White Matter Integrity Pre- and Post Marijuana and Alcohol Initiation in Adolescence
Abstract
:1. Introduction
2. Results and Discussion
2.1. Demographics
| Alcohol initiators (ALC) (n = 8) Mean (SD) | Marijuana initiators (MJ) (n = 8) Mean (SD) | |
|---|---|---|
| 21.2 (19.9–22.1) | 20.6 (19.7–22.7) | |
| Age (SD; range) at baseline | 18.2 (0.7; 16.9–18.8) | 17.5 (0.8; 16.6–19.0) |
| % Caucasian | 75% | 63% |
| GPA at 3-year follow-up | 3.3 (0.6) | 3.1 (0.3) |
| Household Income at 3-year follow-up | 105.9K (57.3) | 163.3K (124.7) |
| Family history alcohol use disorder | 38% | 50% |
| Family history substance use disorder | 25% | 62% |
| Alcohol, lifetime use days at baseline (SD: range) | 26.3 (20.5; 3–60) | 37.1 (33.2; 0–85) |
| Alcohol, days use over follow-up (SD; range) | 205.9 (74.7; 131–312) | 295.0 (197.0; 100–625) |
| Binge Drinking Episodes over follow-up | 88.1 (71.2) | 156.3 (157.4) |
| Marijuana, lifetime use days at baseline (SD; range) | 1.0 (2.1; 0–6) | 3.6 (3.5; 0–9) |
| Marijuana, days use over follow-up (SD; range) * | 3.0 (3.7; 0–10) | 369.1 (168.3; 33–540) |
| Other drug use, lifetime use at baseline (SD; range) | 1.0 (0.7; 0.0–2.0) | 1.9 (4.5; 0.0–13.0) |
| Other drug use, days use over follow-up (SD; range) | 1.0 (0.3; 0.0–2.0) | 20.0 (33.1; 0.0–99.0) † |
| Beck Depression Inventory at baseline | 1.0 (1.0) | 4.6 (3.7) |
| Beck Depression Inventory at 3-year follow-up | 1.0 (1.6) | 5.5 (7.5) |
| State Trait Anxiety Inventory at baseline | 24.3 (2.6) | 29.1 (13.2) |
| State Trait Anxiety Inventory at 3-year follow-up * | 21.3 (2.4) | 29.8 (8.1) |
| Vocabulary T-score at baseline | 62.4 (9.0) | 57.5 (8.6) |
| CBCL Externalizing T-score at baseline * | 38.3 (7.7) | 52.1 (9.8) |
| ASR Externalizing T-score at 3-year follow-up * | 41.3 (12.8) | 59.4 (10.7) |
| CBCL Internalizing T-score at baseline * | 41.0 (8.3) | 50.4 (8.1) |
| ASR Internalizing T-score at 3-year follow-up | 38.5 (11.3) | 49.0 (15.5) |
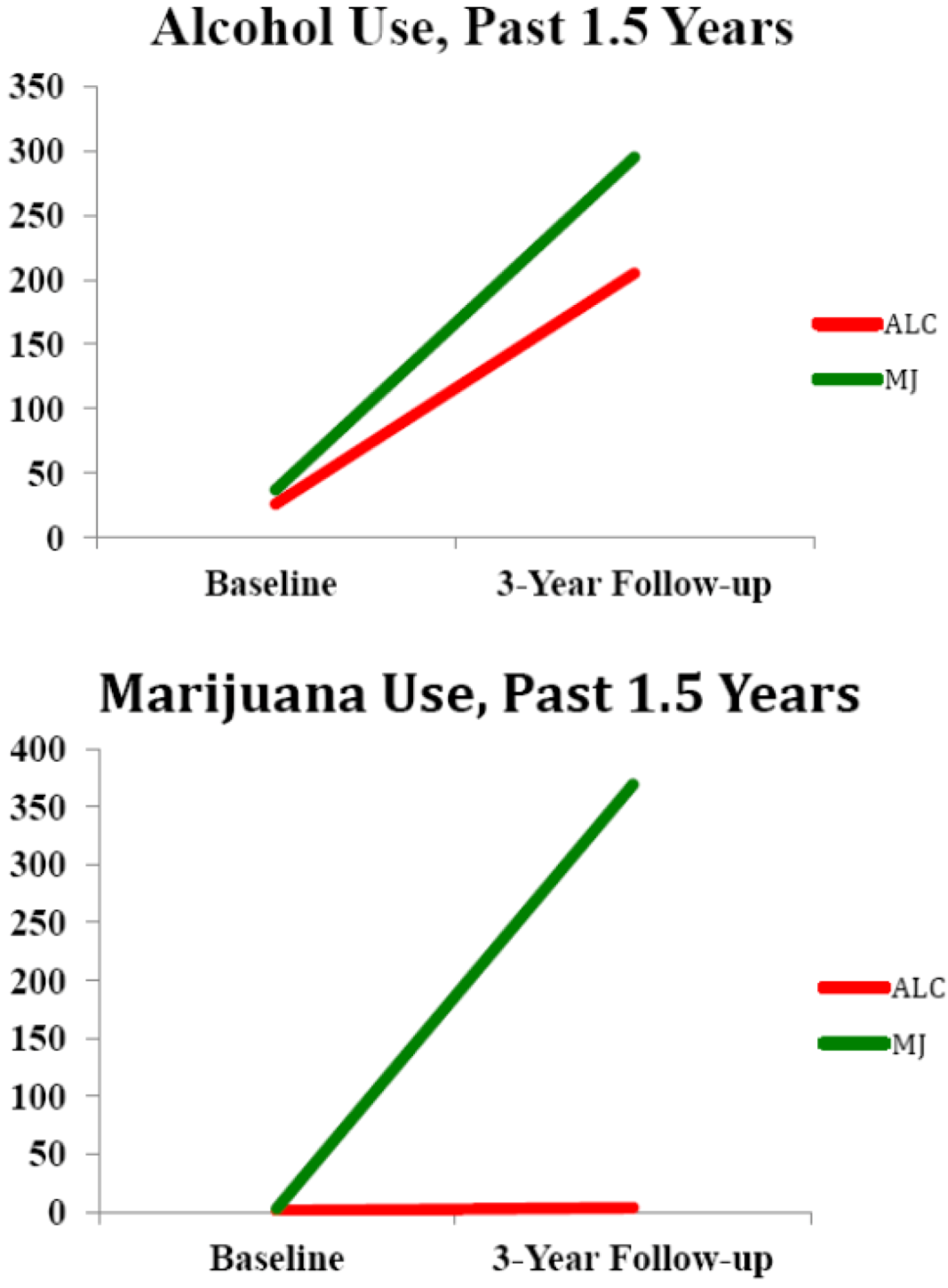
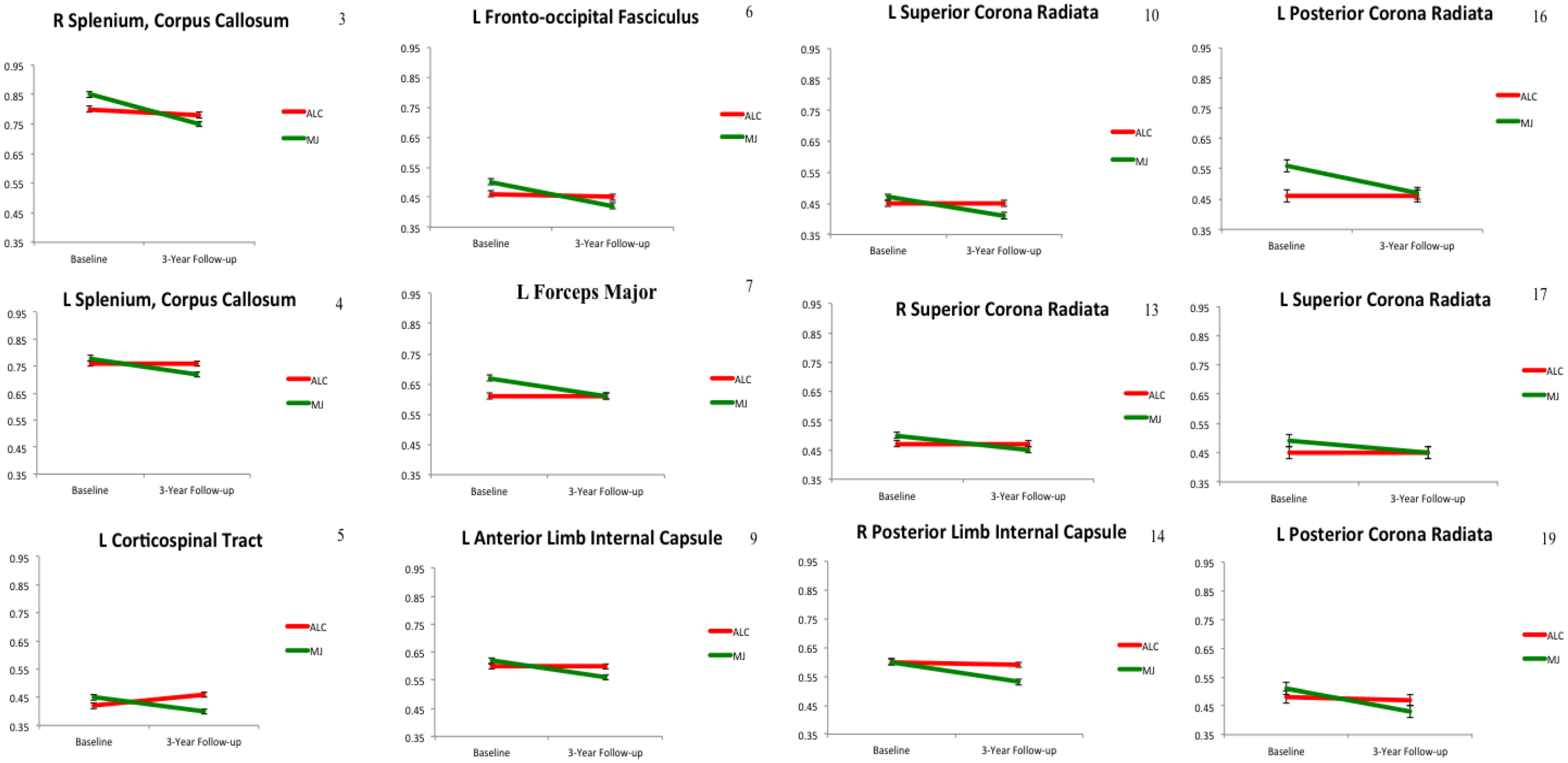
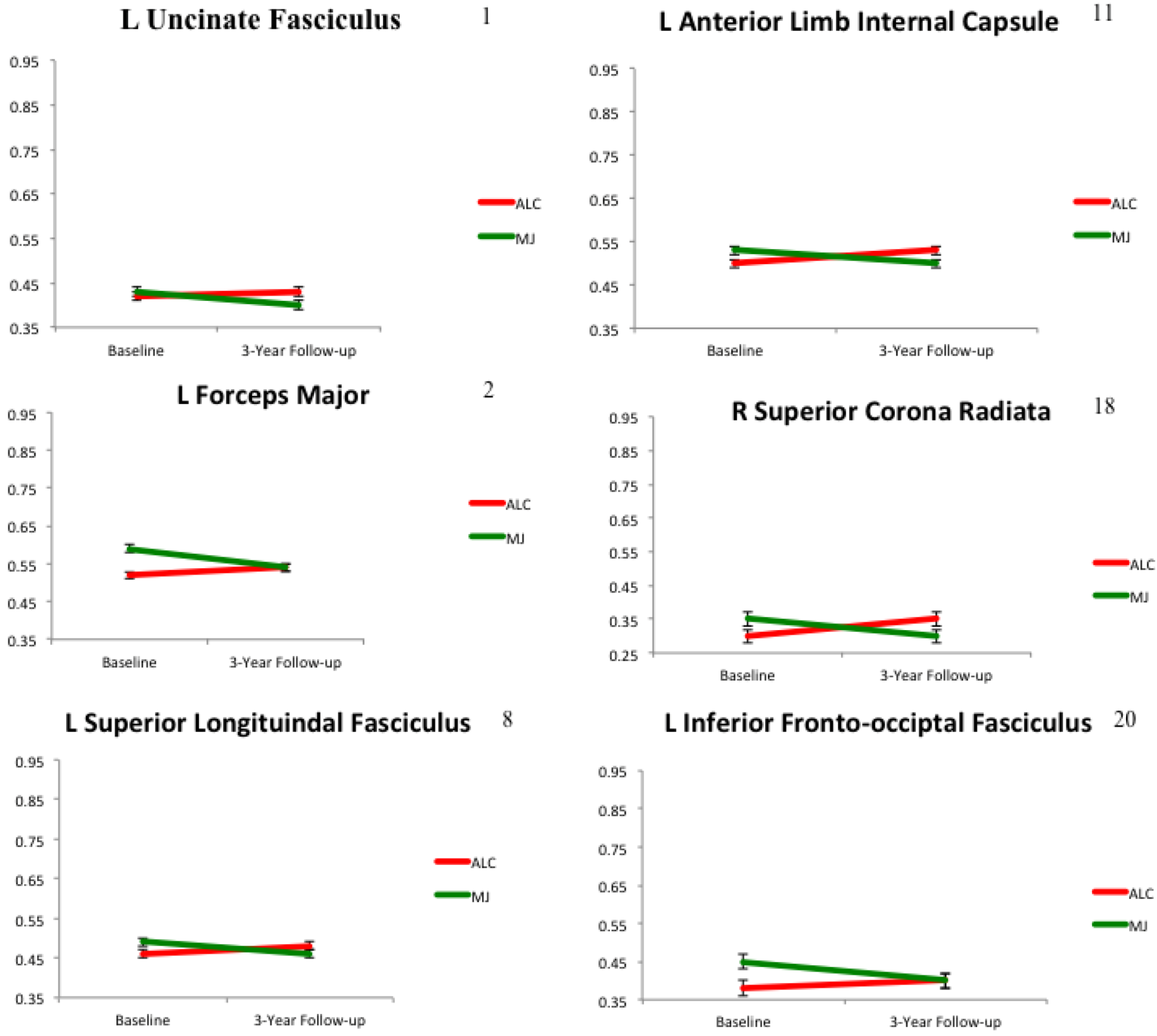
2.2. White Matter Integrity over Time
2.3. Neurocognitive Functioning over Time
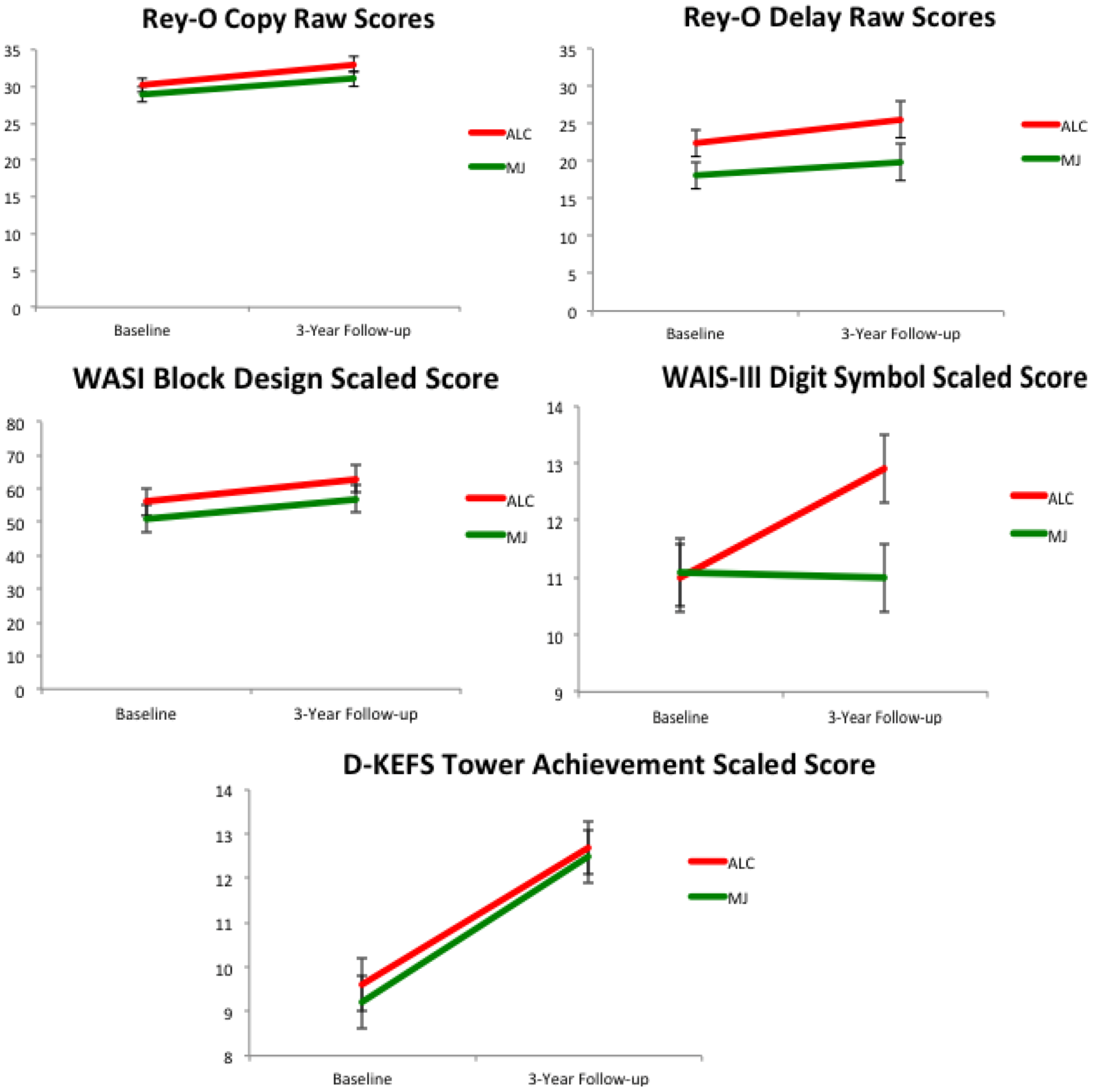
Neurocognitive Correlates with White Matter

2.4. Key Findings and Limitations
3. Experimental Section
3.1. Participants
3.2. Procedures
3.2.1. Substance Use and Mental Health Assessment
3.2.2. Neuropsychological Testing
3.2.3. Diffusion Tensor Imaging Acquisition and Processing
3.3. Data Analyses
3.3.1. Demographic Comparisons.
3.3.2. DTI Statistical Analyses.
3.3.3. Neuropsychological Performance, White Matter Correlates, and Substance Use Severity
4. Conclusions
Conflict of Interest
Acknowledgements
References
- Johnston, L.D.; O’Malley, P.M.; Bachman, J.G.; Schulenberg, J.E. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings, 2012; Institute for Social Research, The University of Michigan: Ann Arbor, MI, USA, 2013. [Google Scholar]
- Gogtay, N.; Giedd, J.N.; Lusk, L.; Hayashi, K.M.; Greenstein, D.; Vaituzis, A.C.; Nugent, T.F., III; Herman, D.H.; Clasen, L.S.; Toga, A.W.; et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. USA 2004, 101, 8174–8179. [Google Scholar] [CrossRef]
- Giedd, J.N.; Blumenthal, J.; Jeffries, N.O.; Castellanos, F.X.; Liu, H.; Zijdenbos, A.; Paus, T.; Evans, A.C.; Rapoport, J.L. Brain development during childhood and adolescence: A longitudinal MRI study. Nat. Neurosci. 1999, 2, 861–863. [Google Scholar]
- Stiles, J.; Jernigan, T.L. The basics of brain development. Neuropsychol. Rev. 2010, 20, 327–348. [Google Scholar] [CrossRef]
- Giedd, J.N. The teen brain: Insights from neuroimaging. J. Adolesc. Health 2008, 42, 335–343. [Google Scholar] [CrossRef]
- Fryer, S.L.; Frank, L.R.; Spadoni, A.D.; Theilmann, R.J.; Nagel, B.J.; Schweinsburg, A.D.; Tapert, S.F. Microstructural integrity of the corpus callosum linked with neuropsychological performance in adolescents. Brain Cogn. 2008, 67, 225–233. [Google Scholar] [CrossRef]
- Nagy, Z.; Westerberg, H.; Klingberg, T. Maturation of white matter is associated with the development of cognitive functions during childhood. J. Cogn. Neurosci. 2004, 16, 1227–1233. [Google Scholar] [CrossRef]
- Mabbott, D.J.; Noseworthy, M.; Bouffet, E.; Laughlin, S.; Rockel, C. White matter growth as a mechanism of cognitive development in children. Neuroimage 2006, 33, 936–946. [Google Scholar] [CrossRef]
- Jacobus, J.; Bava, S.; Cohen-Zion, M.; Mahmood, O.; Tapert, S.F. Functional consequences of marijuana use in adolescents. Pharmacol. Biochem. Behav. 2009, 92, 559–565. [Google Scholar] [CrossRef]
- Schweinsburg, A.D.; Brown, S.A.; Tapert, S.F. The influence of marijuana use on neurocognitive functioning in adolescents. Curr. Drug Abuse Rev. 2008, 1, 99–111. [Google Scholar] [CrossRef]
- Squeglia, L.M.; Jacobus, J.; Tapert, S.F. The influence of substance use on adolescent brain development. Clin. EEG Neurosci. 2009, 40, 31–38. [Google Scholar] [CrossRef]
- Squeglia, L.M.; Spadoni, A.D.; Infante, M.A.; Myers, M.G.; Tapert, S.F. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol. Addict. Behav. 2009, 23, 715–722. [Google Scholar] [CrossRef]
- Tapert, S.F.; Brown, S.A. Neuropsychological correlates of adolescent substance abuse: Four-year outcomes. J. Int. Neuropsychol. Soc. 1999, 5, 481–493. [Google Scholar] [CrossRef]
- Tapert, S.F.; Granholm, E.; Leedy, N.G.; Brown, S.A. Substance use and withdrawal: Neuropsychological functioning over 8 years in youth. J. Int. Neuropsychol. Soc. 2002, 8, 873–883. [Google Scholar]
- Squeglia, L.M.; Pulido, C.; Wetherill, R.R.; Jacobus, J.; Brown, G.G.; Tapert, S.F. Brain response to working memory over three years of adolescence: Influence of initiating heavy drinking. J. Stud. Alcohol Drugs 2012, 73, 749–760. [Google Scholar]
- Squeglia, L.M.; Schweinsburg, A.D.; Pulido, C.; Tapert, S.F. Adolescent binge drinking linked to abnormal spatial working memory brain activation: Differential gender effects. Alcohol. Clin. Exp. Res. 2011, 35, 1831–1841. [Google Scholar] [CrossRef]
- McQueeny, T.; Schweinsburg, B.C.; Schweinsburg, A.D.; Jacobus, J.; Bava, S.; Frank, L.R.; Tapert, S.F. Altered white matter integrity in adolescent binge drinkers. Alcohol. Clin. Exp. Res. 2009, 33, 1278–1285. [Google Scholar] [CrossRef]
- Thoma, R.J.; Monnig, M.A.; Lysne, P.A.; Ruhl, D.A.; Pommy, J.A.; Bogenschutz, M.; Tonigan, J.S.; Yeo, R.A. Adolescent substance abuse: The effects of alcohol and marijuana on neuropsychological performance. Alcohol. Clin. Exp. Res. 2011, 35, 39–46. [Google Scholar] [CrossRef]
- Solowij, N.; Jones, K.A.; Rozman, M.E.; Davis, S.M.; Ciarrochi, J.; Heaven, P.C.; Lubman, D.I.; Yucel, M. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology 2011, 216, 131–144. [Google Scholar] [CrossRef]
- Medina, K.L.; Hanson, K.L.; Schweinsburg, A.D.; Cohen-Zion, M.; Nagel, B.J.; Tapert, S.F. Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after a month of abstinence. J. Int. Neuropsychol. Soc. 2007, 13, 807–820. [Google Scholar]
- Hanson, K.L.; Winward, J.L.; Schweinsburg, A.D.; Medina, K.L.; Brown, S.A.; Tapert, S.F. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict. Behav. 2010, 35, 970–976. [Google Scholar] [CrossRef]
- Meier, M.H.; Caspi, A.; Ambler, A.; Harrington, H.; Houts, R.; Keefe, R.S.; McDonald, K.; Ward, A.; Poulton, R.; Moffitt, T.E. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc. Natl. Acad. Sci. USA 2012. [Google Scholar] [CrossRef]
- Fontes, M.A.; Bolla, K.I.; Cunha, P.J.; Almeida, P.P.; Jungerman, F.; Laranjeira, R.R.; Bressan, R.A.; Lacerda, A.L. Cannabis use before age 15 and subsequent executive functioning. Br. J. Psychiatry 2011, 198, 442–447. [Google Scholar] [CrossRef] [Green Version]
- Gruber, S.A.; Silveri, M.M.; Dahlgren, M.K.; Yurgelun-Todd, D. Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Exp. Clin. Psychopharmcol. 2011, 19, 231–242. [Google Scholar]
- Cheetham, A.; Allen, N.B.; Whittle, S.; Simmons, J.G.; Yucel, M.; Lubman, D.I. Orbitofrontal volumes in early adolescence predict initiation of cannabis use: A 4-year longitudinal and prospective study. Biol. Psychiatry 2012, 71, 684–692. [Google Scholar] [CrossRef]
- Churchwell, J.C.; Lopez-Larson, M.; Yurgelun-Todd, D.A. Altered frontal cortical volume and decision making in adolescent cannabis users. Front. Psychol. 2010, 1, 225. [Google Scholar]
- Lopez-Larson, M.P.; Bogorodzki, P.; Rogowska, J.; McGlade, E.; King, J.B.; Terry, J.; Yurgelun-Todd, D. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behav. Brain Res. 2011, 220, 164–172. [Google Scholar] [CrossRef]
- Schweinsburg, A.D.; Schweinsburg, B.C.; Medina, K.L.; McQueeny, T.; Brown, S.A.; Tapert, S.F. The influence of recency of use on fMRI response during spatial working memory in adolescent marijuana users. J. Psychoactive Drugs 2010, 42, 401–412. [Google Scholar] [CrossRef]
- Jacobus, J.; Goldenberg, D.; Wierenga, C.E.; Tolentino, N.J.; Liu, T.T.; Tapert, S.F. Altered cerebral blood flow and neurocognitive correlates in adolescent cannabis users. Psychopharmacology (Berl.) 2012, 222, 675–684. [Google Scholar]
- Tapert, S.F.; Schweinsburg, A.D.; Drummond, S.P.; Paulus, M.P.; Brown, S.A.; Yang, T.T.; Frank, L.R. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl.) 2007, 194, 173–183. [Google Scholar]
- Jager, G.; Block, R.I.; Luijten, M.; Ramsey, N.F. Cannabis use and memory brain function in adolescent boys: A cross-sectional multicenter functional magnetic resonance imaging study. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 561–572. [Google Scholar]
- Schweinsburg, A.D.; Nagel, B.J.; Schweinsburg, B.C.; Park, A.; Theilmann, R.J.; Tapert, S.F. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res. 2008, 163, 40–51. [Google Scholar] [CrossRef]
- Schweinsburg, A.D.; Schweinsburg, B.C.; Cheung, E.H.; Brown, G.G.; Brown, S.A.; Tapert, S.F. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 2005, 79, 201–210. [Google Scholar] [CrossRef]
- Padula, C.B.; Schweinsburg, A.D.; Tapert, S.F. Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychol. Addict. Behav. 2007, 21, 478–487. [Google Scholar] [CrossRef]
- Bava, S.; Frank, L.R.; McQueeny, T.; Schweinsburg, B.C.; Schweinsburg, A.D.; Tapert, S.F. Altered white matter microstructure in adolescent substance users. Psychiatry Res. 2009, 173, 228–237. [Google Scholar] [CrossRef]
- Bava, S.; Jacobus, J.; Mahmood, O.; Yang, T.T.; Tapert, S.F. Neurocognitive correlates of white matter quality in adolescent substance users. Brain Cogn. 2010, 72, 347–354. [Google Scholar] [CrossRef]
- Jacobus, J.; Thayer, R.E.; Trim, R.S.; Bava, S.; Frank, L.R.; Tapert, S.F. White matter integrity, substance use, and risk taking in adolescence. Psychol. Addict. Behav. 2012. [Google Scholar] [CrossRef]
- Bava, S.; Jacobus, J.; Thayer, R.; Tapert, S.F. Longitudinal changes in white matter integrity among adolescent substance users. Alcohol. Clin. Exp. Res. 2013, 37, E181–E189. [Google Scholar] [CrossRef]
- Jacobus, J.; McQueeny, T.; Bava, S.; Schweinsburg, B.C.; Frank, L.R.; Yang, T.T.; Tapert, S.F. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol. Teratol. 2009, 31, 349–355. [Google Scholar] [CrossRef]
- Lebel, C.; Beaulieu, C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 2011, 31, 10937–10947. [Google Scholar] [CrossRef]
- Delisi, L.E.; Bertisch, H.C.; Szulc, K.U.; Majcher, M.; Brown, K.; Bappal, A.; Ardekani, B.A. A preliminary DTI study showing no brain structural change associated with adolescent cannabis use. Harm Reduct. J. 2006, 3, 17. [Google Scholar] [CrossRef]
- Ashtari, M.; Cervellione, K.; Cottone, J.; Ardekani, B.A.; Sevy, S.; Kumra, S. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. J. Psychiatr. Res. 2009, 43, 189–204. [Google Scholar] [CrossRef]
- Yucel, M.; Zalesky, A.; Takagi, M.J.; Bora, E.; Fornito, A.; Ditchfield, M.; Egan, G.F.; Pantelis, C.; Lubman, D.I. White-matter abnormalities in adolescents with long-term inhalant and cannabis use: A diffusion magnetic resonance imaging study. J. Psychiatry Neurosci. 2010, 35, 409–412. [Google Scholar] [CrossRef]
- Medina, K.L.; McQueeny, T.; Nagel, B.J.; Hanson, K.L.; Yang, T.T.; Tapert, S.F. Prefrontal cortex morphometry in abstinent adolescent marijuana users: Subtle gender effects. Addict. Biol. 2009, 14, 457–468. [Google Scholar] [CrossRef]
- McQueeny, T.; Padula, C.B.; Price, J.; Medina, K.L.; Logan, P.; Tapert, S.F. Gender effects on amygdala morphometry in adolescent marijuana users. Behav. Brain Res. 2011, 224, 128–134. [Google Scholar] [CrossRef]
- Medina, K.L.; Nagel, B.J.; Tapert, S.F. Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Res. 2010, 182, 152–159. [Google Scholar] [CrossRef]
- Cousijn, J.; Wiers, R.W.; Ridderinkhof, K.R.; van den Brink, W.; Veltman, D.J.; Goudriaan, A.E. Grey matter alterations associated with cannabis use: Results of a VBM study in heavy cannabis users and healthy controls. Neuroimage 2012, 59, 3845–3851. [Google Scholar] [CrossRef]
- Rubino, T.; Parolaro, D. Long lasting consequences of cannabis exposure in adolescence. Mol. Cell. Endocrinol. 2008, 286, S108–S113. [Google Scholar] [CrossRef]
- Abush, H.; Akirav, I. Short- and long-term cognitive effects of chronic cannabinoids administration in late-adolescence rats. PloS One 2012, 7, e31731. [Google Scholar] [CrossRef]
- Schneider, M.; Schomig, E.; Leweke, F.M. Acute and chronic cannabinoid treatment differentially affects recognition memory and social behavior in pubertal and adult rats. Addict. Biol. 2008, 13, 345–357. [Google Scholar] [CrossRef]
- Brown, S.A.; Myers, M.G.; Lippke, L.; Tapert, S.F.; Stewart, D.G.; Vik, P.W. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. J. Stud. Alcohol 1998, 59, 427–438. [Google Scholar]
- Achenbach, T.; Rescorla, L. Manual for the Aseba School-Age Forms & Profiles; Research Center for Children, Youth, and Families, University of Vermont: Burlington, VT, USA, 2001. [Google Scholar]
- Beck, A. Beck Depression Inventory (BDI); Psychological Corporation: San Antonio, TX, USA, 1978. [Google Scholar]
- Spielberger, C.; Gorsuch, R.; Lushene, R. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- Rice, J.P.; Reich, T.; Bucholz, K.K.; Neuman, R.J.; Fishman, R.; Rochberg, N.; Hesselbrock, V.M.; Nurnberger, J.I., Jr.; Schuckit, M.A.; Begleiter, H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol. Clin. Exp. Res. 1995, 19, 1018–1023. [Google Scholar] [CrossRef]
- Delis, D.C.; Kramer, J.H.; Kaplan, E.; Ober, B.A. California Verbal Learning Test, 2nd ed; The Psychological Corporation: San Antonio, TX, USA, 2001. [Google Scholar]
- Wechsler, D. Wais-III Manual; Psychological Corporation: New York, NY, USA, 1997. [Google Scholar]
- Gronwall, D.M.A. Paced auditory serial-addition task: A measure of recovery from concussion. Percept. Mot. Skills 1974, 44, 367–373. [Google Scholar] [CrossRef]
- Delis, D.C.; Kaplan, E. Delis-Kaplan Executive Functioning Scale Manual; Psychological Corporation: San Antonio, TX, USA, 2000. [Google Scholar]
- Wechsler, D. Manual for the Wechsler Memory Scale, 3rd ed; Psychological Corporation: New York, NY, USA, 1997. [Google Scholar]
- Corwin, J.; Bylsma, F.W. Translations of excerpts from Andre Rey’s “psychological examiniation of traumatic encephalopathy” and P.A. Osterrieth’s “the complex figure copy test”. Clin. Neuropsychol. 1993, 7, 3–21. [Google Scholar]
- Wechsler, D. Manual for the Wechsler Abbreviated Scale of Intelligence; Psychological Corporation: San Antonio, TX, USA, 1999. [Google Scholar]
- Andersson, J.L.; Kare, S. A model-based method for retrospective correction of geometric distortions in diffusoin weighted EPI. Neuroimage 2002, 16, 177–199. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; de Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004, 23, S208–S219. [Google Scholar] [CrossRef]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef]
- Jenkinson, M. Fast, automated, n-dimensional phase-unwrapping algorithm. Magn. Reson. Med. 2003, 49, 193–197. [Google Scholar]
- Jenkinson, M.; Smith, S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001, 5, 143–156. [Google Scholar] [CrossRef]
- Behrens, T.E.; Woolrich, M.W.; Jenkinson, M.; Johansen-Berg, H.; Nunes, R.G.; Clare, S.; Matthews, P.M.; Brady, J.M.; Smith, S.M. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn. Reson. Med. 2003, 50, 1077–1088. [Google Scholar]
- Smith, S.M.; Jenkinson, M.; Johansen-Berg, H.; Rueckert, D.; Nichols, T.E.; Mackay, C.E.; Watkins, K.E.; Ciccarelli, O.; Cader, M.Z.; Matthews, P.M.; et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 2006, 31, 1487–1505. [Google Scholar] [CrossRef]
- Cox, R.W. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996, 29, 162–173. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jacobus, J.; Squeglia, L.M.; Infante, M.A.; Bava, S.; Tapert, S.F. White Matter Integrity Pre- and Post Marijuana and Alcohol Initiation in Adolescence. Brain Sci. 2013, 3, 396-414. https://doi.org/10.3390/brainsci3010396
Jacobus J, Squeglia LM, Infante MA, Bava S, Tapert SF. White Matter Integrity Pre- and Post Marijuana and Alcohol Initiation in Adolescence. Brain Sciences. 2013; 3(1):396-414. https://doi.org/10.3390/brainsci3010396
Chicago/Turabian StyleJacobus, Joanna, Lindsay M. Squeglia, M. Alejandra Infante, Sunita Bava, and Susan F. Tapert. 2013. "White Matter Integrity Pre- and Post Marijuana and Alcohol Initiation in Adolescence" Brain Sciences 3, no. 1: 396-414. https://doi.org/10.3390/brainsci3010396




