Pattern Recognition of the Multiple Sclerosis Syndrome
Abstract
:1. Introduction
2. Brief Historical Overview of Multiple Sclerosis (MS) Diagnosis
3. Overview of Neuromyelitis Optica Spectrum Disorder
3.1. AQP4-Antibody Positive NMOSD: Pathophysiology and Pathogenicity of Aquaporin 4 Neuromyelitis Optica Spectrum Disorder (AQP4-NMOSD)
3.2. Pathophysiology and Pathogenicity of Myelin Oligodendrocyte Glycoprotein (MOG) Antibodies
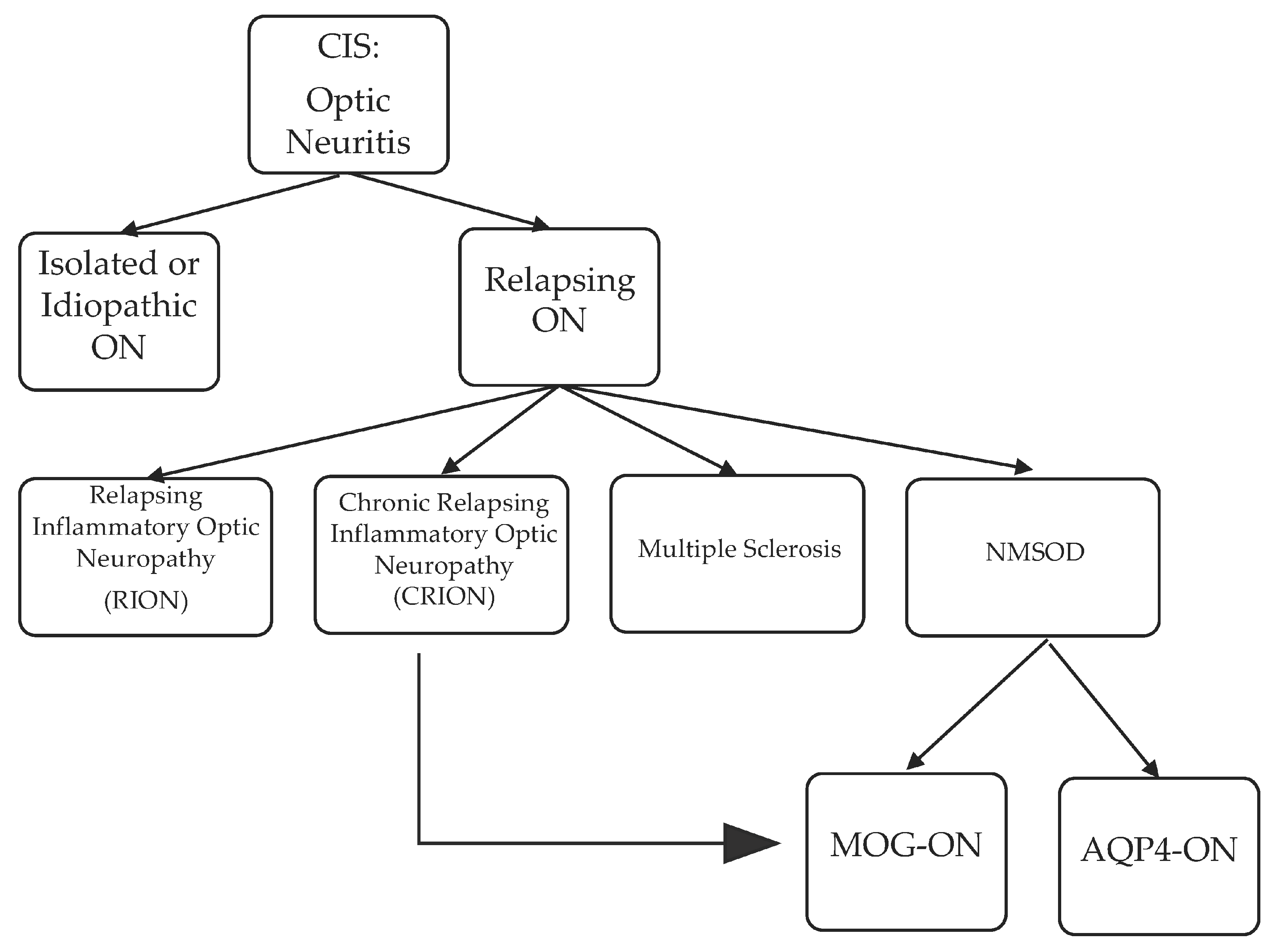
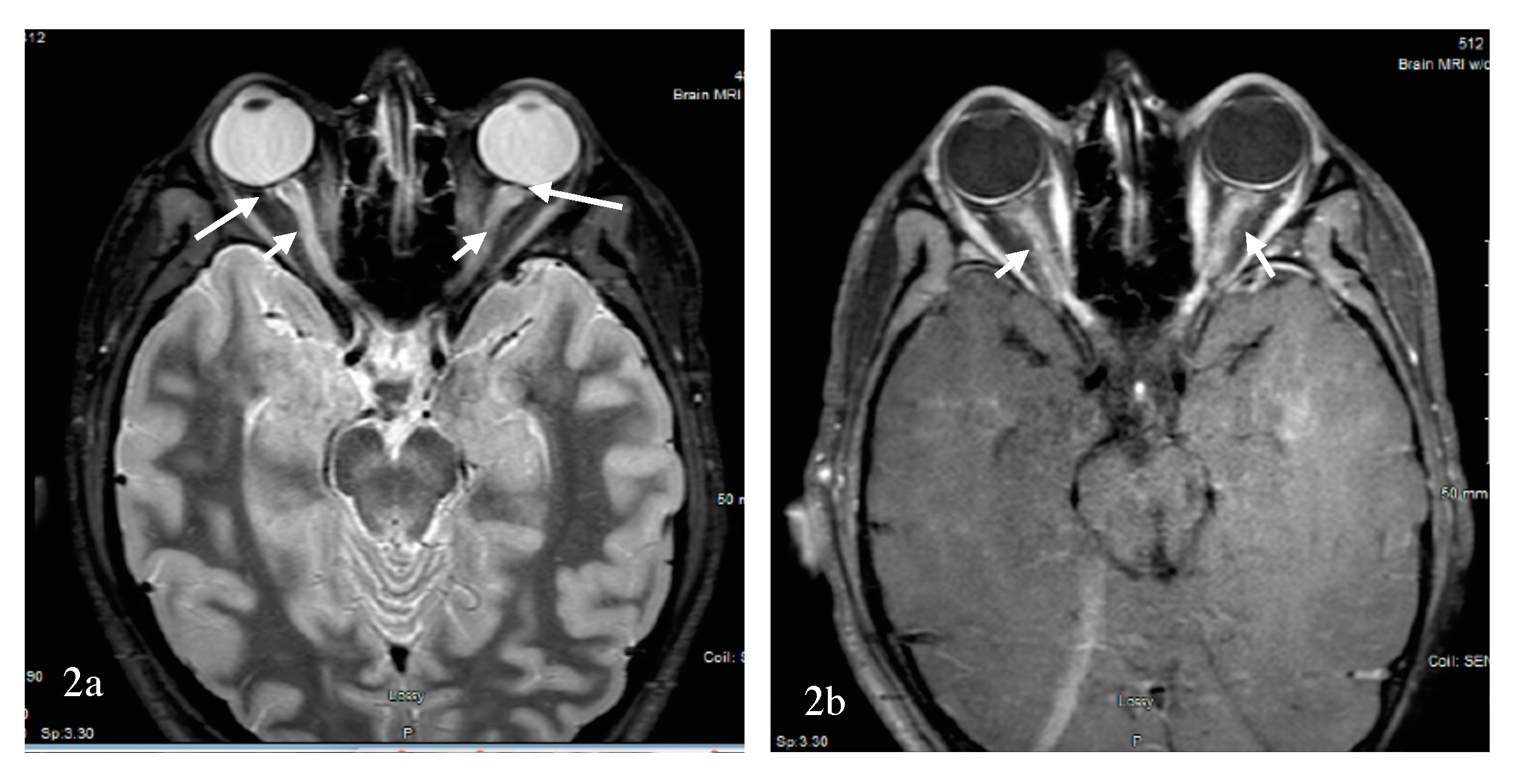
4. Optic Neuritis: From Clinically Isolated Syndrome to MS, NMOSD and Others
4.1. Single Inflammatory Optic Neuritis (SION), Relapsing Inflammatory Optic Neuritis (RION), and Chronic Relapsing Inflammatory Optic Neuropathy (CRION): “Formes frustes” of MS or NMOSD?
4.2. Multiple Sclerosis-Associated Optic Neuritis (MS-ON)
4.3. Neuromyelitis Optica Spectrum Disorder-Associated Optic Neuritis
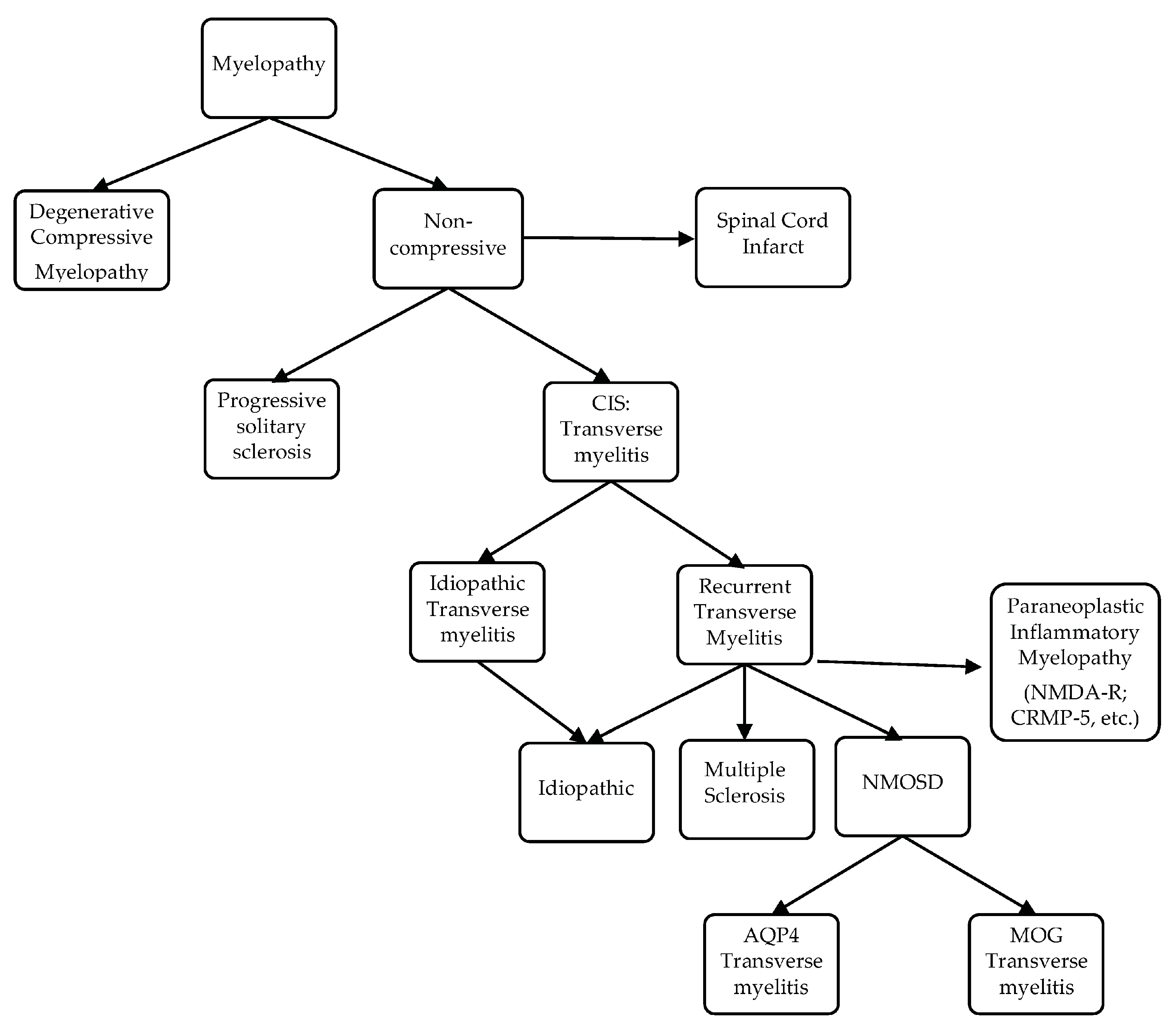
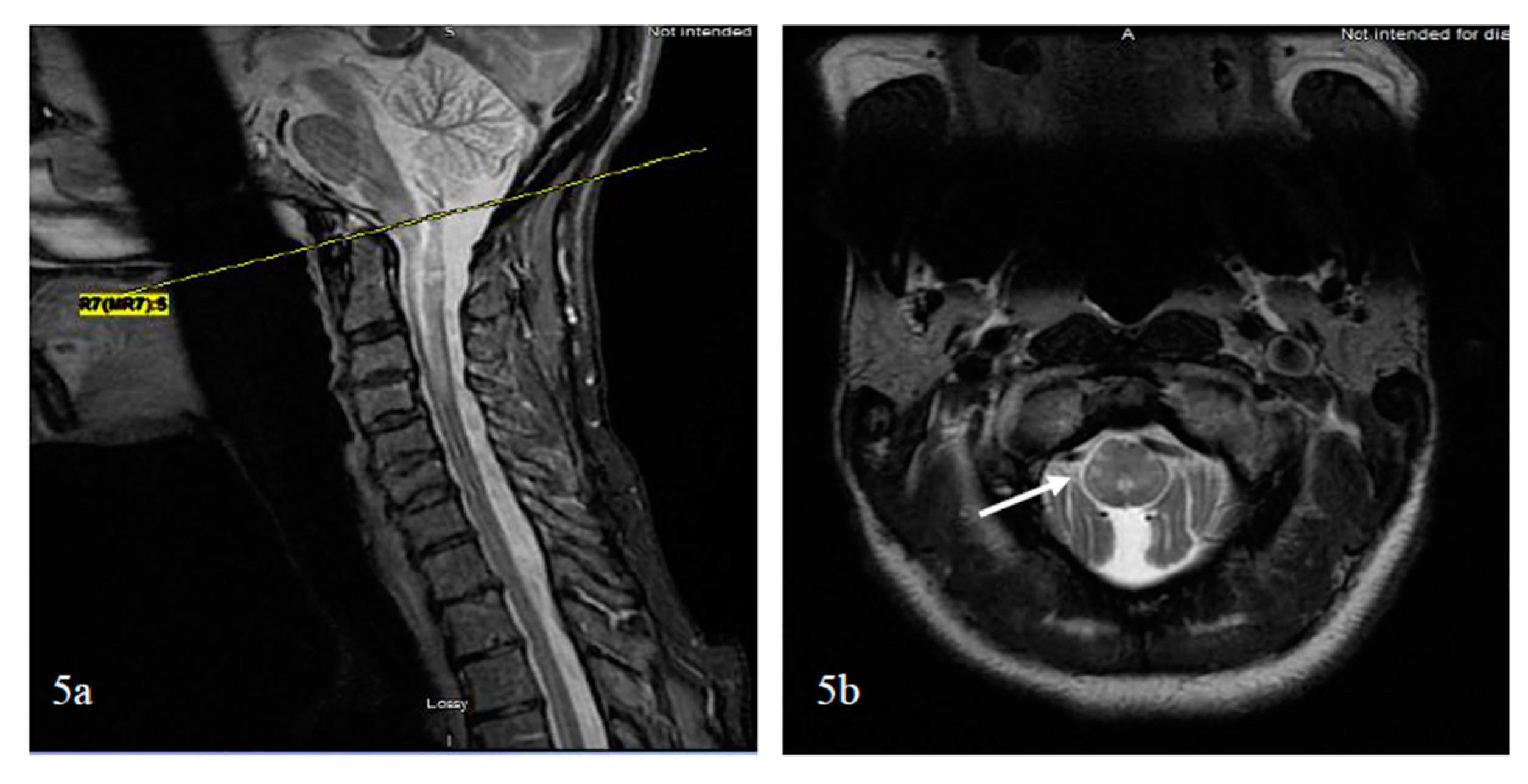
5. Transverse Myelitis Pattern Recognition: From Clinically Isolated Syndrome to MS, NMOSD and Others
5.1. Multiple Sclerosis-Associated Transverse Myelitis (MS-TM) and Myelopathy
5.1.1. Acute Complete Transverse Myelitis (ACTM) versus Acute Partial Transverse Myelitis (APTM)
5.1.2. The Case of Progressive Solitary Sclerosis
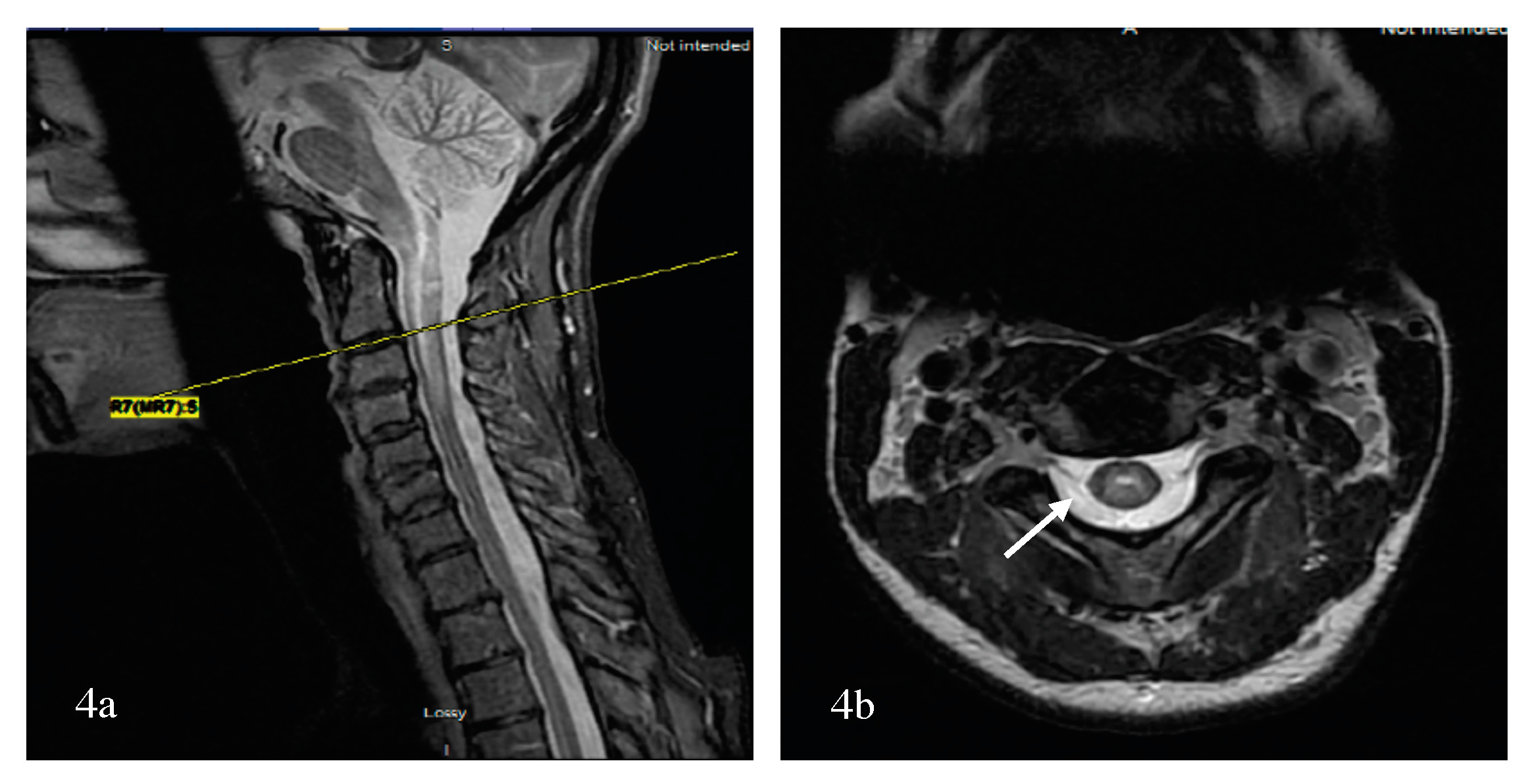
5.2. Neuromyelitis Optica Spectrum Disorder-Associated Transverse Myelitis (NMOSD-TM) and Longitudinally Extensive Transverse Myelitis (LETM)
5.2.1. LETM versus Spinal Cord Infarct versus Spondylotic Myelopathy
5.2.2. Seropositive Versus Seronegative LETM: Does Seronegative LETM Truly Exist?
5.2.3. Short Segment Transverse Myelitis (SSTM) in Neuromyelitis Optica Spectrum Disorder
5.2.4. Imaging Patterns of Neuromyelitis Optica Spectrum Disorder-Associated Transverse Myelitis (NMOSD-TM)
Linear Lesions in NMOSD
Bright Spotty Lesions (BSLs) in Neuromyelitis Optica Spectrum Disorder
6. Brainstem and Cerebellar Pattern Recognition: From Clinically Isolated Syndrome to MS, NMOSD and Others
6.1. Multiple Sclerosis-Associated Brainstem and Cerebellar Symptoms
6.1.1. Trigeminal Neuralgia or Facial Sensory Loss
6.1.2. Oculomotor Abnormalities
6.1.3. Peripheral Type Facial Nerve Palsy
6.1.4. Cerebellar Symptoms
6.2. Acute Brainstem Syndrome Associated with Neuromyelitis Optica Spectrum Disorder
6.2.1. Intractable Hiccups, Nausea and Vomiting
6.2.2. Oculomotor Abnormalities
6.2.3. Other Atypical Brainstem Presentations
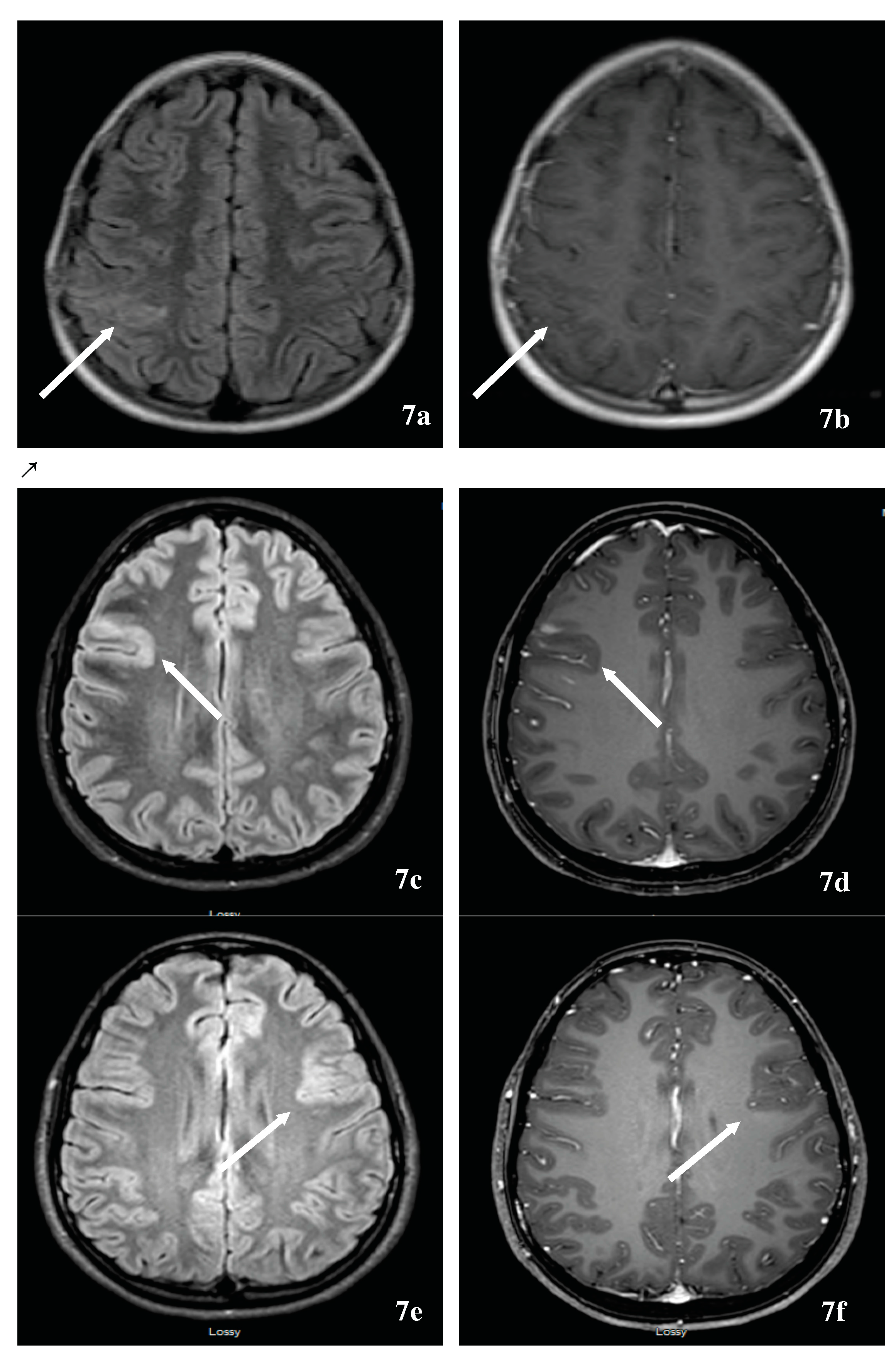
7. Tumefactive Demyelinating Lesion Pattern Recognition: From Clinically Isolated Syndrome to MS, NMOSD and Others
7.1. Multiple Sclerosis-Associated Tumefactive Demyelinating Lesions (MS-TDLs)
7.2. Neuromyelitis Optica Spectrum Disorder-Associated Tumefactive Demyelinating Lesions (NMOSD-TDL) and Hemispheric Presentations
7.3. The relationship of Balo’s Concentric Sclerosis to TDL, MS and NMOSD
8. Clinical Spectrum of MOG-Antibody-Associated-Inflammatory Demyelinating Disorders
8.1. Neuromyelitis Optica Spectrum Disorder-Associated Myelin Oligodendrocyte Glycoprotein Antibody (MOG-NMOSD)
8.2. Pediatric Acute Disseminated Encephalomyelitis (ADEM)
8.3. Overlap Syndrome or Complex Neuromyelitis Optica Spectrum Disorder Encephalitis
8.4. The Myelin Oligodendrocyte Glycoprotein (MOG) Antibody and Its Association to Other Autoantibodies
9. Other Autoantibodies, Diseases, and Biomarkers Associated with Neuromyelitis Optica Spectrum Disorder
9.1. Aquaporin 1-Antibody Associated with NMOSD (-NMOSD)
9.2. Neuromyelitis Optica Spectrum Disorder as a Paraneoplastic Syndrome
9.3. Other Biomarkers Associated with Neuromyelitis Optica Spectrum Disorder
9.3.1. Cerebrospinal Fluid Myelin Basic Protein (CSF MBP)
9.3.2. Cerebrospinal Fluid (CSF) Glial Fibrillary Acid Protein (GFAP) and S100
9.3.3. Interleukin 6 (IL-6) in Neuromyelitis Optica Spectrum Disorder (NMOSD)
10. Tips on Management of Inflammatory Demyelinating Diseases of the Central Nervous System (CNS), with a Focus on Neuromyelitis Optica Spectrum Disorder (NMOSD)
10.1. Management of Acute Demyelinating Relapses Of The Central Nervous System
10.2. Maintenance Therapy of Neuromyelitis Optica Spectrum Disorder (NMOSD)
11. Discussion and Conclusions
Supplementary Materials
Acknowlegments
Author Contributions
Conflicts of Interest
Abbreviations
| ADEM | Acute Disseminated Encephalomyelitis |
| ADEM-ON | Acute Disseminated Encephalomyelitis-Optic Neuritis |
| ATM | Acute Transverse Myelitis |
| APTM | Acute Partial Transverse Myelitis |
| ACTM | Acute complete Transverse Myelitis |
| CRION | Chronic Relapsing Inflammatory Neuropathy |
| GFAP | Glial Fibrillary Acid Protein |
| LETM | Longitudinally Extensive Transverse Myelitis |
| MS | Multiple Sclerosis |
| MS-ON | MS-Associated Optic Neuritis |
| MS-TM | MS-Asociated Transverse Myelitis |
| MS-BS | MS-Asociated Brainstem Syndrome |
| MOG | Myelin Oligodendrocyte Glycoprotein |
| MOG-ON | Myelin Oligodendrocyte Glycoprotein- Associated Optic Neuritis |
| MOG-TM | Myelin Oligodendrocyte Glycoprotein- Associated Transverse Myelitis |
| MDEM-ON | Multiphasic Disseminated Encephalomyelitis-Optic Neuritis |
| MDEM-ON | Multiphasic Disseminated Encephalomyelitis-Optic Neuritis |
| APQ4 | aquaporin 4 |
| NMOSD | Neuromyelitis Optica Spectrum Disorder |
| NMOSD-ON | Neuromyelitis Optica Spectrum Disorder-Associated Optic Neuritis |
| NMOSD-TM | Neuromyelitis Optica Spectrum Disorder-Associated Transverse Myelitis Optic Neuritis |
| NMOSD-BS | Neuromyelitis Optica Spectrum Disorder-Associated Brainstem Syndrome |
| NMDA-R | N-Methyl-D-Aspartate Receptor |
| ON | Optic Neuritis |
| SION | Single Inflammatory or Isolated Optic Neuritis |
| RION | Recurrent Inflammatory or Isolated Optic Neuritis |
| SSTM | Short Segment Transverse Myelitis |
References
- Fang, B.; McKeon, A.; Hinson, S.R.; Kryzer, T.J.; Pittock, S.J.; Aksamit, A.J.; Lennon, V.A. Autoimmune Glial Fibrillary Acidic Protein Astrocytopathy: A Novel Meningoencephalomyelitis. JAMA Neurol. 2016, 73, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Holtje, M.; Mertens, R.; Schou, M.B.; Saether, S.G.; Kochova, E.; Jarius, S.; Pruss, H.; Komorowski, L.; Probst, C.; Paul, F.; et al. Synapsin-antibodies in psychiatric and neurological disorders: Prevalence and clinical findings. Brain Behav. Immun. 2017, 66, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Zamvil, S.S.; Slavin, A.J. Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e62. [Google Scholar] [CrossRef] [PubMed]
- Zekeridou, A.; Lennon, V.A. Aquaporin-4 autoimmunity. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e110. [Google Scholar] [CrossRef] [PubMed]
- Poser, C.M.; Brinar, V.V. Diagnostic criteria for multiple sclerosis: An historical review. Clin. Neurol. Neurosurg. 2004, 106, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, G.A.; Beebe, G.; Kibler, R.F.; Kurland, L.T.; Kurtzke, J.F.; Mcdowell, F.; Nagler, B.; Sibley, W.A.; Tourtellotte, W.W.; Willmon, T.L. Problems of Experimental Trials of Therapy in Multiple Sclerosis: Report by the Panel on the Evaluation of Experimental Trials of Therapy in Multiple Sclerosis. Ann. N. Y. Acad. Sci. 1965, 122, 552–568. [Google Scholar] [CrossRef] [PubMed]
- Poser, C.M.; Paty, D.W.; Scheinberg, L.; McDonald, W.I.; Davis, F.A.; Ebers, G.C.; Johnson, K.P.; Sibley, W.A.; Silberberg, D.H.; Tourtellotte, W.W. New diagnostic criteria for multiple sclerosis: Guidelines for research protocols. Ann. Neurol. 1983, 13, 227–231. [Google Scholar] [CrossRef] [PubMed]
- McDonald, W.I.; Compston, A.; Edan, G.; Goodkin, D.; Hartung, H.P.; Lublin, F.D.; McFarland, H.F.; Paty, D.W.; Polman, C.H.; Reingold, S.C.; et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann. Neurol. 2001, 50, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Edan, G.; Filippi, M.; Hartung, H.P.; Kappos, L.; Lublin, F.D.; Metz, L.M.; McFarland, H.F.; O’Connor, P.W.; et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann. Neurol. 2005, 58, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Rovira, A.; Wattjes, M.P.; Tintore, M.; Tur, C.; Yousry, T.A.; Sormani, M.P.; De Stefano, N.; Filippi, M.; Auger, C.; Rocca, M.A.; et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-clinical implementation in the diagnostic process. Nat. Rev. Neurol. 2015, 11, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Jarius, S.; Wildemann, B.; Paul, F. Neuromyelitis optica: Clinical features, immunopathogenesis and treatment. Clin. Exp. Immunol. 2014, 176, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Asgari, N.; Skejoe, H.P.; Lennon, V.A. Evolution of longitudinally extensive transverse myelitis in an aquaporin-4 IgG-positive patient. Neurology 2013, 81, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.L.; Moonis, G.; Ginat, D.T.; Eisenberg, R.L. Lesions of the corpus callosum. AJR Am. J. Roentgenol. 2013, 200, W1–W16. [Google Scholar] [CrossRef] [PubMed]
- Kazi, A.Z.; Joshi, P.C.; Kelkar, A.B.; Mahajan, M.S.; Ghawate, A.S. MRI evaluation of pathologies affecting the corpus callosum: A pictorial essay. Indian J. Radiol. Imaging 2013, 23, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Bourekas, E.C.; Varakis, K.; Bruns, D.; Christoforidis, G.A.; Baujan, M.; Slone, H.W.; Kehagias, D. Lesions of the corpus callosum: MR imaging and differential considerations in adults and children. AJR Am. J. Roentgenol. 2002, 179, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Jurynczyk, M.; Weinshenker, B.; Akman-Demir, G.; Asgari, N.; Barnes, D.; Boggild, M.; Chaudhuri, A.; D’hooghe, M.; Evangelou, N.; Geraldes, R.; et al. Status of diagnostic approaches to AQP4-IgG seronegative NMO and NMO/MS overlap syndromes. J. Neurol. 2016, 263, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Matthews, L.; Marasco, R.; Jenkinson, M.; Kuker, W.; Luppe, S.; Leite, M.I.; Giorgio, A.; De Stefano, N.; Robertson, N.; Johansen-Berg, H.; et al. Distinction of seropositive NMO spectrum disorder and MS brain lesion distribution. Neurology 2013, 80, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.H.; Tse, C.T.; Chung, C.P.; Lee, R.L.; Kwan, J.S.; Ho, P.W.; Ho, J.W. Brain involvement in neuromyelitis optica spectrum disorders. Arch. Neurol. 2011, 68, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, Y.; Duan, Y.; Li, K. Brain MRI abnormalities in neuromyelitis optica. Eur. J. Radiol. 2011, 80, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Asgari, N.; Lillevang, S.T.; Skejoe, H.P.; Falah, M.; Stenager, E.; Kyvik, K.O. A population-based study of neuromyelitis optica in Caucasians. Neurology 2011, 76, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Jarius, S.; Ruprecht, K.; Kleiter, I.; Borisow, N.; Asgari, N.; Pitarokoili, K.; Pache, F.; Stich, O.; Beume, L.A.; Hummert, M.W.; et al. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J. Neuroinflamm. 2016, 13, 280. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, S.H.; Huh, S.Y.; Kim, H.J. Brain abnormalities in neuromyelitis optica spectrum disorder. Mult. Scler. Int. 2012, 2012, 735486. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Paul, F.; Lana-Peixoto, M.A.; Tenembaum, S.; Asgari, N.; Palace, J.; Klawiter, E.C.; Sato, D.K.; de Seze, J.; Wuerfel, J.; et al. MRI characteristics of neuromyelitis optica spectrum disorder: An international update. Neurology 2015, 84, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Pekcevik, Y.; Orman, G.; Lee, I.H.; Mealy, M.A.; Levy, M.; Izbudak, I. What do we know about brain contrast enhancement patterns in neuromyelitis optica? Clin. Imaging 2016, 40, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Asgari, N.; Flanagan, E.P.; Fujihara, K.; Kim, H.J.; Skejoe, H.P.; Wuerfel, J.; Kuroda, H.; Kim, S.H.; Maillart, E.; Marignier, R.; et al. Disruption of the leptomeningeal blood barrier in neuromyelitis optica spectrum disorder. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e343. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Ito, R.; Tanaka, K.; Tanaka, M. Cortical and leptomeningeal involvement in three cases of neuromyelitis optica. Eur. J. Neurol. 2012, 19, e47–e48. [Google Scholar] [CrossRef] [PubMed]
- Pittock, S.J.; Weinshenker, B.G.; Lucchinetti, C.F.; Wingerchuk, D.M.; Corboy, J.R.; Lennon, V.A. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch. Neurol. 2006, 63, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Jarius, S.; Kleiter, I.; Ruprecht, K.; Asgari, N.; Pitarokoili, K.; Borisow, N.; Hummert, M.W.; Trebst, C.; Pache, F.; Winkelmann, A.; et al. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients. Part 3: Brainstem involvement—frequency, presentation and outcome. J. Neuroinflamm. 2016, 13, 281. [Google Scholar] [CrossRef] [PubMed]
- Wingerchuk, D.M.; Hogancamp, W.F.; O’Brien, P.C.; Weinshenker, B.G. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology 1999, 53, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Lennon, V.A.; Kryzer, T.J.; Pittock, S.J.; Verkman, A.S.; Hinson, S.R. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 2005, 202, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin 4 and neuromyelitis optica. Lancet Neurol. 2012, 11, 535–544. [Google Scholar] [CrossRef]
- Gomes, D.; Agasse, A.; Thiebaud, P.; Delrot, S.; Geros, H.; Chaumont, F. Aquaporins are multifunctional water and solute transporters highly divergent in living organisms. Biochim. Biophys. Acta 2009, 1788, 1213–1228. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Phuan, P.W.; Asavapanumas, N.; Tradtrantip, L. Biology of AQP4 and anti-AQP4 antibody: Therapeutic implications for NMO. Brain Pathol. 2013, 23, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Jarius, S.; Paul, F.; Franciotta, D.; Waters, P.; Zipp, F.; Hohlfeld, R.; Vincent, A.; Wildemann, B. Mechanisms of disease: Aquaporin-4 antibodies in neuromyelitis optica. Nat. Clin. Pract. Neurol. 2008, 4, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Phuan, P.W.; Ratelade, J.; Rossi, A.; Tradtrantip, L.; Verkman, A.S. Complement-dependent cytotoxicity in neuromyelitis optica requires aquaporin-4 protein assembly in orthogonal arrays. J. Biol. Chem. 2012, 287, 13829–13839. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, A.; Yamamoto, T.; Kikuchi, S.; Soeda, T.; Shimizu, K.; Ugawa, Y. Aquaporin-4 expression in distal myopathy with rimmed vacuoles. BMC Neurol. 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lennon, V.A.; Popescu, B.F.; Grouse, C.K.; Topel, J.; Milone, M.; Lassmann, H.; Parisi, J.E.; Pittock, S.J.; Stefoski, D.; et al. Autoimmune aquaporin-4 myopathy in neuromyelitis optica spectrum. JAMA Neurol. 2014, 71, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Li, Y.; Dai, Q.; Zhang, Y.; Xu, Z.; Li, Y.; Cai, G.; Chu, L. Myopathy associated with neuromyelitis optica spectrum disorders. Int. J. Neurosci. 2016, 126, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Ratelade, J.; Zhang, H.; Saadoun, S.; Bennett, J.L.; Papadopoulos, M.C.; Verkman, A.S. Neuromyelitis optica IgG and natural killer cells produce NMO lesions in mice without myelin loss. Acta Neuropathol. 2012, 123, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Ratelade, J.; Bennett, J.L.; Verkman, A.S. Evidence against cellular internalization in vivo of NMO-IgG, aquaporin-4, and excitatory amino acid transporter 2 in neuromyelitis optica. J. Biol. Chem. 2011, 286, 45156–45164. [Google Scholar] [CrossRef] [PubMed]
- Wingerchuk, D.M.; Banwell, B.; Bennett, J.L.; Cabre, P.; Carroll, W.; Chitnis, T.; de Seze, J.; Fujihara, K.; Greenberg, B.; Jacob, A.; et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015, 85, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, M.; Armangue, T.; Martinez-Hernandez, E.; Arrambide, G.; Sola-Valls, N.; Sabater, L.; Tellez, N.; Midaglia, L.; Arino, H.; Peschl, P.; et al. Clinical spectrum associated with MOG autoimmunity in adults: Significance of sharing rodent MOG epitopes. J. Neurol. 2016, 263, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Boyle, L.H.; Traherne, J.A.; Plotnek, G.; Ward, R.; Trowsdale, J. Splice variation in the cytoplasmic domains of myelin oligodendrocyte glycoprotein affects its cellular localisation and transport. J. Neurochem. 2007, 102, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Rubner, P.; Schautzer, F.; Egg, R.; Ulmer, H.; Mayringer, I.; Dilitz, E.; Deisenhammer, F.; Reindl, M. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N. Engl. J. Med. 2003, 349, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Kuhle, J.; Pohl, C.; Mehling, M.; Edan, G.; Freedman, M.S.; Hartung, H.P.; Polman, C.H.; Miller, D.H.; Montalban, X.; Barkhof, F.; et al. Lack of association between antimyelin antibodies and progression to multiple sclerosis. N. Engl. J. Med. 2007, 356, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Reindl, M.; Di Pauli, F.; Rostasy, K.; Berger, T. The spectrum of MOG autoantibody-associated demyelinating diseases. Nat. Rev. Neurol. 2013, 9, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Waters, P.; Woodhall, M.; O’Connor, K.C.; Reindl, M.; Lang, B.; Sato, D.K.; Jurynczyk, M.; Tackley, G.; Rocha, J.; Takahashi, T.; et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e89. [Google Scholar] [CrossRef] [PubMed]
- Brilot, F.; Dale, R.C.; Selter, R.C.; Grummel, V.; Kalluri, S.R.; Aslam, M.; Busch, V.; Zhou, D.; Cepok, S.; Hemmer, B. Antibodies to native myelin oligodendrocyte glycoprotein in children with inflammatory demyelinating central nervous system disease. Ann. Neurol. 2009, 66, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Di Pauli, F.; Mader, S.; Rostasy, K.; Schanda, K.; Bajer-Kornek, B.; Ehling, R.; Deisenhammer, F.; Reindl, M.; Berger, T. Temporal dynamics of anti-MOG antibodies in CNS demyelinating diseases. Clin. Immunol. 2011, 138, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Jarius, S.; Ruprecht, K.; Kleiter, I.; Borisow, N.; Asgari, N.; Pitarokoili, K.; Pache, F.; Stich, O.; Beume, L.A.; Hummert, M.W.; et al. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients. Part 1: Frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J. Neuroinflamm. 2016, 13, 279. [Google Scholar] [CrossRef] [PubMed]
- Kortvelyessy, P.; Breu, M.; Pawlitzki, M.; Metz, I.; Heinze, H.J.; Matzke, M.; Mawrin, C.; Rommer, P.; Kovacs, G.G.; Mitter, C.; et al. ADEM-like presentation, anti-MOG antibodies, and MS pathology: TWO case reports. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e335. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Motomura, M.; Tanaka, K.; Fujikawa, A.; Nakata, R.; Maeda, Y.; Shima, T.; Mukaino, A.; Yoshimura, S.; Miyazaki, T.; et al. Antibodies to myelin oligodendrocyte glycoprotein in idiopathic optic neuritis. BMJ Open 2015, 5, e007766. [Google Scholar] [CrossRef] [PubMed]
- De Seze, J. Inflammatory Optic Neuritis: From Multiple Sclerosis to Neuromyelitis Optica. Neuroophthalmology 2013, 37, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, L.; Woodhall, M.; Tackley, G.; Jurynczyk, M.; Kong, Y.; Domingos, J.; Gore, R.; Vincent, A.; Waters, P.; Leite, M.I.; et al. Isolated new onset ‘atypical’ optic neuritis in the NMO clinic: Serum antibodies, prognoses and diagnoses at follow-up. J. Neurol. 2016, 263, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Benoilid, A.; Tilikete, C.; Collongues, N.; Arndt, C.; Vighetto, A.; Vignal, C.; de Seze, J. Relapsing optic neuritis: A multicentre study of 62 patients. Mult. Scler. 2014, 20, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Pirko, I.; Blauwet, L.A.; Lesnick, T.G.; Weinshenker, B.G. The natural history of recurrent optic neuritis. Arch. Neurol. 2004, 61, 1401–1405. [Google Scholar] [CrossRef] [PubMed]
- Kidd, D.; Burton, B.; Plant, G.T.; Graham, E.M. Chronic relapsing inflammatory optic neuropathy (CRION). Brain 2003, 126, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A.; Plant, G.T. Chronic relapsing inflammatory optic neuropathy: A systematic review of 122 cases reported. J. Neurol. 2014, 261, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Thorne, J.E.; Wittenberg, S.; Kedhar, S.R.; Dunn, J.P.; Jabs, D.A. Optic neuropathy complicating multifocal choroiditis and panuveitis. Am. J. Ophthalmol. 2007, 143, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Chalmoukou, K.; Alexopoulos, H.; Akrivou, S.; Stathopoulos, P.; Reindl, M.; Dalakas, M.C. Anti-MOG antibodies are frequently associated with steroid-sensitive recurrent optic neuritis. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e131. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, R.; Kezuka, T.; Umazume, A.; Okunuki, Y.; Goto, H.; Tanaka, K. Clinical Profile of Anti-Myelin Oligodendrocyte Glycoprotein Antibody Seropositive Cases of Optic Neuritis. Neuroophthalmology 2015, 39, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Confavreux, C.; Vukusic, S.; Moreau, T.; Adeleine, P. Relapses and progression of disability in multiple sclerosis. N. Engl. J. Med. 2000, 343, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Brass, S.D.; Zivadinov, R.; Bakshi, R. Acute demyelinating optic neuritis: A review. Front. Biosci. 2008, 13, 2376–2390. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Siva, A.; Cross, S.A.; O’Brien, P.C.; Kurland, L.T. Optic neuritis: A population-based study in Olmsted County, Minnesota. Neurology 1995, 45, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Galetta, S.L.; Villoslada, P.; Levin, N.; Shindler, K.; Ishikawa, H.; Parr, E.; Cadavid, D.; Balcer, L.J. Acute optic neuritis: Unmet clinical needs and model for new therapies. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e135. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A.; Wattjes, M.P.; Costello, F.; Flores-Rivera, J.; Fraser, C.L.; Fujihara, K.; Leavitt, J.; Marignier, R.; Paul, F.; Schippling, S.; et al. The investigation of acute optic neuritis: A review and proposed protocol. Nat. Rev. Neurol. 2014, 10, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Swartz, N.G.; Beck, R.W.; Savino, P.J.; Sergott, R.C.; Bosley, T.M.; Lam, B.L.; Drucker, M.; Katz, B. Pain in anterior ischemic optic neuropathy. J. Neuroophthalmol. 1995, 15, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.W.; Cleary, P.A.; Anderson, M.M., Jr.; Keltner, J.L.; Shults, W.T.; Kaufman, D.I.; Buckley, E.G.; Corbett, J.J.; Kupersmith, M.J.; Miller, N.R. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N. Engl. J. Med. 1992, 326, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Viana, M.; Sprenger, T.; Andelova, M.; Goadsby, P.J. The typical duration of migraine aura: A systematic review. Cephalalgia 2013, 33, 483–490. [Google Scholar] [CrossRef] [PubMed]
- The clinical profile of optic neuritis. Experience of the Optic Neuritis Treatment Trial. Optic Neuritis Study Group. Arch. Ophthalmol. 1991, 109, 1673–1678. [Google Scholar]
- Beck, R.W. The optic neuritis treatment trial. Implications for clinical practice. Optic Neuritis Study Group. Arch. Ophthalmol. 1992, 110, 331–332. [Google Scholar] [CrossRef] [PubMed]
- Abràm, M.D.; Baker, M.S. New Concepts in Orbital Imaging; Karcioglu, Z., Ed.; Orbital Tumors; Springer: New York, NY, USA, 2014; pp. 111–121. [Google Scholar]
- Akaishi, T.; Nakashima, I.; Takeshita, T.; Mugikura, S.; Sato, D.K.; Takahashi, T.; Nishiyama, S.; Kurosawa, K.; Misu, T.; Nakazawa, T.; et al. Lesion length of optic neuritis impacts visual prognosis in neuromyelitis optica. J. Neuroimmunol. 2016, 293, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Matiello, M.; Lennon, V.A.; Jacob, A.; Pittock, S.J.; Lucchinetti, C.F.; Wingerchuk, D.M.; Weinshenker, B.G. NMO-IgG predicts the outcome of recurrent optic neuritis. Neurology 2008, 70, 2197–2200. [Google Scholar] [CrossRef] [PubMed]
- Buch, D.; Savatovsky, J.; Gout, O.; Vignal, C.; Deschamps, R. Combined brain and anterior visual pathways’ MRIs assist in early identification of neuromyelitis optica spectrum disorder at onset of optic neuritis. Acta Neurol. Belg. 2017, 117, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Storoni, M.; Davagnanam, I.; Radon, M.; Siddiqui, A.; Plant, G.T. Distinguishing optic neuritis in neuromyelitis optica spectrum disease from multiple sclerosis: A novel magnetic resonance imaging scoring system. J. Neuroophthalmol. 2013, 33, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Prelog, K.; Barnes, E.H.; Tantsis, E.M.; Reddel, S.W.; Henderson, A.P.; Vucic, S.; Gorman, M.P.; Benson, L.A.; Alper, G.; et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult. Scler. 2016, 22, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Stiebel-Kalish, H.; Lotan, I.; Brody, J.; Chodick, G.; Bialer, O.; Marignier, R.; Bach, M.; Hellmann, M.A. Retinal Nerve Fiber Layer May Be Better Preserved in MOG-IgG versus AQP4-IgG Optic Neuritis: A Cohort Study. PLoS ONE 2017, 12, e0170847. [Google Scholar] [CrossRef] [PubMed]
- Havla, J.; Kumpfel, T.; Schinner, R.; Spadaro, M.; Schuh, E.; Meinl, E.; Hohlfeld, R.; Outteryck, O. Myelin-oligodendrocyte-glycoprotein (MOG) autoantibodies as potential markers of severe optic neuritis and subclinical retinal axonal degeneration. J. Neurol. 2017, 264, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Pache, F.; Zimmermann, H.; Mikolajczak, J.; Schumacher, S.; Lacheta, A.; Oertel, F.C.; Bellmann-Strobl, J.; Jarius, S.; Wildemann, B.; Reindl, M.; et al. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients. Part 4: Afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients. J. Neuroinflamm. 2016, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.; Zimmermann, H.; Mikolajczak, J.; Oertel, F.C.; Pache, F.; Weinhold, M.; Schinzel, J.; Bellmann-Strobl, J.; Ruprecht, K.; Paul, F.; et al. Severe structural and functional visual system damage leads to profound loss of vision-related quality of life in patients with neuromyelitis optica spectrum disorders. Mult. Scler. Relat. Disord. 2017, 11, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Huang, Y.; Li, H.; Fan, J.; Zhangbao, J.; Yu, H.; Li, Y.; Lu, J.; Zhao, C.; Lu, C.; Wang, M.; Quan, C. MOG-antibody associated demyelinating disease of the CNS: A clinical and pathological study in Chinese Han patients. J. Neuroimmunol. 2017, 305, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Ford, B.; Tampieri, D.; Francis, G. Long-term follow-up of acute partial transverse myelopathy. Neurology 1992, 42, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 2002, 59, 499–505. [Google Scholar]
- Morrissey, S.P.; Miller, D.H.; Kendall, B.E.; Kingsley, D.P.; Kelly, M.A.; Francis, D.A.; MacManus, D.G.; McDonald, W.I. The significance of brain magnetic resonance imaging abnormalities at presentation with clinically isolated syndromes suggestive of multiple sclerosis. A 5-year follow-up study. Brain 1993, 116 Pt 1, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Brex, P.A.; Ciccarelli, O.; O’Riordan, J.I.; Sailer, M.; Thompson, A.J.; Miller, D.H. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N. Engl. J. Med. 2002, 346, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Tintore, M.; Rovira, A.; Hernandez, D.; Rio, J.; Marzo, M.E.; Montalban, X. Optic neuritis, brain stem syndromes and myelitis: Rapid conversion to multiple sclerosis. Med. Clin. 1999, 112, 693–694. [Google Scholar]
- Perumal, J.; Zabad, R.; Caon, C.; MacKenzie, M.; Tselis, A.; Bao, F.; Latif, Z.; Zak, I.; Lisak, R.; Khan, O. Acute transverse myelitis with normal brain MRI: Long-term risk of MS. J. Neurol. 2008, 255, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.F.; Kassab, S.L.; Singh, S. Acute partial transverse myelitis with normal cerebral magnetic resonance imaging: Transition rate to clinically definite multiple sclerosis. Mult. Scler. 2005, 11, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Schmalstieg, W.F.; Keegan, B.M.; Weinshenker, B.G. Solitary sclerosis: Progressive myelopathy from solitary demyelinating lesion. Neurology 2012, 78, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Keegan, B.M.; Kaufmann, T.J.; Weinshenker, B.G.; Kantarci, O.H.; Schmalstieg, W.F.; Paz Soldan, M.M.; Flanagan, E.P. Progressive solitary sclerosis: Gradual motor impairment from a single CNS demyelinating lesion. Neurology 2016, 87, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Ayrignac, X.; Carra-Dalliere, C.; Homeyer, P.; Labauge, P. Solitary sclerosis: Progressive myelopathy from solitary demyelinating lesion. A new entity? Acta Neurol. Belg. 2013, 113, 533–534. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, C.; Cohen, M.; Mondot, L.; Ayrignac, X.; Labauge, P. A Case Report of Solitary Sclerosis: This is Really Multiple Sclerosis. Neurol. Ther. 2017. [Google Scholar] [CrossRef] [PubMed]
- Rathnasabapathi, D.; Elsone, L.; Krishnan, A.; Young, C.; Larner, A.; Jacob, A. Solitary sclerosis: Progressive neurological deficit from a spatially isolated demyelinating lesion: A further report. J. Spinal Cord Med. 2015, 38, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, S.; Logullo, F.; Di Bella, P.; Silvestrini, M.; Provinciali, L. Multiple sclerosis, solitary sclerosis or something else? Mult. Scler. 2014, 20, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Stuve, O. Idiopathic transverse myelitis and neuromyelitis optica: Clinical profiles, pathophysiology and therapeutic choices. Curr. Neuropharmacol. 2011, 9, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, M.; Armangue, T.; Sola-Valls, N.; Arrambide, G.; Meca-Lallana, J.E.; Oreja-Guevara, C.; Mendibe, M.; Alvarez de Arcaya, A.; Aladro, Y.; Casanova, B.; et al. Neuromyelitis optica spectrum disorders: Comparison according to the phenotype and serostatus. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e225. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.F. Nosology of idiopathic transverse myelitis syndromes. Acta Neurol. Scand. 2007, 115, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Tobin, W.O.; Weinshenker, B.G.; Lucchinetti, C.F. Longitudinally extensive transverse myelitis. Curr. Opin. Neurol. 2014, 27, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Trebst, C.; Raab, P.; Voss, E.V.; Rommer, P.; Abu-Mugheisib, M.; Zettl, U.K.; Stangel, M. Longitudinal extensive transverse myelitis--it’s not all neuromyelitis optica. Nat. Rev. Neurol. 2011, 7, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Kister, I.; Johnson, E.; Raz, E.; Babb, J.; Loh, J.; Shepherd, T.M. Specific MRI findings help distinguish acute transverse myelitis of Neuromyelitis Optica from spinal cord infarction. Mult. Scler. Relat. Disord. 2016, 9, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, E.P.; Krecke, K.N.; Marsh, R.W.; Giannini, C.; Keegan, B.M.; Weinshenker, B.G. Specific pattern of gadolinium enhancement in spondylotic myelopathy. Ann. Neurol. 2014, 76, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.W.; Kim, S.H.; Huh, S.Y.; Kim, W.; Yun, J.; Joung, A.; Sato, D.K.; Fujihara, K.; Kim, H.J. Idiopathic aquaporin-4 antibody negative longitudinally extensive transverse myelitis. Mult. Scler. 2015, 21, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Iorio, R.; Damato, V.; Mirabella, M.; Evoli, A.; Marti, A.; Plantone, D.; Frisullo, G.; Batocchi, A.P. Distinctive clinical and neuroimaging characteristics of longitudinally extensive transverse myelitis associated with aquaporin-4 autoantibodies. J. Neurol. 2013, 260, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- Kitley, J.; Leite, M.I.; Kuker, W.; Quaghebeur, G.; George, J.; Waters, P.; Woodhall, M.; Vincent, A.; Palace, J. Longitudinally extensive transverse myelitis with and without aquaporin 4 antibodies. JAMA Neurol. 2013, 70, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Cobo-Calvo, A.; Sepulveda, M.; Bernard-Valnet, R.; Ruiz, A.; Brassat, D.; Martinez-Yelamos, S.; Saiz, A.; Marignier, R. Antibodies to myelin oligodendrocyte glycoprotein in aquaporin 4 antibody seronegative longitudinally extensive transverse myelitis: Clinical and prognostic implications. Mult. Scler. 2016, 22, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Sato, D.K.; Callegaro, D.; Lana-Peixoto, M.A.; Waters, P.J.; de Haidar Jorge, F.M.; Takahashi, T.; Nakashima, I.; Apostolos-Pereira, S.L.; Talim, N.; Simm, R.F.; et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology 2014, 82, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Kitley, J.; Waters, P.; Woodhall, M.; Leite, M.I.; Murchison, A.; George, J.; Kuker, W.; Chandratre, S.; Vincent, A.; Palace, J. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: A comparative study. JAMA Neurol. 2014, 71, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, E.P.; Weinshenker, B.G.; Krecke, K.N.; Lennon, V.A.; Lucchinetti, C.F.; McKeon, A.; Wingerchuk, D.M.; Shuster, E.A.; Jiao, Y.; Horta, E.S.; et al. Short myelitis lesions in aquaporin-4-IgG-positive neuromyelitis optica spectrum disorders. JAMA Neurol. 2015, 72, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Jarius, S.; Ruprecht, K.; Wildemann, B.; Kuempfel, T.; Ringelstein, M.; Geis, C.; Kleiter, I.; Kleinschnitz, C.; Berthele, A.; Brettschneider, J.; et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: A multicentre study of 175 patients. J. Neuroinflamm. 2012, 9, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, S.Y.; Kim, S.H.; Hyun, J.W.; Jeong, I.H.; Park, M.S.; Lee, S.H.; Kim, H.J. Short segment myelitis as a first manifestation of neuromyelitis optica spectrum disorders. Mult. Scler. 2017, 23, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Tan, S.; Zhang, L.; Shan, Y.; Wang, Y.; Lin, Y.; Zhou, F.; Zhang, B.; Chen, X.; Zhou, L.; et al. Linear lesions may assist early diagnosis of neuromyelitis optica and longitudinally extensive transverse myelitis, two subtypes of NMOSD. J. Neurol. Sci. 2016, 360, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Yonezu, T.; Ito, S.; Mori, M.; Ogawa, Y.; Makino, T.; Uzawa, A.; Kuwabara, S. “Bright spotty lesions” on spinal magnetic resonance imaging differentiate neuromyelitis optica from multiple sclerosis. Mult. Scler. 2014, 20, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Pekcevik, Y.; Mitchell, C.H.; Mealy, M.A.; Orman, G.; Lee, I.H.; Newsome, S.D.; Thompson, C.B.; Pardo, C.A.; Calabresi, P.A.; Levy, M.; et al. Differentiating neuromyelitis optica from other causes of longitudinally extensive transverse myelitis on spinal magnetic resonance imaging. Mult. Scler. 2016, 22, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Gass, A.; Filippi, M.; Rodegher, M.E.; Schwartz, A.; Comi, G.; Hennerici, M.G. Characteristics of chronic MS lesions in the cerebrum, brainstem, spinal cord, and optic nerve on T1-weighted MRI. Neurology 1998, 50, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Matthews, L.A.; Palace, J.A. The role of imaging in diagnosing neuromyelitis optica spectrum disorder. Mult. Scler. Relat. Disord. 2014, 3, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Zaffaroni, M.; Baldini, S.M.; Ghezzi, A. Cranial nerve, brainstem and cerebellar syndromes in the differential diagnosis of multiple sclerosis. Neurol. Sci. 2001, 22 (Suppl. 2), S74–S78. [Google Scholar] [CrossRef] [PubMed]
- Renaud, M.; Aupy, J.; Camuset, G.; Collongues, N.; Chanson, J.B.; de Seze, J.; Blanc, F. Chronic Bickerstaff’s encephalitis with cognitive impairment, a reality? BMC Neurol. 2014, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Gass, A.; Filippi, M.; Grossman, R.I. The contribution of MRI in the differential diagnosis of posterior fossa damage. J. Neurol. Sci. 2000, 172 (Suppl. 1), S43–S49. [Google Scholar] [CrossRef]
- Hooge, J.P.; Redekop, W.K. Trigeminal neuralgia in multiple sclerosis. Neurology 1995, 45, 1294–1296. [Google Scholar] [CrossRef] [PubMed]
- Van der Meijs, A.H.; Tan, I.L.; Barkhof, F. Incidence of enhancement of the trigeminal nerve on MRI in patients with multiple sclerosis. Mult. Scler. 2002, 8, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.J.; Young, C.A.; Smith, E.T. Central trigeminal involvement in multiple sclerosis using high-resolution MRI at 3 T. Br. J. Radiol. 2010, 83, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.J.; da Rocha, A.J.; Mendes, M.F.; Maia, A.C., Jr.; Braga, F.T.; Tilbery, C.P. Trigeminal involvement in multiple sclerosis: Magnetic resonance imaging findings with clinical correlation in a series of patients. Mult. Scler. 2005, 11, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, C.; Lunskens, S.; Deryck, O.; Casselman, J.; Vanopdenbosch, L. MRI characteristics of trigeminal nerve involvement in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2013, 2, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.; Kupersmith, M.J.; Turbin, R.; Bose, S.; Roth, R. Isolated sixth nerve palsy: An uncommon presenting sign of multiple sclerosis. J. Neurol. 2000, 247, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, D.M.; Moster, M.L.; Eggenberger, E.R.; Galetta, S.L.; Liu, G.T. Isolated trochlear nerve palsy in patients with multiple sclerosis. Neurology 1999, 53, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Nerrant, E.; Tilikete, C. Ocular Motor Manifestations of Multiple Sclerosis. J. Neuroophthalmol. 2017, 33, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Naegelin, Y.; Weier, K.; Amann, M.; Hirsch, J.; von Felten, S.; Yaldizli, O.; Sprenger, T.; Radue, E.W.; Kappos, L.; et al. MRI characteristics of periaqueductal lesions in multiple sclerosis. Mult. Scler. Relat. Disord. 2014, 3, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, T.; Moriwaka, F.; Hamada, K.; Hamada, T.; Tashiro, K. Facial palsy in multiple sclerosis. J. Neurol. 1997, 244, 631–633. [Google Scholar] [CrossRef] [PubMed]
- Saleh, C.; Patsi, O.; Mataigne, F.; Beyenburg, S. Peripheral (Seventh) Nerve Palsy and Multiple Sclerosis: A Diagnostic Dilemma—A Case Report. Case Rep. Neurol. 2016, 8, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Kutzelnigg, A.; Faber-Rod, J.C.; Bauer, J.; Lucchinetti, C.F.; Sorensen, P.S.; Laursen, H.; Stadelmann, C.; Bruck, W.; Rauschka, H.; Schmidbauer, M.; et al. Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol. 2007, 17, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Tornes, L.; Conway, B.; Sheremata, W. Multiple sclerosis and the cerebellum. Neurol. Clin. 2014, 32, 957–977. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Miyazawa, I.; Misu, T.; Takano, R.; Nakashima, I.; Fujihara, K.; Tobita, M.; Itoyama, Y. Intractable hiccup and nausea in neuromyelitis optica with anti-aquaporin-4 antibody: A herald of acute exacerbations. J. Neurol. Neurosurg. Psychiatry 2008, 79, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Apiwattanakul, M.; Popescu, B.F.; Matiello, M.; Weinshenker, B.G.; Lucchinetti, C.F.; Lennon, V.A.; McKeon, A.; Carpenter, A.F.; Miller, G.M.; Pittock, S.J. Intractable vomiting as the initial presentation of neuromyelitis optica. Ann. Neurol. 2010, 68, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Jiang, Y.; Lu, X.; Gu, F.; Kang, Z.; Dai, Y.; Lu, Z.; Hu, X. The role of anti-aquaporin 4 antibody in the conversion of acute brainstem syndrome to neuromyelitis optica. BMC Neurol. 2016, 16, 203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Zhang, B.; Dai, Y.; Kang, Z.; Lu, C.; Qiu, W.; Hu, X.; Lu, Z. Comparative clinical characteristics of neuromyelitis optica spectrum disorders with and without medulla oblongata lesions. J. Neurol. 2014, 261, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, K.; Matsushita, T.; Furuta, K.; Isobe, N.; Yonekawa, T.; Ohyagi, Y.; Kira, J. Wall-eyed bilateral internuclear ophthalmoplegia (WEBINO) syndrome in a patient with neuromyelitis optica spectrum disorder and anti-aquaporin-4 antibody. Mult. Scler. 2011, 17, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeong, S.H.; Park, S.M.; Sohn, E.H.; Lee, A.Y.; Kim, J.M.; Jo, H.J.; Lee, Y.H.; Kim, J.S. Anti-aquaporin-4 antibody-positive dorsal midbrain syndrome. Mult. Scler. 2015, 21, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Hage, R., Jr.; Merle, H.; Jeannin, S.; Cabre, P. Ocular oscillations in the neuromyelitis optica spectrum. J. Neuroophthalmol. 2011, 31, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, M.; Sakai, T.; Kono, Y.; Shikishima, K.; Tsuneoka, H. A limited form of neuromyelitis optica with a lesion of the fourth nerve nucleus. J. Neuroophthalmol. 2013, 33, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Rizek, P.; Nicolle, D.; Yeow Tay, K.; Kremenchutzky, M. Bilateral trochlear nerve palsy in a patient with neuromyelitis optica. Mult. Scler. Relat. Disord. 2014, 3, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Uludag, I.F.; Sariteke, A.; Ocek, L.; Zorlu, Y.; Sener, U.; Tokucoglu, F.; Uludag, B. Neuromyelitis optica presenting with horner syndrome: A case report and review of literature. Mult. Scler. Relat. Disord. 2017, 14, 32–34. [Google Scholar] [CrossRef] [PubMed]
- De Seze, J.; Vukusic, S.; Viallet-Marcel, M.; Tilikete, C.; Zephir, H.; Delalande, S.; Stojkovic, T.; Defoort-Dhellemmes, S.; Confavreux, C.; Vermersch, P. Unusual ocular motor findings in multiple sclerosis. J. Neurol. Sci. 2006, 243, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Giles, C.L.; Henderson, J.W. Horner’s syndrome: An analysis of 216 cases. Am. J. Ophthalmol. 1958, 46, 289–296. [Google Scholar] [CrossRef]
- Walton, K.A.; Buono, L.M. Horner syndrome. Curr. Opin. Ophthalmol. 2003, 14, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Lana-Peixoto, M.A.; Callegaro, D.; Talim, N.; Talim, L.E.; Pereira, S.A.; Campos, G.B.; Brazilian Committee for Treatment and Research in Multiple Sclerosis. Pathologic yawning in neuromyelitis optica spectrum disorders. Mult. Scler. Relat. Disord. 2014, 3, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Shan, F.; Zhong, R.; Wu, L.; Fan, Y.; Long, Y.; Gao, C. Neuromyelitis optica spectrum disorders may be misdiagnosed as Wernicke’s encephalopathy. Int. J. Neurosci. 2016, 126, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Jarius, S.; Kleffner, I.; Dorr, J.M.; Sastre-Garriga, J.; Illes, Z.; Eggenberger, E.; Chalk, C.; Ringelstein, M.; Aktas, O.; Montalban, X.; et al. Clinical, paraclinical and serological findings in Susac syndrome: An international multicenter study. J. Neuroinflamm. 2014, 11, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleffner, I.; Dorr, J.; Ringelstein, M.; Gross, C.C.; Bockenfeld, Y.; Schwindt, W.; Sundermann, B.; Lohmann, H.; Wersching, H.; Promesberger, J.; et al. Diagnostic criteria for Susac syndrome. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Given, C.A., 2nd; Stevens, B.S.; Lee, C. The MRI appearance of tumefactive demyelinating lesions. AJR Am. J. Roentgenol. 2004, 182, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Cha, S. Update on brain tumor imaging: From anatomy to physiology. AJNR Am. J. Neuroradiol. 2006, 27, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Jia, G.E.; Wang, X.; Zhang, M.; Ma, Z. Cerebral tumefactive demyelinating lesions. Oncol. Lett. 2015, 10, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Kister, I.; Herbert, J.; Zhou, Y.; Ge, Y. Ultrahigh-Field MR (7 T) Imaging of Brain Lesions in Neuromyelitis Optica. Mult. Scler. Int. 2013, 2013, 398259. [Google Scholar] [CrossRef] [PubMed]
- Sinnecker, T.; Dorr, J.; Pfueller, C.F.; Harms, L.; Ruprecht, K.; Jarius, S.; Bruck, W.; Niendorf, T.; Wuerfel, J.; Paul, F. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology 2012, 79, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Mabray, M.C.; Cohen, B.A.; Villanueva-Meyer, J.E.; Valles, F.E.; Barajas, R.F.; Rubenstein, J.L.; Cha, S. Performance of Apparent Diffusion Coefficient Values and Conventional MRI Features in Differentiating Tumefactive Demyelinating Lesions From Primary Brain Neoplasms. AJR Am. J. Roentgenol. 2015, 205, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, S.; Hara, M.; Morita, A.; Teramoto, H.; Momose, M.; Takahashi, T.; Kamei, S. Tumefactive Demyelinating Lesion Differentiated from a Brain Tumor Using a Combination of Magnetic Resonance Imaging and (11)C-methionine Positron Emission Tomography. Intern. Med. 2015, 54, 1411–1414. [Google Scholar] [CrossRef] [PubMed]
- Kiriyama, T.; Kataoka, H.; Taoka, T.; Tonomura, Y.; Terashima, M.; Morikawa, M.; Tanizawa, E.; Kawahara, M.; Furiya, Y.; Sugie, K.; et al. Characteristic neuroimaging in patients with tumefactive demyelinating lesions exceeding 30 mm. J. Neuroimaging 2011, 21, e69–e77. [Google Scholar] [CrossRef] [PubMed]
- Parks, N.E.; Bhan, V.; Shankar, J.J. Perfusion Imaging of Tumefactive Demyelinating Lesions Compared to High Grade Gliomas. Can. J. Neurol. Sci. 2016, 43, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Kebir, S.; Gaertner, F.C.; Mueller, M.; Nelles, M.; Simon, M.; Schafer, N.; Stuplich, M.; Schaub, C.; Niessen, M.; Mack, F.; et al. 18F-fluoroethyl-L-tyrosine positron emission tomography for the differential diagnosis of tumefactive multiple sclerosis versus glioma: A case report. Oncol. Lett. 2016, 11, 2195–2198. [Google Scholar] [CrossRef] [PubMed]
- Kalus, S.; Di Muzio, B.; Gaillard, F. Demyelination preceding a diagnosis of central nervous system lymphoma. J. Clin. Neurosci. 2016, 24, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Jeong, I.H.; Kim, S.H.; Hyun, J.W.; Joung, A.; Cho, H.J.; Kim, H.J. Tumefactive demyelinating lesions as a first clinical event: Clinical, imaging, and follow-up observations. J. Neurol. Sci. 2015, 358, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Kim, B.J.; Lee, K.H. Development of extensive brain lesions following fingolimod (FTY720) treatment in a patient with neuromyelitis optica spectrum disorder. Mult. Scler. 2012, 18, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Faissner, S.; Hoepner, R.; Lukas, C.; Chan, A.; Gold, R.; Ellrichmann, G. Tumefactive multiple sclerosis lesions in two patients after cessation of fingolimod treatment. Ther. Adv. Neurol. Disord. 2015, 8, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Totaro, R.; di Carmine, C.; Carolei, A. Tumefactive demyelinating lesion in a patient with relapsing remitting multiple sclerosis treated with fingolimod. J. Neurol. Neurophysiol. 2014, S12, 1–2. [Google Scholar]
- Hellmann, M.A.; Lev, N.; Lotan, I.; Mosberg-Galili, R.; Inbar, E.; Luckman, J.; Fichman-Horn, S.; Yakimov, M.; Steiner, I. Tumefactive demyelination and a malignant course in an MS patient during and following fingolimod therapy. J. Neurol. Sci. 2014, 344, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Naismith, R.T. Natalizumab to fingolimod washout in patients at risk of PML: When good intentions yield bad outcomes. Neurology 2014, 82, 1196–1197. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, F.; Seppi, D.; Calabrese, M.; Perini, P.; Gallo, P. Switching therapy from natalizumab to fingolimod in relapsing-remitting multiple sclerosis: Clinical and magnetic resonance imaging findings. Mult. Scler. 2012, 18, 1640–1643. [Google Scholar] [CrossRef] [PubMed]
- Jander, S.; Turowski, B.; Kieseier, B.C.; Hartung, H.P. Emerging tumefactive multiple sclerosis after switching therapy from natalizumab to fingolimod. Mult. Scler. 2012, 18, 1650–1652. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, H.; Shirah, B.; Alassiri, A. Tumefactive demyelinating lesions: A comprehensive review. Mult. Scler. Relat. Disord. 2017, 14, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Sinnecker, T.; Kuchling, J.; Dusek, P.; Dorr, J.; Niendorf, T.; Paul, F.; Wuerfel, J. Ultrahigh field MRI in clinical neuroimmunology: A potential contribution to improved diagnostics and personalised disease management. EPMA J. 2015, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Jiang, Y.; Chen, X.; Dai, Y.; Kang, Z.; Lu, Z.; Peng, F.; Hu, X. Clinical, radiographic characteristics and immunomodulating changes in neuromyelitis optica with extensive brain lesions. BMC Neurol. 2013, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Saiki, S.; Ueno, Y.; Moritani, T.; Sato, T.; Sekine, T.; Kawajiri, S.; Adachi, S.; Yokoyama, K.; Tomizawa, Y.; Motoi, Y.; et al. Extensive hemispheric lesions with radiological evidence of blood-brain barrier integrity in a patient with neuromyelitis optica. J. Neurol. Sci. 2009, 284, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Roy, U.; Saini, D.S.; Pan, K.; Pandit, A.; Ganguly, G.; Panwar, A. Neuromyelitis Optica Spectrum Disorder with Tumefactive Demyelination mimicking Multiple Sclerosis: A Rare Case. Front. Neurol. 2016, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Isobe, N.; Matsuoka, T.; Ishizu, T.; Kawano, Y.; Yoshiura, T.; Ohyagi, Y.; Kira, J. Extensive vasogenic edema of anti-aquaporin-4 antibody-related brain lesions. Mult. Scler. 2009, 15, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Magana, S.M.; Matiello, M.; Pittock, S.J.; McKeon, A.; Lennon, V.A.; Rabinstein, A.A.; Shuster, E.; Kantarci, O.H.; Lucchinetti, C.F.; Weinshenker, B.G. Posterior reversible encephalopathy syndrome in neuromyelitis optica spectrum disorders. Neurology 2009, 72, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, C.; Ludwin, S.; Tabira, T.; Guseo, A.; Lucchinetti, C.F.; Leel-Ossy, L.; Ordinario, A.T.; Bruck, W.; Lassmann, H. Tissue preconditioning may explain concentric lesions in Balo’s type of multiple sclerosis. Brain 2005, 128, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Hardy, T.A.; Corboy, J.R.; Weinshenker, B.G. Balo concentric sclerosis evolving from apparent tumefactive demyelination. Neurology 2017, 88, 2150–2152. [Google Scholar] [CrossRef] [PubMed]
- Hardy, T.A.; Tobin, W.O.; Lucchinetti, C.F. Exploring the overlap between multiple sclerosis, tumefactive demyelination and Balo’s concentric sclerosis. Mult. Scler. 2016, 22, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Graber, J.J.; Kister, I.; Geyer, H.; Khaund, M.; Herbert, J. Neuromyelitis optica and concentric rings of Balo in the brainstem. Arch. Neurol. 2009, 66, 274–275. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Mori, M.; Katayama, K.; Kikkawa, Y.; Kuwabara, S. Anti-aquaporin-4 antibody-seronegative NMO spectrum disorder with Balo’s concentric lesions. Intern. Med. 2013, 52, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.W.; Woodhall, M.R.; Kim, S.H.; Jeong, I.H.; Kong, B.; Kim, G.; Kim, Y.; Park, M.S.; Irani, S.R.; Waters, P.; et al. Longitudinal analysis of myelin oligodendrocyte glycoprotein antibodies in CNS inflammatory diseases. J. Neurol. Neurosurg. Psychiatry 2017, 88, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Rostasy, K.; Mader, S.; Schanda, K.; Huppke, P.; Gartner, J.; Kraus, V.; Karenfort, M.; Tibussek, D.; Blaschek, A.; Bajer-Kornek, B.; et al. Anti-myelin oligodendrocyte glycoprotein antibodies in pediatric patients with optic neuritis. Arch. Neurol. 2012, 69, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kim, S.J.; Lee, H.J.; Kuroda, H.; Palace, J.; Fujihara, K. Differential diagnosis of neuromyelitis optica spectrum disorders. Ther. Adv. Neurol. Disord. 2017, 10, 265–289. [Google Scholar] [CrossRef] [PubMed]
- Probstel, A.K.; Rudolf, G.; Dornmair, K.; Collongues, N.; Chanson, J.B.; Sanderson, N.S.; Lindberg, R.L.; Kappos, L.; de Seze, J.; Derfuss, T. Anti-MOG antibodies are present in a subgroup of patients with a neuromyelitis optica phenotype. J. Neuroinflamm. 2015, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Waters, P.; Woodhall, M.; Vincent, A. Recurrent Optic Neuritis Associated With MOG Antibody Seropositivity. Neurologist 2017, 22, 101–102. [Google Scholar] [CrossRef] [PubMed]
- Reindl, M.; Linington, C.; Brehm, U.; Egg, R.; Dilitz, E.; Deisenhammer, F.; Poewe, W.; Berger, T. Antibodies against the myelin oligodendrocyte glycoprotein and the myelin basic protein in multiple sclerosis and other neurological diseases: A comparative study. Brain 1999, 122 Pt 11, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Huppke, P.; Rostasy, K.; Karenfort, M.; Huppke, B.; Seidl, R.; Leiz, S.; Reindl, M.; Gartner, J. Acute disseminated encephalomyelitis followed by recurrent or monophasic optic neuritis in pediatric patients. Mult. Scler. 2013, 19, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, A.; Monden, Y.; Watanabe, M.; Sugie, H.; Morita, M.; Kezuka, T.; Momoi, M.; Yamagata, T. Persistent presence of the anti-myelin oligodendrocyte glycoprotein autoantibody in a pediatric case of acute disseminated encephalomyelitis followed by optic neuritis. Neuropediatrics 2014, 45, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Ching, B.H.; Mohamed, A.R.; Khoo, T.B.; Ismail, H.I. Multiphasic disseminated encephalomyelitis followed by optic neuritis in a child with gluten sensitivity. Mult. Scler. 2015, 21, 1209–1211. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Hennes, E.M.; Schanda, K.; Karenfort, M.; Kornek, B.; Seidl, R.; Diepold, K.; Lauffer, H.; Marquardt, I.; Strautmanis, J.; et al. Children with multiphasic disseminated encephalomyelitis and antibodies to the myelin oligodendrocyte glycoprotein (MOG): Extending the spectrum of MOG antibody positive diseases. Mult. Scler. 2016, 22, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R.; Nakashima, I.; Takahashi, T.; Kaneko, K.; Akaishi, T.; Takai, Y.; Sato, D.K.; Nishiyama, S.; Misu, T.; Kuroda, H.; et al. MOG antibody-positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e322. [Google Scholar] [CrossRef] [PubMed]
- Kruer, M.C.; Koch, T.K.; Bourdette, D.N.; Chabas, D.; Waubant, E.; Mueller, S.; Moscarello, M.A.; Dalmau, J.; Woltjer, R.L.; Adamus, G. NMDA receptor encephalitis mimicking seronegative neuromyelitis optica. Neurology 2010, 74, 1473–1475. [Google Scholar] [CrossRef] [PubMed]
- Hacohen, Y.; Absoud, M.; Woodhall, M.; Cummins, C.; De Goede, C.G.; Hemingway, C.; Jardine, P.E.; Kneen, R.; Pike, M.G.; Whitehouse, W.P.; et al. Autoantibody biomarkers in childhood-acquired demyelinating syndromes: Results from a national surveillance cohort. J. Neurol. Neurosurg. Psychiatry 2014, 85, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Hacohen, Y.; Absoud, M.; Hemingway, C.; Jacobson, L.; Lin, J.P.; Pike, M.; Pullaperuma, S.; Siddiqui, A.; Wassmer, E.; Waters, P.; et al. NMDA receptor antibodies associated with distinct white matter syndromes. Neurol. Neuroimmunol. Neuroinflamm. 2014, 1, e2. [Google Scholar] [CrossRef] [PubMed]
- Splendiani, A.; Felli, V.; Di Sibio, A.; Gennarelli, A.; Patriarca, L.; Stratta, P.; Di Cesare, E.; Rossi, A.; Massimo, G. Magnetic resonance imaging and magnetic resonance spectroscopy in a young male patient with anti-N-methyl-D-aspartate receptor encephalitis and uncommon cerebellar involvement: A case report with review of the literature. Neuroradiol. J. 2016, 29, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Titulaer, M.J.; Hoftberger, R.; Iizuka, T.; Leypoldt, F.; McCracken, L.; Cellucci, T.; Benson, L.A.; Shu, H.; Irioka, T.; Hirano, M.; et al. Overlapping demyelinating syndromes and anti-N-methyl-D-aspartate receptor encephalitis. Ann. Neurol. 2014, 75, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Lekoubou, A.; Viaccoz, A.; Didelot, A.; Anastasi, A.; Marignier, R.; Ducray, F.; Rogemond, V.; Honnorat, J. Anti-N-methyl-D-aspartate receptor encephalitis with acute disseminated encephalomyelitis-like MRI features. Eur. J. Neurol. 2012, 19, e16–e17. [Google Scholar] [CrossRef] [PubMed]
- Zoccarato, M.; Saddi, M.V.; Serra, G.; Pelizza, M.F.; Rosellini, I.; Peddone, L.; Ticca, A.; Giometto, B.; Zuliani, L. Aquaporin-4 antibody neuromyelitis optica following anti-NMDA receptor encephalitis. J. Neurol. 2013, 260, 3185–3187. [Google Scholar] [CrossRef] [PubMed]
- Outteryck, O.; Baille, G.; Hodel, J.; Giroux, M.; Lacour, A.; Honnorat, J.; Zephir, H.; Vermersch, P. Extensive myelitis associated with anti-NMDA receptor antibodies. BMC Neurol. 2013, 13, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kezuka, T.; Usui, Y.; Yamakawa, N.; Matsunaga, Y.; Matsuda, R.; Masuda, M.; Utsumi, H.; Tanaka, K.; Goto, H. Relationship between NMO-antibody and anti-MOG antibody in optic neuritis. J. Neuroophthalmol. 2012, 32, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Kezuka, T.; Tanaka, K.; Matsunaga, Y.; Goto, H. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology 2014, 83, 475. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Hernandez, E.; Sepulveda, M.; Rostasy, K.; Hoftberger, R.; Graus, F.; Harvey, R.J.; Saiz, A.; Dalmau, J. Antibodies to aquaporin 4, myelin-oligodendrocyte glycoprotein, and the glycine receptor alpha1 subunit in patients with isolated optic neuritis. JAMA Neurol. 2015, 72, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Popescu, B.F.; Bunyan, R.F.; Guo, Y.; Parisi, J.E.; Lennon, V.A.; Lucchinetti, C.F. Evidence of aquaporin involvement in human central pontine myelinolysis. Acta Neuropathol. Commun. 2013, 1, 40. [Google Scholar] [CrossRef] [PubMed]
- Tzartos, J.S.; Stergiou, C.; Kilidireas, K.; Zisimopoulou, P.; Thomaidis, T.; Tzartos, S.J. Anti-aquaporin-1 autoantibodies in patients with neuromyelitis optica spectrum disorders. PLoS ONE 2013, 8, e74773. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Gomar, I.; Diaz Sanchez, M.; Ucles Sanchez, A.J.; Casado Chocan, J.L.; Suarez-Luna, N.; Ramirez-Lorca, R.; Villadiego, J.; Toledo-Aral, J.J.; Echevarria, M. Comparative Analysis for the Presence of IgG Anti-Aquaporin-1 in Patients with NMO-Spectrum Disorders. Int. J. Mol. Sci. 2016, 17, 1195. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Zheng, Y.; Shan, F.; Chen, M.; Fan, Y.; Zhang, B.; Gao, C.; Gao, Q.; Yang, N. Development of a cell-based assay for the detection of anti-aquaporin 1 antibodies in neuromyelitis optica spectrum disorders. J. Neuroimmunol. 2014, 273, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Schanda, K.; Waters, P.; Holzer, H.; Aboulenein-Djamshidian, F.; Leite, M.I.; Palace, J.; Vukusic, S.; Marignier, R.; Berger, T.; Reindl, M. Antibodies to aquaporin-1 are not present in neuromyelitis optica. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e160. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Yamasaki, R.; Kawano, Y.; Imamura, Y.; Kira, J. Anti-KIR4.1 antibodies in Japanese patients with idiopathic central nervous system demyelinating diseases. Clin. Exp. Neuroimmunol. 2013, 4, 241–242. [Google Scholar] [CrossRef]
- Srivastava, R.; Aslam, M.; Kalluri, S.R.; Schirmer, L.; Buck, D.; Tackenberg, B.; Rothhammer, V.; Chan, A.; Gold, R.; Berthele, A.; et al. Potassium channel KIR4.1 as an immune target in multiple sclerosis. N. Engl. J. Med. 2012, 367, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.; Srivastava, R.; Kalluri, S.R.; Seidel, U.; Schuelke, M.; Schimmel, M.; Rostasy, K.; Leiz, S.; Hosie, S.; Grummel, V.; et al. Potassium channel KIR4.1-specific antibodies in children with acquired demyelinating CNS disease. Neurology 2014, 82, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Brickshawana, A.; Hinson, S.R.; Romero, M.F.; Lucchinetti, C.F.; Guo, Y.; Buttmann, M.; McKeon, A.; Pittock, S.J.; Chang, M.H.; Chen, A.P.; et al. Investigation of the KIR4.1 potassium channel as a putative antigen in patients with multiple sclerosis: A comparative study. Lancet Neurol. 2014, 13, 795–806. [Google Scholar] [CrossRef]
- Brill, L.; Goldberg, L.; Karni, A.; Petrou, P.; Abramsky, O.; Ovadia, H.; Ben-Hur, T.; Karussis, D.; Vaknin-Dembinsky, A. Increased anti-KIR4.1 antibodies in multiple sclerosis: Could it be a marker of disease relapse? Mult. Scler. 2015, 21, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, O.; Nakane, S.; Sakai, W.; Maeda, Y.; Niino, M.; Takahashi, T.; Fukazawa, T.; Kikuchi, S.; Fujihara, K.; Matsuo, H. Lack of KIR4.1 autoantibodies in Japanese patients with MS and NMO. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e263. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, B. Antibodies to the inward rectifying potassium channel 4.1 in multiple sclerosis: Different methodologies--conflicting results? Mult. Scler. 2015, 21, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Pittock, S.J.; Lennon, V.A. Aquaporin-4 autoantibodies in a paraneoplastic context. Arch. Neurol. 2008, 65, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Armagan, H.; Tuzun, E.; Icoz, S.; Birisik, O.; Ulusoy, C.; Demir, G.; Altintas, A.; Akman-Demir, G. Long extensive transverse myelitis associated with aquaporin-4 antibody and breast cancer: Favorable response to cancer treatment. J. Spinal Cord Med. 2012, 35, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Moussawi, K.; Lin, D.J.; Matiello, M.; Chew, S.; Morganstern, D.; Vaitkevicius, H. Brainstem and limbic encephalitis with paraneoplastic neuromyelitis optica. J. Clin. Neurosci. 2016, 23, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.; Guo, Y.; Tselis, A.; Pittock, S.J.; Lennon, V.A.; Lucchinetti, C.F.; Lisak, R.P. Paraneoplastic neuromyelitis optica spectrum disorder associated with metastatic carcinoid expressing aquaporin-4. JAMA Neurol. 2014, 71, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Soelberg, K.; Larsen, S.R.; Moerch, M.T.; Thomassen, M.; Brusgaard, K.; Paul, F.; Smith, T.J.; Godballe, C.; Grauslund, J.; Lillevang, S.T.; et al. Aquaporin-4 IgG autoimmune syndrome and immunoreactivity associated with thyroid cancer. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e252. [Google Scholar] [CrossRef] [PubMed]
- Jarius, S.; Wandinger, K.P.; Borowski, K.; Stoecker, W.; Wildemann, B. Antibodies to CV2/CRMP5 in neuromyelitis optica-like disease: Case report and review of the literature. Clin. Neurol. Neurosurg. 2012, 114, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Faissner, S.; Lukas, C.; Reinacher-Schick, A.; Tannapfel, A.; Gold, R.; Kleiter, I. Amphiphysin-positive paraneoplastic myelitis and stiff-person syndrome. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e285. [Google Scholar] [CrossRef] [PubMed]
- Melamed, E.; Levy, M.; Waters, P.J.; Sato, D.K.; Bennett, J.L.; John, G.R.; Hooper, D.C.; Saiz, A.; Bar-Or, A.; Kim, H.J.; et al. Update on biomarkers in neuromyelitis optica. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e134. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Kiyota, N.; Kuroda, H.; Sato, D.K.; Nishiyama, S.; Takahashi, T.; Misu, T.; Nakashima, I.; Fujihara, K.; Aoki, M. Severe demyelination but no astrocytopathy in clinically definite neuromyelitis optica with anti-myelin-oligodendrocyte glycoprotein antibody. Mult. Scler. 2015, 21, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Sato, D.K.; Nakashima, I.; Nishiyama, S.; Tanaka, S.; Marignier, R.; Hyun, J.W.; Oliveira, L.M.; Reindl, M.; Seifert-Held, T.; et al. Myelin injury without astrocytopathy in neuroinflammatory disorders with MOG antibodies. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1257–1259. [Google Scholar] [CrossRef] [PubMed]
- Misu, T.; Takano, R.; Fujihara, K.; Takahashi, T.; Sato, S.; Itoyama, Y. Marked increase in cerebrospinal fluid glial fibrillar acidic protein in neuromyelitis optica: An astrocytic damage marker. J. Neurol. Neurosurg. Psychiatry 2009, 80, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Misu, T.; Fujihara, K.; Kakita, A.; Konno, H.; Nakamura, M.; Watanabe, S.; Takahashi, T.; Nakashima, I.; Takahashi, H.; Itoyama, Y. Loss of aquaporin 4 in lesions of neuromyelitis optica: Distinction from multiple sclerosis. Brain 2007, 130, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Takano, R.; Misu, T.; Takahashi, T.; Sato, S.; Fujihara, K.; Itoyama, Y. Astrocytic damage is far more severe than demyelination in NMO: A clinical CSF biomarker study. Neurology 2010, 75, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Uzawa, A.; Mori, M.; Kuwabara, S. Cytokines and chemokines in neuromyelitis optica: Pathogenetic and therapeutic implications. Brain Pathol. 2014, 24, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Icoz, S.; Tuzun, E.; Kurtuncu, M.; Durmus, H.; Mutlu, M.; Eraksoy, M.; Akman-Demir, G. Enhanced IL-6 production in aquaporin-4 antibody positive neuromyelitis optica patients. Int. J. Neurosci. 2010, 120, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012, 8, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Van Wagoner, N.J.; Oh, J.W.; Repovic, P.; Benveniste, E.N. Interleukin-6 (IL-6) production by astrocytes: Autocrine regulation by IL-6 and the soluble IL-6 receptor. J. Neurosci. 1999, 19, 5236–5244. [Google Scholar] [PubMed]
- Sherman, E.; Han, M.H. Acute and Chronic Management of Neuromyelitis Optica Spectrum Disorder. Curr. Treat. Options Neurol. 2015, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.A.; Mealy, M.A.; Levy, M. Treatment of Neuromyelitis Optica Spectrum Disorder: Acute, Preventive, and Symptomatic. Curr. Treat. Options Neurol. 2016, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Weinshenker, B.G.; O’Brien, P.C.; Petterson, T.M.; Noseworthy, J.H.; Lucchinetti, C.F.; Dodick, D.W.; Pineda, A.A.; Stevens, L.N.; Rodriguez, M. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann. Neurol. 1999, 46, 878–886. [Google Scholar] [CrossRef]
- Kleiter, I.; Gahlen, A.; Borisow, N.; Fischer, K.; Wernecke, K.D.; Wegner, B.; Hellwig, K.; Pache, F.; Ruprecht, K.; Havla, J.; et al. Neuromyelitis optica: Evaluation of 871 attacks and 1,153 treatment courses. Ann. Neurol. 2016, 79, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Perumal, J.S.; Caon, C.; Hreha, S.; Zabad, R.; Tselis, A.; Lisak, R.; Khan, O. Oral prednisone taper following intravenous steroids fails to improve disability or recovery from relapses in multiple sclerosis. Eur. J. Neurol. 2008, 15, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Multiple Sclerosis Therapy Consensus Group (MSTCG); Wiendl, H.; Toyka, K.V.; Rieckmann, P.; Gold, R.; Hartung, H.P.; Hohlfeld, R. Basic and escalating immunomodulatory treatments in multiple sclerosis: Current therapeutic recommendations. J. Neurol. 2008, 255, 1449–1463. [Google Scholar] [PubMed]
- Cortese, I.; Chaudhry, V.; So, Y.T.; Cantor, F.; Cornblath, D.R.; Rae-Grant, A. Evidence-based guideline update: Plasmapheresis in neurologic disorders: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2011, 76, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Gold, C.; Krenzer, M.; Klinker, E.; Mansouri-Thalegani, B.; Mullges, W.; Toyka, K.V.; Gold, R. Immunoadsorption versus plasma exchange versus combination for treatment of myasthenic deterioration. Ther. Adv. Neurol. Disord. 2016, 9, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, I.; Takahashi, T.; Cree, B.A.; Kim, H.J.; Suzuki, C.; Genain, C.P.; Vincent, T.; Fujihara, K.; Itoyama, Y.; Bar-Or, A. Transient increases in anti-aquaporin-4 antibody titers following rituximab treatment in neuromyelitis optica, in association with elevated serum BAFF levels. J. Clin. Neurosci. 2011, 18, 997–998. [Google Scholar] [CrossRef] [PubMed]
- Perumal, J.S.; Kister, I.; Howard, J.; Herbert, J. Disease exacerbation after rituximab induction in neuromyelitis optica. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e61. [Google Scholar] [CrossRef] [PubMed]
- Stellmann, J.P.; Krumbholz, M.; Friede, T.; Gahlen, A.; Borisow, N.; Fischer, K.; Hellwig, K.; Pache, F.; Ruprecht, K.; Havla, J.; et al. Immunotherapies in neuromyelitis optica spectrum disorder: Efficacy and predictors of response. J. Neurol. Neurosurg. Psychiatry 2017, 88, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Kleiter, I.; Hellwig, K.; Berthele, A.; Kumpfel, T.; Linker, R.A.; Hartung, H.P.; Paul, F.; Aktas, O.; Neuromyelitis Optica Study Group. Failure of natalizumab to prevent relapses in neuromyelitis optica. Arch. Neurol. 2012, 69, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, J.M.; Cotter, J.; Klingman, J.; Huang, E.J.; Cree, B.A. Massive CNS monocytic infiltration at autopsy in an alemtuzumab-treated patient with NMO. Neurol. Neuroimmunol. Neuroinflamm. 2014, 1, e34. [Google Scholar] [CrossRef] [PubMed]
- Ayzenberg, I.; Schollhammer, J.; Hoepner, R.; Hellwig, K.; Ringelstein, M.; Aktas, O.; Kumpfel, T.; Krumbholz, M.; Trebst, C.; Paul, F.; et al. Efficacy of glatiramer acetate in neuromyelitis optica spectrum disorder: A multicenter retrospective study. J. Neurol. 2016, 263, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Gahlen, A.; Trampe, A.K.; Haupeltshofer, S.; Ringelstein, M.; Aktas, O.; Berthele, A.; Wildemann, B.; Gold, R.; Jarius, S.; Kleiter, I. Aquaporin-4 antibodies in patients treated with natalizumab for suspected MS. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e363. [Google Scholar] [CrossRef] [PubMed]
- Kira, J.I. Unexpected exacerbations following initiation of disease-modifying drugs in neuromyelitis optica spectrum disorder: Which factor is responsible, anti-aquaporin 4 antibodies, B cells, Th1 cells, Th2 cells, Th17 cells, or others? Mult. Scler. 2017, 23, 1300–1302. [Google Scholar] [CrossRef] [PubMed]
- Yamout, B.I.; Beaini, S.; Zeineddine, M.M.; Akkawi, N. Catastrophic relapses following initiation of dimethyl fumarate in two patients with neuromyelitis optica spectrum disorder. Mult. Scler. 2017, 23, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
- Ringelstein, M.; Ayzenberg, I.; Harmel, J.; Lauenstein, A.S.; Lensch, E.; Stogbauer, F.; Hellwig, K.; Ellrichmann, G.; Stettner, M.; Chan, A.; et al. Long-term Therapy With Interleukin 6 Receptor Blockade in Highly Active Neuromyelitis Optica Spectrum Disorder. JAMA Neurol. 2015, 72, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Chugai Pharmaceutical A Multicenter, Randomized, Double-blind, Placebo-controlled, Phase 3 Study to Evaluate the Efficacy and Safety of SA237 as Monotherapy in Patients With Neuromyelitis Optica (NMO) and NMO Spectrum Disorder (NMOSD). Available online: https://clinicaltrials.gov/ct2/show/NCT02073279?term=SA237&cond=NMO+Spectrum+Disorder&rank=1 (accessed on 16 September 2017).
- Chugai Pharmaceutical A Multicenter, Randomized, Addition to Baseline Treatment, Double-blind, Placebo-controlled, Phase 3 Study to Evaluate the Efficacy and Safety of SA237 in Patients With Neuromyelitis Optica (NMO) and NMO Spectrum Disorder (NMOSD). Available online: https://clinicaltrials.gov/ct2/show/NCT02028884?term=SA237&cond=NMO+Spectrum+Disorder&rank=2 (accessed on 16 September 2017).
- Kang, S.; Tanaka, T.; Kishimoto, T. Therapeutic uses of anti-interleukin-6 receptor antibody. Int. Immunol. 2015, 27, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Pandit, L.; Asgari, N.; Apiwattanakul, M.; Palace, J.; Paul, F.; Leite, M.I.; Kleiter, I.; Chitnis, T.; GJCF International Clinical Consortium & Biorepository for Neuromyelitis Optica. Demographic and clinical features of neuromyelitis optica: A review. Mult. Scler. 2015, 21, 845–853. [Google Scholar] [PubMed]
- Reindl, M.; Rostasy, K. MOG antibody-associated diseases. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e60. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, M.; Gerdes, L.A.; Krumbholz, M.; Ertl-Wagner, B.; Thaler, F.S.; Schuh, E.; Metz, I.; Blaschek, A.; Dick, A.; Bruck, W.; et al. Autoantibodies to MOG in a distinct subgroup of adult multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e257. [Google Scholar] [CrossRef] [PubMed]
| MRI | MS | AQP4-NMOSD | MOG-NMOSD |
|---|---|---|---|
| Optic nerve (Range) [74,76,77] |
|
|
|
| MRI | MS | AQP4-NMOSD | MOG-NMOSD |
|---|---|---|---|
| Spinal Cord (Range) [113,115,117] |
|
|
|
| Spanish Study [76] Sepulveda et al., 2016; n = 56 | German Study [60] Jarius et al., 2016; n = 50 | |
|---|---|---|
| MOG assay | CBA | CBA |
| Gender (Female:Male) | 1.67:1 | 2.8:1 |
| Race | 54 Caucasian; 2 others | 49 Caucasian; 1 Asian |
| Median age disease onset (Range) | 37 years (18–70) | 31 years (6–70) |
| Clinical syndrome at onset (N) |
|
|
| Median time to second attack | No data | 5 month |
| Clinical syndrome at last FU (N) |
|
|
| Relapsing% versus Monophasic % | 71% versus 29% | 80% versus 20% |
| Median follow-up (range), months | 43 (4–554) months | 75 (1–507) months |
| MOG titers, serum (range) MOG titers, CSF | 1/960 (1/160–1/10240) No data | 1/160–1/20480 1/2–1/64 |
CSF
| Mean= 41 (SD = 70) 3/53 | Median = 33 (IQR 13–125) 6/45 |
| Relapse number | 125
|
|
| Annualized relapse rate | 1.11 | 0.83 |
| Outcome | Data on VA in 46 patients
| Data on VA in 38 patients
|
| Concomitant autoimmune antibodies Concomitant autoimmune disorders | No data n = 7 (13%) | n = 19/45 (42.2%) n = 4/47 (8.5%) |
| Relapse management |
|
|
| Maintenance Therapy Medications | 46% No data | Monoclonal B-cell therapies No data |
| Hupke et al., 2012 [188] | Baumann et al., 2016 [191] | |
|---|---|---|
| N of patients | 7 | 8 |
| Median Age (range) years | 6 (4–8) | 3 (1–7) |
| Gender (F/M) | 6/1 | 5/3 |
Clinical presentation
| n = 7 n = 3/7 Unilateral in n = 6; Bilateral in n = 1 Always following ADEM/MDEM N of attacks: 1–7 Inter-attack intervals: 3 weeks-2 years | n = 7 Unilateral/Bilateral: ND; n = 2 |
| Median inter-attack interval | Minimum 4 weeks for ON | 4 months for MDEM |
| Preceding febrile illness; N; (weeks prior) | ND | Yes; n = 4; 4 weeks prior |
| Median follow up (range) years | 6 for n = 4/7 | 4 (1–8) |
| Autoantibody | ADEM stage: MOG-antibody (+) 3/7 and ND 4/7 MOG- antibody (+) with ON AQP4-antibody (-) | MDEM stage: MOG- antibody (+) 8/8 AQP4-antibody (-) |
| CSF | Pleocytosis Negative OCB | Pleocytosis Negative OCB in n = 7/8 |
| MRI Brain MRI spinal cord | Classical ADEM findings New lesions with MDEM No new brain MRI lesions during ON ND | Classical ADEM findings with TDL and cortical GM lesions. New lesions with MDEM LETM n = 2 SSTM n =2 |
| Treatment | Improvement with corticosteroids Azathioprine: Partial effectiveness IFN-beta and GA: Not effective | Corticosteroids during attacks (n = 8) IVIG (n = 1) during attack PLEX (n = 1) during attack IVIG Monthly (n = 4) |
| Outcome | Minimal to no relapses, n = 2 Continuous relapses, n = 2 Mild vision loss, n = 4 | Normal n = 4 Mild-moderate deficit n = 4 (psychomotor and/or seizures) |
| Demographics | Presentation | MRI | Treatment/Response | Serum Ab (Number of Patients) | CSF/an Cillary Testing (Number of Patients) | Final Outcome | |
|---|---|---|---|---|---|---|---|
| Kruer et al., 2010 [193] | n = 1 F, 15 years | Seizures, encephalopathy, movement disorder, myelopathy, unilateral ON | Multifocal CEL; LETM | Prednisone: improvement. Interferon-B therapy, cyclophosphamide: failed therapy Multi-modality therapy *: drastic improvement | AQP4: - | Pleocytosis: + Protein: 2x NL Glucose: low OCB: + NMDAR: + Pan CT scan: - | Asymptomatic |
| Lekoubou et al., 2012 [198] | n = 1 F, 34 years | Encephalopathy, movement disorder, myelopathy | Widespread, multifocal white matter lesions, right frontal contrast enhancing lesion, LETM | IVIG, steroids: failed therapy Immunosuppressant: dramatic improvement | AQP4: - | Pleocytosis: + Protein: elevation Glucose: low OCB: + NMDAR: + Viral panel: - Pan CT scan: - | Minor residual cognitive impairment |
| Zoccarato et al., 2013 [199] | n = 1 F, 50 years | Encephalopathy, movement disorder, myelopathy, unilateral ON | T2 hyperintense lesions in cortical medial temporal lobe, pons, hypothalamus, medulla, cervical & dorsal spine | Hystero-adnexectomy, oral steroids: improvement PLEX, steroids: improvement Immunosuppressant: stable disease | AQP4: + NMDAR: + | Pleocytosis: ND Protein: ND Glucose: ND OCB: + NMDAR: + EEG: - Pan CT scan: ovarian teratoma | Stable disease |
| Outteryck et al., 2013 [200] | n = 1 F, 65 years | Encephalopathy, movement disorder, myelopathy, subclinical unilateral ON | LETM with gadolinium enhancement, T2 hyperintensities in insular regions, medial temporal lobes & thalamus, gadolinium enhancement in meninges & ventricles | IV steroids: paraparesis worsened PLEX, immunosuppressant, oral steroids: significant improvement | AQP4: - NMDAR: + | Pleocytosis: + Protein: elevation Glucose: ND OCB: + NMDAR: + Pan CT scan: - | Death: due to rapidly evolving pneumocystis pneumonia |
| Hacohen et al., 2014 [194] | n = 15 M (6), 3–15 years F (9), 2–15 years | Seizures, encephalopathy, movement disorder, myelopathy, unilateral/bilateral ON | Normal, optic nerve signal changes, multiple white matter abnormalities, spinal cord involvement, periventricular/juxtacortical, brainstem lesions | ND | AQP4: +(3) NMDAR: +(2) MOG: +(7) VGKC: +(3) GlyR: +(1) | Pleocytosis: + (3) Protein: ND Glucose: ND OCB: +(3)/-(7)/ND (5) NMDAR: ND Viral panel: ND MOG: ND EEG: ND Pan CT scan: ND | Full recovery (4), EDSS 1 (6), EDSS 3 (2), EDSS 4 (2), EDSS 7 (1), seizures (2), Visual loss (1) |
| Hacohen et al., 2014 [195] | n = 10 M (5), 2–18 years F (5), 1.33–11 years | Brainstem encephalitis HSV encephalitis Unilateral/bilateral ON ADEM Recurrent hyperventilation, dizziness, double vision | Normal (2), brainstem signal change (2), white matter changes (7), multiple cranial nerve enhancement (1), gray matter changes (1) | No treatment: (1) Multimodality treatment with IV, oral steroids, IVIG and immunosuppressants | AQP4: ND NMDAR: +(10) MOG: ND VGKC: ND GlyR: ND | Pleocytosis: ND Protein: ND Glucose: NDOCB: +(1)/-(2)/ ND (7) NMDAR: +(4)/ ND (6) MOG: + (3)/ND (7) | Full recovery (4) Incomplete (5) Unchanged (1) |
| Titulaer et al., 2014 [197] | n = 12 M (5), 10–38 years F (7), 8–55 years | Seizures, encephalopathy, movement disorder, myelopathy (LETM) unilateral/bilateral ON | ND (2), Normal (1), optic nerve signal changes (1), white matter lesions (4), T2/FLAIR abnormalities in various aspects of the brain (7), spinal cord involvement (4), Gd enhanced lesions (6), brainstem lesions (4) | No treatment: (1) Multimodality treatment with steroids, IVIG, PLEX, interferon, imunosuppressanT | AQP4: +(3), -(5), ND (4) NMDAR: +(2), -(5), ND (5) MOG: +(6), -(3), ND (3) VGKC: ND GlyR: ND | Pleocytosis: +(8)/-(4) Protein and glucose ND OCB: +(5)/-(5)/ND (2) NMDAR: +(12) MOG: +(7)/-(4)/ND (1) | ND |
| Splendiani et al., 2016 [196] | n = 1 M, 17 years | Seizures, encephalopathy, movement disorder, myelopathy | T2 & FLAIR cortical-subcortical hyperintensities in right cerebellar hemisphere, ipsilateral cerebellar tonsil, with faint T1 hypointensity | Olanzapine: progression of symptoms IV steroids: gradual improvement | AQP4: ND NMDAR: + MOG: ND VGKC: ND GlyR: ND | CSF findings: ND NMDAR: + Viral panel: - EEG: - Pan CT scan: - | Asymptomatic |
| Ogawa et al., 2017 [192] | n = 4 patients M, 23–39 years | Seizures, encephalopathy, ON | T2 & FLAIR unilateral cortical hyperintensities; no ADEM-like lesions. | IV steroids with significant improvement 2–3 antiepileptics/patient | AQP4: - MOG: +(3) NMDAR: -(4) AMPA: -(4) LGI1: -(4) CASPR2: -(4) GABAb: -(4) | Pleocytosis + Protein: NL-1.5xNL OCB: ND MBP: NL (3); ND (1) MOG: +(3); ND (1) | Remain on antiepileptics Otherwise full recovery |
| Medication Name | Mechanism of Action (MOA) | Dosage | Treatment Response | Side Effects |
|---|---|---|---|---|
| Azathioprine | Thiopurine antagonist of endogenous purines in DNA and RNA, interferes with lymphocyte proliferation | Initial: 2–3 mg/kg/day with concomitant prednisone (5–60 mg daily) for 6–12 months Maintenance: 2–3 mg/kg/day | Approximately 50/50 chance of preventing additional relapse | Nausea, diarrhea, rash, recurrent infections, leukopenia, transaminase elevation, increased risk of lymphoma |
| Cyclophosphamide | Cytotoxic alkylating agent, inhibits mitosis | Initial: 1000 mg every 2 months with associated steroid Maintenance: same as initial dosing | Specific treatment response unavailable – only recommended when other immunosuppressive therapies fail or are not available due to contradictory preliminary findings. | GI symptoms, hyponatremia, heart block, pancytopenia, opportunistic infections |
| Eculizumab | Binds to the complement protein C5 specifically, inhibiting its cleavage to C5a and C5b and subsequent generation of the terminal complement complex C5b-9 | Standard dose: IV 600 mg weekly for four weeks, then IV 900 mg every two weeks | Specific treatment response unavailable at this time | Headache, increased risk of infection with encapsulated organisms, especially meningococcal infections |
| Methotrexate | Folic acid antagonist | Initial: start with 7.5 mg weekly with upward titration and concomitant prednisone (5–60 mg daily) Maintenance: 7.5–15 mg weekly with concurrent prednisone (5–10 mg daily for at least sixmonths) | Remission rates in up to 2/3 of subjects when used as monotherapy or in conjunction with corticosteroids | Pneumonitis, GI upset, cytopenia, hepatotoxicity |
| Mitoxatrone | Causes DNA cross-linking and strand breaks, interferes with DNA repair | Initial: 12 mg/m² for 3–6 months Maintenance: 6–12 mg/m² every 3 months | Remission in up to 70% of subjects when dosed appropriately | Nausea, transaminase elevation, leukopenia, hair loss, amenorrhea, minor infections including UTI and URI, rarely heart failure and acute leukemia |
| Mycophenolate mofetil | Inhibits inosine monophosphate dehydrogenase, impairs B- and T-cell synthesis | Initial: 1000–2000 mg daily with concurrent prednisone (5–60 mg daily) Maintenance: 1000–2000 mg | Approximately 60–75% achieve remission with fewer side effects and adverse effects | Photosensitivity, recurrent infections, headache, constipation, abdominal pain, leukopenia, PML is rare |
| Rituximab | Removal of B cells as antigen presenting cells and reduction in the CD20+ early plasmablast population generating anti-quaporin-4 antibodies | Initial: 1000 mg weekly for two weeks or 375 mg/m² weekly for four weeks Maintenance: 375 mg/m² or 1000 mg weekly for 2 weeks when CD19 count >1% on flow cytometry | Remission rates up to 83% were achieved with persistent B cell depletion | Sepsis, infections (Herpes zoster, UTIs, URIs), leukopenia, transaminase elevation, PML is rare |
| Tocilizumab | Directed against the IL-6 receptor reducing plasmablast survival, inhibiting AQP4 antibody production | Standard dose: 8 mg/kg every four weeks | Specific treatment response unavailable at this time | GI disturbance, fatigue, UTIs, neutropenia, leukopenia, elevation of cholesterol, transient mild transaminase elevation, DVT, TB reactivation |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabad, R.K.; Stewart, R.; Healey, K.M. Pattern Recognition of the Multiple Sclerosis Syndrome. Brain Sci. 2017, 7, 138. https://doi.org/10.3390/brainsci7100138
Zabad RK, Stewart R, Healey KM. Pattern Recognition of the Multiple Sclerosis Syndrome. Brain Sciences. 2017; 7(10):138. https://doi.org/10.3390/brainsci7100138
Chicago/Turabian StyleZabad, Rana K., Renee Stewart, and Kathleen M. Healey. 2017. "Pattern Recognition of the Multiple Sclerosis Syndrome" Brain Sciences 7, no. 10: 138. https://doi.org/10.3390/brainsci7100138




