamine D2,3 receptors in pig brain. Neuroimage 2003, 19, 1127–1136. [Google Scholar] [CrossRef]
- Di Chiara, G. Drug addiction as dopamine-dependent associative learning disorder. Eur. J. Pharmacol. 1999, 375, 13–30. [Google Scholar]
- Koob, G.; LeMoal, M. Drug abuse: Hedonic homeostatic dysregulation. Science 1997, 278, 52–58. [Google Scholar] [CrossRef]
- Diagnostic and Statistical Manual of Mental Disorders, 4th ed; American Psychiatric Association: Washington, DC, USA, 1994.
- WHO, ICD-10 Symptom Checkliste für Psychische Störungen; Verlag Hans Huber: Bern, Switzerland, 1995.
- CDC. Cigarette smoking among adults and trends in smoking cessation-united states, 2008. Morb. Mortal. Wkly. Rep. 2009, 58, 1227–1232.
- Hughes, J.R.; Keely, J.; Naud, S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004, 99, 29–38. [Google Scholar] [CrossRef]
- Piasecki, T.M. Relapse to smoking. Clin. Psychol. Rev. 2006, 26, 196–215. [Google Scholar]
- Martin-Soelch, C. Neurobiologische und neuropsychologische modelle der substanzabhängigkeit. Z. Neuropsychol. 2010, 21, 153–166. [Google Scholar] [CrossRef]
- Uhl, G.; Blum, K.; Noble, E.; Smith, S. Substance abuse vulnerability and D2 receptor genes. Trends Neurosci. 1993, 16, 83–88. [Google Scholar] [CrossRef]
- Kendler, K.S.; Jacobson, K.C.; Prescott, C.A.; Neale, M.C. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am. J. Psychiatry 2003, 160, 687–695. [Google Scholar] [CrossRef]
- Li, M.D.; Burmeister, M. New insights into the genetics of addiction. Nat. Rev. Genet. 2009, 10, 225–231. [Google Scholar]
- True, W.R.; Xian, H.; Scherrer, J.F.; Madden, P.A.; Bucholz, K.K.; Heath, A.C.; Eisen, S.A.; Lyons, M.J.; Goldberg, J.; Tsuang, M. Common genetic vulnerability for nicotine and alcohol dependence in men. Arch. Gen. Psychiatry 1999, 56, 655–661. [Google Scholar]
- Nurco, D.N.; Kinlock, T.W.; O’Grady, K.E.; Hanlon, T.E. Early family adversity as a precursor to narcotic addiction. Drug Alcohol Depend. 1996, 43, 103–113. [Google Scholar] [CrossRef]
- Sinha, R. Chronic stress, drug use, and vulnerability to addiction. Ann. N. Y. Acad. Sci. 1141, 105–130. [Google Scholar]
- Volkow, N.D.; Fowler, J.S.; Wang, G.J.; Swanson, J.M.; Telang, F. Dopamine in drug abuse and addiction: Results of imaging studies and treatment implications. Arch. Neurol. 2007, 64, 1575–1579. [Google Scholar] [CrossRef]
- Anthony, J.C.; Petronis, K.R. Early-onset drug use and risk of later drug problems. Drug Alcohol Depend. 1995, 40, 9–15. [Google Scholar] [CrossRef]
- Steinberg, L.; Albert, D.; Cauffman, E.; Banich, M.; Graham, S.; Woolard, J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Dev. Psychol. 2008, 44, 1764–1778. [Google Scholar]
- Martin-Soelch, C. Reward and Dependence; Peter Lang: Bern, Germany, 2002. [Google Scholar]
- Schultz, W. Multiple reward signals in the brain. Nat. Rev. Neurosci. 2000, 1, 199–207. [Google Scholar] [CrossRef]
- Solomon, R.L. The opponent-process theory of acquired motivation: The costs of pleasure and the benefits of pain. Am. Psychol. 1980, 35, 691–712. [Google Scholar] [CrossRef]
- Koob, G.F.; Le Moal, M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 3113–3123. [Google Scholar] [CrossRef]
- Smith, R.; Aston-Jones, G. Noradrenergic transmission in the extended amygdala: Role in increased drug-seekingand relapse during protracted drug abstinence. Brain Struct. Funct. 2008, 213, 43–61. [Google Scholar]
- Koob, G. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology 2009, 56, 18–31. [Google Scholar] [CrossRef]
- Robinson, T.; Berridge, K. The psychology and neurobiology of addiction: An incentive-sensitization view. Addiction 2000, 95, 91–117. [Google Scholar]
- Everitt, B.J.; Belin, D.; Economidou, D.; Pelloux, Y.; Dalley, J.W.; Robbins, T.W. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 3125–3135. [Google Scholar] [CrossRef]
- Porrino, L.J.; Daunais, J.B.; Smith, H.R.; Nader, M.A. The expanding effects of cocaine: Studies in a nonhuman primate model of cocaine self-administration. Neurosci. Biobehav. Rev. 2004, 27, 813–820. [Google Scholar] [CrossRef]
- Yalachkov, Y.; Kaiser, J.; Naumer, M.J. Brain regions related to tool use and action knowledge reflect nicotine dependence. J. Neurosci. 2009, 29, 4922–4929. [Google Scholar] [CrossRef]
- Baxter, B.W.; Hinson, R.E. Is smoking automatic? Demands of smoking behavior on attentional resources. J. Abnorm. Psychol. 2001, 110, 59–66. [Google Scholar]
- Field, M.; Mogg, K.; Bradley, B.P. Automaticity of smoking behavior: The relationship between dual-task performance, daily cigarette intake and subjective nicotine effects. J. Psychopharmacol. 2006, 20, 799–805. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Telang, F.; Fowler, J.S.; Logan, J.; Childress, A.R.; Jayne, M.; Ma, Y.; Wong, C. Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. J. Neurosci. 2006, 26, 6583–6588. [Google Scholar]
- Wong, D.F.; Kuwabara, H.; Schretlen, D.J.; Bonson, K.R.; Zhou, Y.; Nandi, A.; Brasic, J.R.; Kimes, A.S.; Maris, M.A.; Kumar, A.; et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology 2006, 31, 2716–2727. [Google Scholar] [CrossRef]
- Vollstadt-Klein, S.; Wichert, S.; Rabinstein, J.; Buhler, M.; Klein, O.; Ende, G.; Hermann, D.; Mann, K. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction 2010, 105, 1741–1749. [Google Scholar] [CrossRef]
- Böning, J.A. Neurobiology of an addiction memory. J. Neural Transm. 2001, 108, 755–765. [Google Scholar]
- Robbins, T.W.; Everitt, B.J. Limbic-striatal memory systems and drug addiction. Neurobiol. Learn. Mem. 2002, 78, 625–636. [Google Scholar] [CrossRef]
- Koob, G.F. Dynamics of neuronal circuits in addiction: Reward, antireward, and emotional memory. Pharmacopsychiatry 2009, 42, 32–41. [Google Scholar] [CrossRef]
- Martin-Soelch, C.; Chevalley, A.F.; Künig, G.; Missimer, J.; Magyar, S.; Mino, A.; Schultz, W.; Leenders, K.L. Changes in reward-induced brain activation in opiate addicts. Eur. J. Neurosci. 2001, 14, 1360–1368. [Google Scholar] [CrossRef]
- Martin-Soelch, C.; Magyar, S.; Künig, G.; Missimer, J.; Schultz, W.; Leenders, K.L. Changes in brain activation associated with reward processing in smokers and nonsmokers: A pet study. Exp. Brain Res. 2001, 139, 278–386. [Google Scholar] [CrossRef]
- Martin-Soelch, C.; Missimer, J.; Leenders, K.; Schultz, W. Neural activity related to the processing of increasing monetary reward in smokers and nonsmokers. Eur. J. Neurosci. 2003, 18, 680–688. [Google Scholar]
- Martin-Soelch, C.; Kobel, M.; Stoecklin, M.; Michael, T.; Weber, S.; Krebs, B.; Opwis, K. Reduced response to reward in smokers and cannabis users. Neuropsychobiology 2009, 60, 94–103. [Google Scholar] [CrossRef]
- Bechara, A. Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nat. Neurosci. 2005, 8, 1458–1463. [Google Scholar] [CrossRef]
- Bechara, A. Risky business: Emotion, decision-making, and addiction. J. Gambl. Stud. 2003, 19, 23–51. [Google Scholar] [CrossRef]
- Bechara, A.; Damasio, A.R.; Damasio, H.; Anderson, S.W. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 1994, 50, 7–15. [Google Scholar] [CrossRef]
- Feil, J.; Sheppard, D.; Fitzgerald, P.B.; Yucel, M.; Lubman, D.I.; Bradshaw, J.L. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci. Biobehav. Rev. 2010. [Google Scholar]
- Bayard, S.; Raffard, S.; Gely-Nargeot, M.C. Do facets of self-reported impulsivity predict decision-making under ambiguity and risk? Evidence from a community sample. Psychiatry Res. 2011, 190, 322–326. [Google Scholar] [CrossRef]
- Goldstein, R.Z.; Craig, A.D.; Bechara, A.; Garavan, H.; Childress, A.R.; Paulus, M.P.; Volkow, N.D. The neurocircuitry of impaired insight in drug addiction. Trends Cogn. Sci. 2009, 13, 372–380. [Google Scholar]
- McLennan, J.D.; Shaw, E.; Shema, S.J.; Gardner, W.P.; Pope, S.K.; Kelleher, K.J. Adolescents’ insight in heavy drinking. J. Adolesc. Health 1998, 22, 409–416. [Google Scholar] [CrossRef]
- Craig, A.D. How do you feel—now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef]
- Garavan, H.; Stout, J.C. Neurocognitive insights into substance abuse. Trends Cogn. Sci. 2005, 9, 195–201. [Google Scholar] [CrossRef]
- Hester, R.; Nestor, L.; Garavan, H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology 2009, 34, 2450–2458. [Google Scholar] [CrossRef]
- Naqvi, N.H.; Rudrauf, D.; Damasio, H.; Bechara, A. Damage to the insula disrupts addiction to cigarette smoking. Science 2007, 315, 531–534. [Google Scholar]
- Brody, A.L.; Mandelkern, M.A.; Olmstead, R.E.; Scheibal, D.; Hahn, E.; Shiraga, S.; Zamora-Paja, E.; Farahi, J.; Saxena, S.; London, E.D.; et al. Gene variants of brain dopamine pathways and smoking-induced dopamine release in the ventral caudate/nucleus accumbens. Arch. Gen. Psychiatry 2006, 63, 808–816. [Google Scholar] [CrossRef]
- Brody, A.L.; Olmstead, R.E.; London, E.D.; Farahi, J.; Meyer, J.H.; Grossman, P.; Lee, G.S.; Huang, J.; Hahn, E.L.; Mandelkern, M.A. Smoking-induced ventral striatum dopamine release. Am. J. Psychiatry 2004, 161, 1211–1218. [Google Scholar] [CrossRef]
- Scott, D.J.; Domino, E.F.; Heitzeg, M.M.; Koeppe, R.A.; Ni, L.; Guthrie, S.; Zubieta, J.K. Smoking modulation of mu opioid and dopamine D2 receptor-mediated neurotransmission in humans. Neuropsychopharmacology 2007, 32, 450–457. [Google Scholar] [CrossRef]
- Barrett, S.P.; Boileau, I.; Okker, J.; Pihl, R.O.; Dagher, A. The hedonic response to cigarette smoking is proportional to dopamine release in the human striatum as measured by positron emission tomography and [11C]raclopride. Synapse 2004, 54, 65–71. [Google Scholar] [CrossRef]
- Montgomery, A.J.; Lingford-Hughes, A.R.; Egerton, A.; Nutt, D.J.; Grasby, P.M. The effect of nicotine on striatal dopamine release in man: A [11C]raclopride pet study. Synapse 2007, 61, 637–645. [Google Scholar]
- Brody, A.L.; Mandelkern, M.A.; Olmstead, R.E.; Allen-Martinez, Z.; Scheibal, D.; Abrams, A.L.; Costello, M.R.; Farahi, J.; Saxena, S.; Monterosso, J.; et al. Ventral striatal dopamine release in response to smoking a regular vs a denicotinized cigarette. Neuropsychopharmacology 2009, 34, 282–289. [Google Scholar] [CrossRef]
- Scott, D.J.; Stohler, C.S.; Egnatuk, C.M.; Wang, H.; Koeppe, R.A.; Zubieta, J.K. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron 2007, 55, 325–336. [Google Scholar] [CrossRef]
- Fehr, C.; Yakushev, I.; Hohmann, N.; Buchholz, H.G.; Landvogt, C.; Deckers, H.; Eberhardt, A.; Klager, M.; Smolka, M.N.; Scheurich, A.; et al. Association of low striatal dopamine D2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am. J. Psychiatry 2008, 165, 507–514. [Google Scholar] [CrossRef]
- Brown, A.K.; Mandelkern, M.A.; Farahi, J.; Robertson, C.; Ghahremani, D.G.; Sumerel, B.; Moallem, N.; London, E.D. Sex differences in striatal dopamine D2/D3 receptor availability in smokers and non-smokers. Int. J. Neuropsychopharmacol. 2012, 15, 989–994. [Google Scholar] [CrossRef]
- Leroy, C.; Karila, L.; Martinot, J.L.; Lukasiewicz, M.; Duchesnay, E.; Comtat, C.; Dolle, F.; Benyamina, A.; Artiges, E.; Ribeiro, M.J.; et al. Striatal and extrastriatal dopamine transporter in cannabis and tobacco addiction: A high-resolution PET study. Addict. Biol. 2011, 17, 981–990. [Google Scholar]
- Newberg, A.; Lerman, C.; Wintering, N.; Ploessl, K.; Mozley, P.D. Dopamine transporter binding in smokers and nonsmokers. Clin. Nucl. Med. 2007, 32, 452–455. [Google Scholar]
- Yang, Y.K.; Yao, W.J.; Yeh, T.L.; Lee, I.H.; Chen, P.S.; Lu, R.B.; Chiu, N.T. Decreased dopamine transporter availability in male smokers—a dual isotope spect study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 274–279. [Google Scholar] [CrossRef]
- Das, D.; Cherbuin, N.; Anstey, K.J.; Sachdev, P.S.; Easteal, S. Lifetime cigarette smoking is associated with striatal volume measures. Addict. Biol. 2012, 17, 817–825. [Google Scholar] [CrossRef]
- Gallinat, J.; Meisenzahl, E.; Jacobsen, L.K.; Kalus, P.; Bierbrauer, J.; Kienast, T.; Witthaus, H.; Leopold, K.; Seifert, F.; Schubert, F.; et al. Smoking and structural brain deficits: A volumetric mr investigation. Eur. J. Neurosci. 2006, 24, 1744–1750. [Google Scholar] [CrossRef]
- Hong, L.E.; Gu, H.; Yang, Y.; Ross, T.J.; Salmeron, B.J.; Buchholz, B.; Thaker, G.K.; Stein, E.A. Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch. Gen. Psychiatry 2009, 66, 431–441. [Google Scholar] [CrossRef]
- Janes, A.C.; Nickerson, L.D.; Frederick, B.; Kaufman, M.J. Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug Alcohol Depend. 2012, 125, 252–259. [Google Scholar]
- Fagerström, K.-O. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict. Behav. 1978, 3, 235–241. [Google Scholar] [CrossRef]
- Hong, L.E.; Hodgkinson, C.A.; Yang, Y.; Sampath, H.; Ross, T.J.; Buchholz, B.; Salmeron, B.J.; Srivastava, V.; Thaker, G.K.; Goldman, D.; et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc. Natl. Acad. Sci. USA 2010, 107, 13509–13514. [Google Scholar]
- Bierut, L.J.; Stitzel, J.A.; Wang, J.C.; Hinrichs, A.L.; Grucza, R.A.; Xuei, X.; Saccone, N.L.; Saccone, S.F.; Bertelsen, S.; Fox, L.; et al. Variants in nicotinic receptors and risk for nicotine dependence. Am. J. Psychiatry 2008, 165, 1163–1171. [Google Scholar] [CrossRef]
- Sutherland, M.T.; McHugh, M.J.; Pariyadath, V.; Stein, E.A. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage 2012, 62, 2281–2295. [Google Scholar] [CrossRef]
- Shiffman, S.; Balabanis, M.H.; Gwaltney, C.J.; Paty, J.A.; Gnys, M.; Kassel, J.D.; Hickcox, M.; Paton, S.M. Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug Alcohol Depend. 2007, 91, 159–168. [Google Scholar] [CrossRef]
- Powell, J.; Dawkins, L.; Davis, R.E. Smoking, reward responsiveness, and response inhibition: Tests of an incentive motivational model. Biol. Psychiatry 2002, 51, 151–163. [Google Scholar] [CrossRef]
- Peters, J.; Bromberg, U.; Schneider, S.; Brassen, S.; Menz, M.; Banaschewski, T.; Conrod, P.J.; Flor, H.; Gallinat, J.; Garavan, H.; et al. Lower ventral striatal activation during reward anticipation in adolescent smokers. Am. J. Psychiatry 2011, 168, 540–549. [Google Scholar]
- Rose, E.J.; Ross, T.J.; Salmeron, B.J.; Lee, M.; Shakleya, D.M.; Huestis, M.; Stein, E.A. Chronic exposure to nicotine is associated with reduced reward-related activity in the striatum but not the midbrain. Biol. Psychiatry 2012, 71, 206–213. [Google Scholar] [CrossRef]
- David, S.P.; Munafo, M.R.; Johansen-Berg, H.; Smith, S.M.; Rogers, R.D.; Matthews, P.M.; Walton, R.T. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: A functional magnetic resonance imaging study. Biol. Psychiatry 2005, 58, 488–494. [Google Scholar] [CrossRef]
- Due, D.L.; Huettel, S.A.; Hall, W.G.; Rubin, D.C. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: Evidence from functional magnetic resonance imaging. Am. J. Psychiatry 2002, 159, 954–960. [Google Scholar] [CrossRef]
- Franklin, T.R.; Wang, Z.; Wang, J.; Sciortino, N.; Harper, D.; Li, Y.; Ehrman, R.; Kampman, K.; O'Brien, C.P.; Detre, J.A.; et al. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: A perfusion fmri study. Neuropsychopharmacology 2007, 32, 2301–2309. [Google Scholar]
- McClernon, F.J.; Kozink, R.V.; Lutz, A.M.; Rose, J.E. 24-h smoking abstinence potentiates fMRI-bold activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berl.) 2009, 204, 25–35. [Google Scholar] [CrossRef]
- Franklin, T.R.; Wang, Z.; Li, Y.; Suh, J.J.; Goldman, M.; Lohoff, F.W.; Cruz, J.; Hazan, R.; Jens, W.; Detre, J.A.; et al. Dopamine transporter genotype modulation of neural responses to smoking cues: Confirmation in a new cohort. Addict. Biol. 2011, 16, 308–322. [Google Scholar] [CrossRef]
- Franklin, T.; Wang, Z.; Suh, J.J.; Hazan, R.; Cruz, J.; Li, Y.; Goldman, M.; Detre, J.A.; O’Brien, C.P.; Childress, A.R. Effects of varenicline on smoking cue-triggered neural and craving responses. Arch. Gen. Psychiatry 2011, 68, 516–526. [Google Scholar] [CrossRef]
- Kang, O.S.; Chang, D.S.; Jahng, G.H.; Kim, S.Y.; Kim, H.; Kim, J.W.; Chung, S.Y.; Yang, S.I.; Park, H.J.; Lee, H.; et al. Individual differences in smoking-related cue reactivity in smokers: An eye-tracking and fmri study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 38, 285–293. [Google Scholar] [CrossRef]
- Freeman, T.P.; Morgan, C.J.; Beesley, T.; Curran, H.V. Drug cue induced overshadowing: Selective disruption of natural reward processing by cigarette cues amongst abstinent but not satiated smokers. Psychol. Med. 2012, 42, 161–171. [Google Scholar] [CrossRef]
- Versace, F.; Lam, C.Y.; Engelmann, J.M.; Robinson, J.D.; Minnix, J.A.; Brown, V.L.; Cinciripini, P.M. Beyond cue reactivity: Blunted brain responses to pleasant stimuli predict long-term smoking abstinence. Psychol. Med. 2012, 42, 161–171. [Google Scholar]
- Smolka, M.N.; Buhler, M.; Klein, S.; Zimmermann, U.; Mann, K.; Heinz, A.; Braus, D.F. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berl.) 2006, 184, 577–588. [Google Scholar] [CrossRef]
- Janes, A.C.; Frederick, B.; Richardt, S.; Burbridge, C.; Merlo-Pich, E.; Renshaw, P.F.; Evins, A.E.; Fava, M.; Kaufman, M.J. Brain fmri reactivity to smoking-related images before and during extended smoking abstinence. Exp. Clin. Psychopharmacol. 2009, 17, 365–373. [Google Scholar] [CrossRef]
- Janes, A.C.; Pizzagalli, D.A.; Richardt, S.; de, B.F.B.; Chuzi, S.; Pachas, G.; Culhane, M.A.; Holmes, A.J.; Fava, M.; Evins, A.E.; et al. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol. Psychiatry 2010, 67, 722–729. [Google Scholar] [CrossRef]
- Heishman, S.J.; Kleykamp, B.A.; Singleton, E.G. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl.) 2010, 210, 453–469. [Google Scholar] [CrossRef]
- Nestor, L.; McCabe, E.; Jones, J.; Clancy, L.; Garavan, H. Differences in “bottom-up” and “top-down” neural activity in current and former cigarette smokers: Evidence for neural substrates which may promote nicotine abstinence through increased cognitive control. Neuroimage 2011, 56, 2258–2275. [Google Scholar]
- Lessov-Schlaggar, C.N.; Lepore, R.L.; Kristjansson, S.D.; Schlaggar, B.L.; Barnes, K.A.; Petersen, S.E.; Madden, P.A.; Heath, A.C.; Barch, D.M. Functional neuroimaging study in identical twin pairs discordant for regular cigarette smoking. Addict. Biol. 2012, 18, 98–108. [Google Scholar]
- Addicott, M.A.; Baranger, D.A.; Kozink, R.V.; Smoski, M.J.; Dichter, G.S.; McClernon, F.J. Smoking withdrawal is associated with increases in brain activation during decision making and reward anticipation: A preliminary study. Psychopharmacology (Berl.) 2012, 219, 563–573. [Google Scholar] [CrossRef]
- Brody, A.L.; Mandelkern, M.A.; Jarvik, M.E.; Lee, G.S.; Smith, E.C.; Huang, J.C.; Bota, R.G.; Bartzokis, G.; London, E.D. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol. Psychiatry 2004, 55, 77–84. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Martin-Soelch, C. Neuroadaptive Changes Associated with Smoking: Structural and Functional Neural Changes in Nicotine Dependence. Brain Sci. 2013, 3, 159-176. https://doi.org/10.3390/brainsci3010159
Martin-Soelch C. Neuroadaptive Changes Associated with Smoking: Structural and Functional Neural Changes in Nicotine Dependence. Brain Sciences. 2013; 3(1):159-176. https://doi.org/10.3390/brainsci3010159
Chicago/Turabian StyleMartin-Soelch, Chantal. 2013. "Neuroadaptive Changes Associated with Smoking: Structural and Functional Neural Changes in Nicotine Dependence" Brain Sciences 3, no. 1: 159-176. https://doi.org/10.3390/brainsci3010159



