Surgical Neurostimulation for Spinal Cord Injury
Abstract
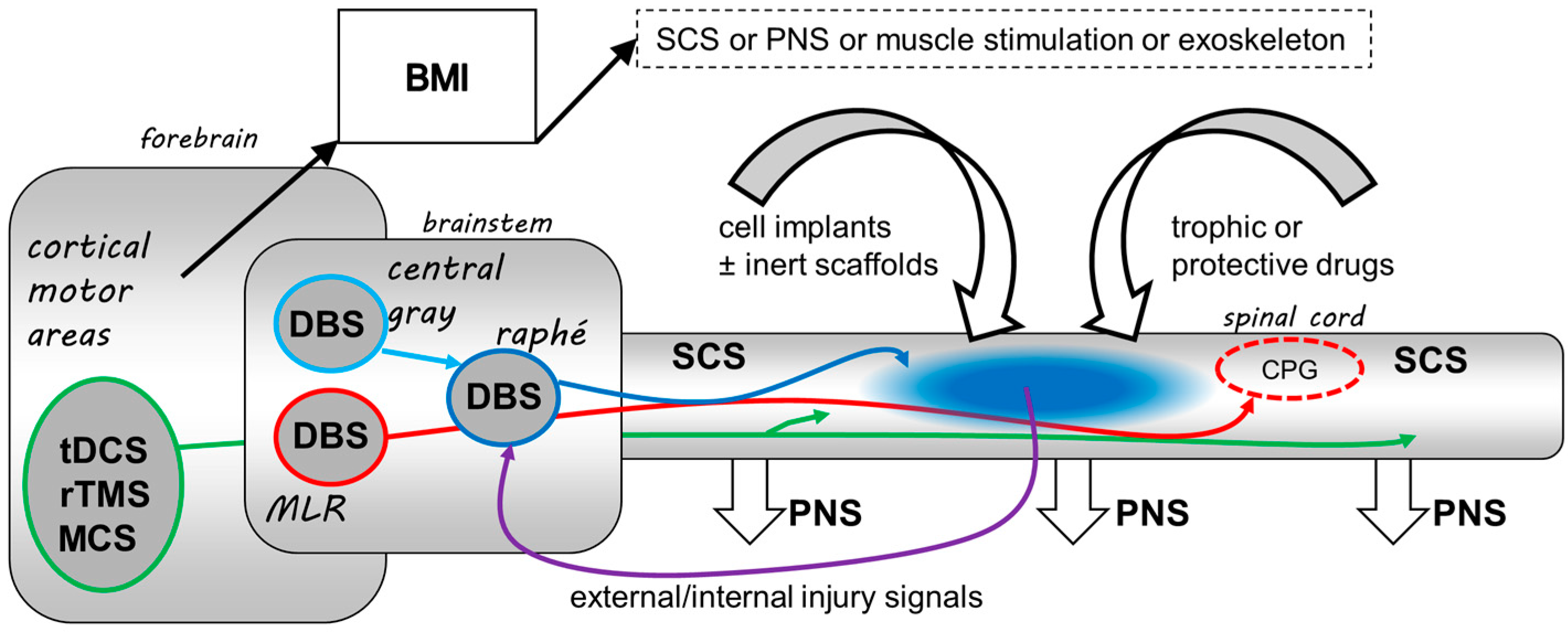
:1. Introduction
2. Current Management of Acute Spinal Cord Injury
3. Current Management of Chronic Spinal Cord Injury
4. The Potential Roles for Neurostimulation in SCI
5. Neurostimulation for Pain Following SCI
6. Neurostimulation for Sensorimotor Recovery Following SCI
7. Neurostimulation for Autonomic Recovery Following SCI
8. The Future of Neurostimulation in SCI
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A.; Fehlings, M.G. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 2014, 6, 309–331. [Google Scholar] [PubMed]
- Jazayeri, S.B.; Beygi, S.; Shokraneh, F.; Hagen, E.M.; Rahimi-Movaghar, V. Incidence of traumatic spinal cord injury worldwide: A systematic review. Eur. Spine J. 2015, 24, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Rahimi-Movaghar, V.; Sayyah, M.K.; Akbari, H.; Khorramirouz, R.; Rasouli, M.R.; Moradi-Lakeh, M.; Shokraneh, F.; Vaccaro, A.R. Epidemiology of traumatic spinal cord injury in developing countries: A systematic review. Neuroepidemiology 2013, 41, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Cripps, R.A.; Lee, B.B.; Wing, P.; Weerts, E.; Mackay, J.; Brown, D. A global map for traumatic spinal cord injury epidemiology: Towards a living data repository for injury prevention. Spinal Cord 2011, 49, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.A.; Krassioukov, A.V. contemporary cardiovascular concerns after spinal cord injury: Mechanisms, maladaptations, and management. J. Neurotrauma 2015, 32, 1927–1942. [Google Scholar] [CrossRef] [PubMed]
- Krueger, H.; Noonan, V.K.; Trenaman, L.M.; Joshi, P.; Rivers, C.S. The economic burden of traumatic spinal cord injury in Canada. Chronic Dis. Inj. Can. 2013, 33, 113–122. [Google Scholar] [PubMed]
- Hawryluk, G.; Whetstone, W.; Saigal, R.; Ferguson, A.; Talbott, J.; Bresnahan, J.; Dhall, S.; Pan, J.; Beattie, M.; Manley, G. Mean arterial blood pressure correlates with neurological recovery after human spinal cord injury: Analysis of high frequency physiologic data. J. Neurotrauma 2015, 32, 1958–1967. [Google Scholar] [CrossRef] [PubMed]
- Walters, B.C.; Hadley, M.N.; Hurlbert, R.J.; Aarabi, B.; Dhall, S.S.; Gelb, D.E.; Harrigan, M.R.; Rozelle, C.J.; Ryken, T.C.; Theodore, N. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery 2013, 60, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.K.; Tetreault, L.; Shamji, M.F.; Singh, A.; Vukas, R.R.; Harrop, J.S.; Fehlings, M.G.; Vaccaro, A.R.; Hilibrand, A.S.; Arnold, P.M. Optimal timing of surgical decompression for acute traumatic central cord syndrome: A systematic review of the literature. Neurosurgery 2015, 77, S15–S32. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Vaccaro, A.; Wilson, J.R.; Singh, A.; David, W.C.; Harrop, J.S.; Aarabi, B.; Shaffrey, C.; Dvorak, M.; Fisher, C.; et al. Early versus delayed decompression for traumatic cervical spinal cord injury: Results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS ONE 2012, 7, e32037. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.M.; Long, X.H.; Zhou, Y.; Peng, H.W.; Liu, Z.L.; Huang, S.H. Is Urgent decompression superior to delayed surgery for traumatic spinal cord injury? A meta-analysis. World Neurosurg. 2016, 87, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Phang, I.; Papadopoulos, M.C. Intraspinal pressure monitoring in a patient with spinal cord injury reveals different intradural compartments: Injured Spinal Cord Pressure Evaluation (ISCoPE) study. Neurocrit. Care 2015, 23, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Chen, S.; Papadopoulos, M.C. Intraspinal pressure and spinal cord perfusion pressure predict neurological outcome after traumatic spinal cord injury. J. Neurol. Neurosurg. Psychiatry 2016. [Google Scholar] [CrossRef] [PubMed]
- Werndle, M.C.; Saadoun, S.; Phang, I.; Czosnyka, M.; Varsos, G.; Czosnyka, Z.; Smielewski, P.; Jamous, A.; Bell, B.A.; Zoumprouli, A.; et al. Measurement of intraspinal pressure after spinal cord injury: technical note from the injured spinal cord pressure evaluation study. Acta Neurochir. Suppl. 2016, 122, 323–328. [Google Scholar] [PubMed]
- Werndle, M.C.; Saadoun, S.; Phang, I.; Czosnyka, M.; Varsos, G.V.; Czosnyka, Z.H.; Smielewski, P.; Jamous, A.; Bell, B.A.; Zoumprouli, A.; et al. Monitoring of spinal cord perfusion pressure in acute spinal cord injury: Initial findings of the injured spinal cord pressure evaluation study. Crit. Care Med. 2014, 42, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Kopjar, B.; Grossman, R.G. 329 efficacy and safety of riluzole in acute spinal cord injury: Rationale and design of aospine phase III multicenter double-blinded Randomized Controlled Trial (RISCIS). Neurosurgery 2016, 63, 196. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Nakashima, H.; Nagoshi, N.; Chow, D.S.; Grossman, R.G.; Kopjar, B. Rationale, design and critical end points for the Riluzole in Acute Spinal Cord Injury Study (RISCIS): A randomized, double-blinded, placebo-controlled parallel multi-center trial. Spinal Cord 2016, 54, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Grossman, R.G.; Fehlings, M.G.; Frankowski, R.F.; Burau, K.D.; Chow, D.S.; Tator, C.; Teng, A.; Toups, E.G.; Harrop, J.S.; Aarabi, B.; et al. A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J. Neurotrauma 2014, 31, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Waxman, S.G. Aminopyridines and the treatment of spinal cord injury. J. Neurotrauma 1993, 10, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Domingo, A.; Al-Yahya, A.A.; Asiri, Y.; Eng, J.J.; Lam, T. A systematic review of the effects of pharmacological agents on walking function in people with spinal cord injury. J. Neurotrauma 2012, 29, 865–879. [Google Scholar] [CrossRef] [PubMed]
- DeForge, D.; Nymark, J.; Lemaire, E.; Gardner, S.; Hunt, M.; Martel, L.; Curran, D.; Barbeau, H. Effect of 4-aminopyridine on gait in ambulatory spinal cord injuries: A double-blind, placebo-controlled, crossover trial. Spinal Cord 2004, 42, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, S.S.; Pugh, S.L.; Perez-Espejo, M.A.; Oro, J.J. Effect of 4-aminopyridine in acute spinal cord injury. Surg. Neurol. 1995, 43, 443–447. [Google Scholar] [CrossRef]
- Hurlbert, R.J. Methylprednisolone for acute spinal cord injury: An inappropriate standard of care. J. Neurosurg. 2000, 93, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Levi, A.D.; Green, B.A.; Wang, M.Y.; Dietrich, W.D.; Brindle, T.; Vanni, S.; Casella, G.; Elhammady, G.; Jagid, J. Clinical application of modest hypothermia after spinal cord injury. J. Neurotrauma 2009, 26, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Madden, L.K.; DeVon, H.A. A systematic review of the effects of body temperature on outcome after adult traumatic brain injury. J. Neurosci. Nurs. 2015, 47, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.I.; Bullock, M.R.; Dietrich, W.D. Hypothermia in traumatic brain injury. Neurosurg. Clin. N. Am. 2016, 27, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Ramer, L.M.; Ramer, M.S.; Bradbury, E.J. Restoring function after spinal cord injury: Towards clinical translation of experimental strategies. Lancet Neurol. 2014, 13, 1241–1256. [Google Scholar] [CrossRef]
- Tabakow, P.; Jarmundowicz, W.; Czapiga, B.; Fortuna, W.; Miedzybrodzki, R.; Czyz, M.; Huber, J.; Szarek, D.; Okurowski, S.; Szewczyk, P.; et al. Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury. Cell Transplant. 2013, 22, 1591–1612. [Google Scholar] [CrossRef] [PubMed]
- Tabakow, P.; Raisman, G.; Fortuna, W.; Czyz, M.; Huber, J.; Li, D.; Szewczyk, P.; Okurowski, S.; Miedzybrodzki, R.; Czapiga, B.; et al. Functional regeneration of supraspinal connections in a patient with transected spinal cord following transplantation of bulbar olfactory ensheathing cells with peripheral nerve bridging. Cell Transplant. 2014, 23, 1631–1655. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, E.J.; Moon, L.D.; Popat, R.J.; King, V.R.; Bennett, G.S.; Patel, P.N.; Fawcett, J.W.; McMahon, S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002, 416, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Popovich, P.G.; Tovar, C.A.; Lemeshow, S.; Yin, Q.; Jakeman, L.B. Independent evaluation of the anatomical and behavioral effects of Taxol in rat models of spinal cord injury. Exp. Neurol. 2014, 261, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Deumens, R.; Joosten, E.A.; Waxman, S.G.; Hains, B.C. Locomotor dysfunction and pain: The scylla and charybdis of fiber sprouting after spinal cord injury. Mol. Neurobiol. 2008, 37, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Dlouhy, B.J.; Awe, O.; Rao, R.C.; Kirby, P.A.; Hitchon, P.W. Autograft-derived spinal cord mass following olfactory mucosal cell transplantation in a spinal cord injury patient: Case report. J. Neurosurg. Spine 2014, 21, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Tang, C.; Rao, M.S.; Weissman, I.L.; Wu, J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 2013, 19, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Hariz, M.; Blomstedt, P.; Zrinzo, L. Future of brain stimulation: New targets, new indications, new technology. Mov. Disord. 2013, 28, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.A.; Green, A.L.; Nandi, D.; Aziz, T.Z. Deep brain stimulation: Indications and evidence. Expert Rev. Med. Devices 2007, 4, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.D. Targeting recovery: Priorities of the spinal cord-injured population. J. Neurotrauma 2004, 21, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Simpson, L.A.; Eng, J.J.; Hsieh, J.T.; Wolfe, D.L. The health and life priorities of individuals with spinal cord injury: A systematic review. J. Neurotrauma 2012, 29, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, L.C.; Matis, A.; Lindau, N.T.; Felder, P.; Gullo, M.; Schwab, M.E. Deep brain stimulation of the midbrain locomotor region improves paretic hindlimb function after spinal cord injury in rats. Sci. Transl. Med. 2013, 5, 208ra146. [Google Scholar] [CrossRef] [PubMed]
- Donati, A.R.; Shokur, S.; Morya, E.; Campos, D.S.; Moioli, R.C.; Gitti, C.M.; Augusto, P.B.; Tripodi, S.; Pires, C.G.; Pereira, G.A.; et al. Long-term training with a brain-machine interface-based gait protocol induces partial neurological recovery in paraplegic patients. Sci. Rep. 2016, 6, 30383. [Google Scholar] [CrossRef] [PubMed]
- Capogrosso, M.; Milekovic, T.; Borton, D.; Wagner, F.; Moraud, E.M.; Mignardot, J.B.; Buse, N.; Gandar, J.; Barraud, Q.; Xing, D.; et al. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature 2016, 539, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Groothuis, J.; Ramsey, N.F.; Ramakers, G.M.; van der Plasse, G. Physiological challenges for intracortical electrodes. Brain Stimul. 2014, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Q.; Zaaimi, B.; Martin, J.H. competition with primary sensory afferents drives remodeling of corticospinal axons in mature spinal motor circuits. J. Neurosci. 2016, 36, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Carballosa-Gonzalez, M.M.; Blaya, M.O.; Alonso, O.F.; Bramlett, H.M.; Hentall, I.D. Midbrain raphe stimulation improves behavioral and anatomical recovery from fluid-percussion brain injury. J. Neurotrauma 2013, 30, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Carballosa-Gonzalez, M.M.; Vitores, A.; Hentall, I.D. Hindbrain raphe stimulation boosts cyclic adenosine monophosphate and signaling proteins in the injured spinal cord. Brain Res. 2014, 1543, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Hentall, I.D.; Burns, S.B. Restorative effects of stimulating medullary raphe after spinal cord injury. J. Rehabil. Res. Dev. 2009, 46, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Hentall, I.D.; Gonzalez, M.M. Promotion of recovery from thoracic spinal cord contusion in rats by stimulation of medullary raphe or its midbrain input. Neurorehabil. Neural Repair 2012, 26, 374–384. [Google Scholar] [CrossRef] [PubMed]
- International Association for the Study of Pain. IASP Taxonomy. 2012. Available online: http://www.iasp-pain.org/Taxonomy (accessed on 5 January 2017).
- Bryce, T.N.; Biering-Sorensen, F.; Finnerup, N.B.; Cardenas, D.D.; Defrin, R.; Lundeberg, T.; Norrbrink, C.; Richards, J.S.; Siddall, P.; Stripling, T.; et al. International spinal cord injury pain classification: Part I. Background and description. March 6–7, 2009. Spinal Cord 2012, 50, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Bryce, T.N.; Biering-Sorensen, F.; Finnerup, N.B.; Cardenas, D.D.; Defrin, R.; Ivan, E.; Lundeberg, T.; Norrbrink, C.; Richards, J.S.; Siddall, P.; et al. International Spinal Cord Injury Pain (ISCIP) Classification: Part 2. Initial validation using vignettes. Spinal Cord 2012, 50, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Adriaansen, J.J.; Post, M.W.; de Groot, S.; van Asbeck, F.W.; Stolwijk-Swuste, J.M.; Tepper, M.; Lindeman, E. Secondary health conditions in persons with spinal cord injury: A longitudinal study from one to five years post-discharge. J. Rehabil. Med. 2013, 45, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Adriaansen, J.J.; van Asbeck, F.W.; Lindeman, E.; van der Woude, L.H.; de Groot, S.; Post, M.W. Secondary health conditions in persons with a spinal cord injury for at least 10 years: Design of a comprehensive long-term cross-sectional study. Disabil. Rehabil. 2013, 35, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, S.; Iwarsson, S.; Lexell, J. Secondary health conditions, activity limitations, and life satisfaction in older adults with long-term spinal cord injury. PM&R 2016. [Google Scholar] [CrossRef]
- Turner, J.A.; Cardenas, D.D.; Warms, C.A.; McClellan, C.B. Chronic pain associated with spinal cord injuries: A community survey. Arch. Phys. Med. Rehabil. 2001, 82, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.; Fullen, B.M.; Stokes, D.; Lennon, O. Neuropathic pain prevalence following spinal cord injury: A systematic review and meta-analysis. Eur. J. Pain 2017, 21, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Nardone, R.; Holler, Y.; Brigo, F.; Seidl, M.; Christova, M.; Bergmann, J.; Golaszewski, S.; Trinka, E. Functional brain reorganization after spinal cord injury: Systematic review of animal and human studies. Brain Res. 2013, 1504, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Nardone, R.; Holler, Y.; Leis, S.; Holler, P.; Thon, N.; Thomschewski, A.; Golaszewski, S.; Brigo, F.; Trinka, E. Invasive and non-invasive brain stimulation for treatment of neuropathic pain in patients with spinal cord injury: A review. J. Spinal Cord Med. 2014, 37, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Saulino, M.; Averna, J.F. Evaluation and Management of SCI-Associated Pain. Curr. Pain Headache Rep. 2016, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Duarte, I.; Morse, L.R.; Alam, M.; Bikson, M.; Zafonte, R.; Fregni, F. Targeted therapies using electrical and magnetic neural stimulation for the treatment of chronic pain in spinal cord injury. Neuroimage 2014, 85 Pt. 3, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; O’Connor, A.B.; Kent, J.; Mackey, S.C.; Raja, S.N.; Stacey, B.R.; Levy, R.M.; Backonja, M.; Baron, R.; Harke, H.; et al. Interventional management of neuropathic pain: NeuPSIG recommendations. Pain 2013, 154, 2249–2261. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.G.; Mickle, W.A. Evaluation of seven years’ experience with depth electrode studies in human patients. In Electrical Studies on the Unanesthetized Brain; PB Hoeber: New York, NY, USA, 2013; pp. 214–247. [Google Scholar]
- Melzack, R.; Wall, P.D. Pain mechanisms: A new theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Boccard, S.G.; Pereira, E.A.; Moir, L.; Aziz, T.Z.; Green, A.L. Long-term outcomes of deep brain stimulation for neuropathic pain. Neurosurgery 2013, 72, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Toth, C.; Nath, R.K. Deep brain stimulation for intractable pain: A 15-year experience. Neurosurgery 1997, 40, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, P.; Esposito, V. The functional anatomy of neuropathic pain. Neurosurg. Clin. N. Am. 2004, 15, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Canavero, S.; Bonicalzi, V. Neuromodulation for central pain. Expert Rev. Neurother. 2003, 3, 591–607. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.A.; Boccard, S.G.; Aziz, T.Z. Deep brain stimulation for pain: Distinguishing dorsolateral somesthetic and ventromedial affective targets. Neurosurgery 2014, 61, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Im, S.H.; Ha, S.W.; Kim, D.R.; Son, B.C. Long-term results of motor cortex stimulation in the treatment of chronic, intractable neuropathic pain. Stereotact. Funct. Neurosurg. 2015, 93, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Previnaire, J.G.; Nguyen, J.P.; Perrouin-Verbe, B.; Fattal, C. Chronic neuropathic pain in spinal cord injury: Efficiency of deep brain and motor cortex stimulation therapies for neuropathic pain in spinal cord injury patients. Ann. Phys. Rehabil. Med. 2009, 52, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.M.; Lamb, S.; Adams, J.E. Treatment of chronic pain by deep brain stimulation: Long term follow-up and review of the literature. Neurosurgery 1987, 21, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Rasche, D.; Rinaldi, P.C.; Young, R.F.; Tronnier, V.M. Deep brain stimulation for the treatment of various chronic pain syndromes. Neurosurg. Focus 2006, 21, E8. [Google Scholar] [CrossRef] [PubMed]
- Hamani, C.; Schwalb, J.M.; Rezai, A.R.; Dostrovsky, J.O.; Davis, K.D.; Lozano, A.M. Deep brain stimulation for chronic neuropathic pain: Long-term outcome and the incidence of insertional effect. Pain 2006, 125, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.L.; Green, A.L.; Nandi, D.; Bittar, R.G.; Wang, S.; Aziz, T.Z. Deep brain stimulation for neuropathic pain. Neuromodulation 2006, 9, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Yamamoto, T.; Kobayashi, K.; Kasai, M.; Oshima, H.; Fukaya, C. Motor cortex stimulation for phantom limb pain: Comprehensive therapy with spinal cord and thalamic stimulation. Stereotact. Funct. Neurosurg. 2001, 77, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Hentall, I.D.; Luca, C.C.; Widerstrom-Noga, E.; Vitores, A.; Fisher, L.D.; Martinez-Arizala, A.; Jagid, J.R. The midbrain central gray best suppresses chronic pain with electrical stimulation at very low pulse rates in two human cases. Brain Res. 2016, 1632, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.A.; Wang, S.; Owen, S.L.; Aziz, T.Z.; Green, A.L. Human periventricular grey somatosensory evoked potentials suggest rostrocaudally inverted somatotopy. Stereotact. Funct. Neurosurg. 2013, 91, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.A.; Green, A.L.; Aziz, T.Z. Deep brain stimulation for pain. Handb. Clin. Neurol. 2013, 116, 277–294. [Google Scholar] [PubMed]
- Spooner, J.; Yu, H.; Kao, C.; Sillay, K.; Konrad, P. Neuromodulation of the cingulum for neuropathic pain after spinal cord injury. Case report. J. Neurosurg. 2007, 107, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Boccard, S.G.; Pereira, E.A.; Moir, L.; Van Hartevelt, T.J.; Kringelbach, M.L.; FitzGerald, J.J.; Baker, I.W.; Green, A.L.; Aziz, T.Z. Deep brain stimulation of the anterior cingulate cortex: Targeting the affective component of chronic pain. Neuroreport 2014, 25, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Coffey, R.J. Deep brain stimulation for chronic pain: Results of two multicenter trials and a structured review. Pain Med. 2001, 2, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Mekhail, N.; Petersen, E.; Krames, E.; Staats, P.; Pope, J.; Saweris, Y.; Lad, S.P.; Diwan, S.; Falowski, S.; et al. The appropriate use of neurostimulation: Stimulation of the intracranial and extracranial space and head for chronic pain. Neuromodulation Appropriateness Consensus Committee. Neuromodulation 2014, 17, 551–570. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Yoshimine, T. Stimulation of primary motor cortex for intractable deafferentation pain. Acta Neurochir. Suppl. 2007, 97 Pt. 2, 51–56. [Google Scholar] [PubMed]
- Nashold, B.S., Jr.; Friedman, H. Dorsal column stimulation for control of pain. Preliminary report on 30 patients. J. Neurosurg. 1972, 36, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Spiegelmann, R.; Friedman, W.A. Spinal cord stimulation: A contemporary series. Neurosurgery 1991, 28, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Toth, C.; Nath, R.K.; Laing, P. Epidural spinal cord stimulation for treatment of chronic pain—Some predictors of success. A 15-year experience. Surg. Neurol. 1998, 50, 110–120. [Google Scholar] [CrossRef]
- Taylor, R.S.; van Buyten, J.P.; Buchser, E. Spinal cord stimulation for chronic back and leg pain and failed back surgery syndrome: A systematic review and analysis of prognostic factors. Spine (Phila Pa 1976) 2005, 30, 152–160. [Google Scholar] [CrossRef]
- Strafella, A.P.; Vanderwerf, Y.; Sadikot, A.F. Transcranial magnetic stimulation of the human motor cortex influences the neuronal activity of subthalamic nucleus. Eur. J. Neurosci. 2004, 20, 2245–2249. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.; Siebner, H.R.; Ward, N.S.; Lee, L.; Nitsche, M.A.; Paulus, W.; Rothwell, J.C.; Lemon, R.N.; Frackowiak, R.S. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur. J. Neurosci. 2005, 22, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chu, H.; Li, J.; Yang, M.; Du, L.; Li, J.; Chen, L.; Yang, D.; Zhang, H.; Chan, C. Repetitive transcranial magnetic stimulation for pain after spinal cord injury: A systematic review and meta-analysis. J. Neurosurg. Sci. 2016. Available online: https://www.ncbi.nlm.nih.gov/labs/articles/27603408/ (accessed on 5 January 2017). [Google Scholar]
- Cruccu, G.; Garcia-Larrea, L.; Hansson, P.; Keindl, M.; Lefaucheur, J.P.; Paulus, W.; Taylor, R.; Tronnier, V.; Truini, A.; Attal, N. EAN guidelines on central neurostimulation therapy in chronic pain conditions. Eur. J. Neurol. 2016, 23, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Cruccu, G.; Aziz, T.Z.; Garcia-Larrea, L.; Hansson, P.; Jensen, T.S.; Lefaucheur, J.P.; Simpson, B.A.; Taylor, R.S. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur. J. Neurol. 2007, 14, 952–970. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Andre-Obadia, N.; Antal, A.; Ayache, S.S.; Baeken, C.; Benninger, D.H.; Cantello, R.M.; Cincotta, M.; de Carvalho, M.; De Ridder, D.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol. 2014, 125, 2150–2206. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Drouot, X.; Menard-Lefaucheur, I.; Zerah, F.; Bendib, B.; Cesaro, P.; Keravel, Y.; Nguyen, J.P. Neurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of pain. J. Neurol. Neurosurg. Psychiatry 2004, 75, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Osenbach, R.K. Motor cortex stimulation for intractable pain. Neurosurg. Focus 2006, 21, E7. [Google Scholar] [CrossRef] [PubMed]
- Nardone, R.; Holler, Y.; Langthaler, P.B.; Lochner, P.; Golaszewski, S.; Schwenker, K.; Brigo, F.; Trinka, E. rTMS of the prefrontal cortex has analgesic effects on neuropathic pain in subjects with spinal cord injury. Spinal Cord 2016, 55, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P. Cortical neurostimulation for neuropathic pain: State of the art and perspectives. Pain 2016, 157, S81–S89. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.J.; Kim, Y.K.; Kim, H.R.; Kim, S.E.; Lee, Y.; Shin, H.I. Transcranial direct current stimulation to lessen neuropathic pain after spinal cord injury: A mechanistic PET study. Neurorehabil. Neural Repair 2014, 28, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Guy, S.D.; Mehta, S.; Casalino, A.; Cote, I.; Kras-Dupuis, A.; Moulin, D.E.; Parrent, A.G.; Potter, P.; Short, C.; Teasell, R.; et al. The CanPain SCI Clinical Practice Guidelines for Rehabilitation Management of Neuropathic Pain after Spinal Cord: Recommendations for treatment. Spinal Cord 2016, 54, S14–S23. [Google Scholar] [CrossRef] [PubMed]
- Widerstrom-Noga, E.; Biering-Sorensen, F.; Bryce, T.N.; Cardenas, D.D.; Finnerup, N.B.; Jensen, M.P.; Richards, J.S.; Richardson, E.J.; Siddall, P.J. The international spinal cord injury pain extended data set (Version 1.0). Spinal Cord 2016, 54, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Widerstrom-Noga, E.; Biering-Sorensen, F.; Bryce, T.; Cardenas, D.D.; Finnerup, N.B.; Jensen, M.P.; Richards, J.S.; Siddall, P.J. The international spinal cord injury pain basic data set. Spinal Cord 2008, 46, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M. Deep brain stimulation for locomotor recovery following spinal cord injury. Neurosurgery 2014, 74, N18–N19. [Google Scholar] [CrossRef] [PubMed]
- Wenger, N.; Moraud, E.M.; Raspopovic, S.; Bonizzato, M.; DiGiovanna, J.; Musienko, P.; Morari, M.; Micera, S.; Courtine, G. Closed-loop neuromodulation of spinal sensorimotor circuits controls refined locomotion after complete spinal cord injury. Sci. Transl. Med. 2014, 6, 255ra133. [Google Scholar] [CrossRef] [PubMed]
- Courtine, G.; Gerasimenko, Y.; van den Brand, R.; Yew, A.; Musienko, P.; Zhong, H.; Song, B.; Ao, Y.; Ichiyama, R.M.; Lavrov, I.; et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat. Neurosci. 2009, 12, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Holinski, B.J.; Everaert, D.G.; Mushahwar, V.K.; Stein, R.B. Real-time control of walking using recordings from dorsal root ganglia. J. Neural Eng. 2013, 10, 056008. [Google Scholar] [CrossRef] [PubMed]
- Fenelon, G.; Goujon, C.; Gurruchaga, J.M.; Cesaro, P.; Jarraya, B.; Palfi, S.; Lefaucheur, J.P. Spinal cord stimulation for chronic pain improved motor function in a patient with Parkinson’s disease. Parkinsonism Relat. Disord. 2012, 18, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, R.; Petersson, P.; Siesser, W.B.; Caron, M.G.; Nicolelis, M.A. Spinal cord stimulation restores locomotion in animal models of Parkinson’s disease. Science 2009, 323, 1578–1582. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.W. Electrical stimulation in multiple sclerosis. Hosp. Pract. 1976, 11, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.W.; Weinstein, S.P. Chronic dorsal column stimulation in multiple sclerosis. Preliminary report. N. Y. State J. Med. 1973, 73, 2868–2872. [Google Scholar] [PubMed]
- Angeli, C.A.; Edgerton, V.R.; Gerasimenko, Y.P.; Harkema, S.J. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 2014, 137 Pt. 5, 1394–1409. [Google Scholar] [CrossRef] [PubMed]
- Sayenko, D.G.; Angeli, C.; Harkema, S.J.; Edgerton, V.R.; Gerasimenko, Y.P. Neuromodulation of evoked muscle potentials induced by epidural spinal-cord stimulation in paralyzed individuals. J. Neurophysiol. 2014, 111, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Harkema, S.; Gerasimenko, Y.; Hodes, J.; Burdick, J.; Angeli, C.; Chen, Y.; Ferreira, C.; Willhite, A.; Rejc, E.; Grossman, R.G.; et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 2011, 377, 1938–1947. [Google Scholar] [CrossRef]
- Minassian, K.; McKay, W.B.; Binder, H.; Hofstoetter, U.S. Targeting lumbar spinal neural circuitry by epidural stimulation to restore motor function after spinal cord injury. Neurotherapeutics 2016, 13, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Mayr, W.; Krenn, M.; Dimitrijevic, M.R. Epidural and transcutaneous spinal electrical stimulation for restoration of movement after incomplete and complete spinal cord injury. Curr. Opin. Neurol. 2016, 29, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Amer, A.; Ryan, D.; Martin, J.H. Combined motor cortex and spinal cord neuromodulation promotes corticospinal system functional and structural plasticity and motor function after injury. Exp. Neurol. 2016, 277, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Squair, J.W.; Bjerkefors, A.; Inglis, J.T.; Lam, T.; Carpenter, M.G. Cortical and vestibular stimulation reveal preserved descending motor pathways in individuals with motor-complete spinal cord injury. J. Rehabil. Med. 2016, 48, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.G.; Ji, S.G.; Kim, M.K. Effect of high-frequency repetitive transcranial magnetic stimulation on motor cortical excitability and sensory nerve conduction velocity in subacute-stage incomplete spinal cord injury patients. J. Phys. Ther. Sci. 2016, 28, 2002–2004. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Rabchevsky, A.G. Autonomic consequences of spinal cord injury. Compr. Physiol. 2014, 4, 1419–1453. [Google Scholar] [PubMed]
- Wyndaele, J.J. The management of neurogenic lower urinary tract dysfunction after spinal cord injury. Nat. Rev. Urol. 2016, 13, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Elliott, S.; Noonan, V.K.; Thorogood, N.P.; Fallah, N.; Aludino, A.; Dvorak, M.F. Impact of bladder, bowel and sexual dysfunction on health status of people with thoracolumbar spinal cord injuries living in the community. J. Spinal Cord Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Cobo Cuenca, A.I.; Sampietro-Crespo, A.; Virseda-Chamorro, M.; Martin-Espinosa, N. Psychological impact and sexual dysfunction in men with and without spinal cord injury. J. Sex. Med. 2015, 12, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Krassioukov, A. Autonomic function following cervical spinal cord injury. Respir. Physiol. Neurobiol. 2009, 169, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, A.K. Autonomic dysfunction in spinal cord injury: Clinical presentation of symptoms and signs. Prog. Brain Res. 2006, 152, 1–8. [Google Scholar] [PubMed]
- Trivedi, P.M.; Kumar, L.; Emmanuel, A.V. Altered colorectal compliance and anorectal physiology in upper and lower motor neurone spinal injury may explain bowel symptom pattern. Am. J. Gastroenterol. 2016, 111, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Garshick, E.; Kelley, A.; Cohen, S.A.; Garrison, A.; Tun, C.G.; Gagnon, D.; Brown, R. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord 2005, 43, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Partida, E.; Mironets, E.; Hou, S.; Tom, V.J. Cardiovascular dysfunction following spinal cord injury. Neural Regen. Res. 2016, 11, 189–194. [Google Scholar] [PubMed]
- Dance, D.L.; Chopra, A.; Campbell, K.; Ditor, D.S.; Hassouna, M.; Craven, B.C. Exploring daily blood pressure fluctuations and cardiovascular risk among individuals with motor complete spinal cord injury: A pilot study. J. Spinal Cord Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Phillips, A.A.; Krassioukov, A.V. Increased central arterial stiffness after spinal cord injury: contributing factors, implications and possible interventions. J. Neurotrauma 2016. [Google Scholar] [CrossRef] [PubMed]
- West, C.R.; Squair, J.W.; McCracken, L.; Currie, K.D.; Somvanshi, R.; Yuen, V.; Phillips, A.A.; Kumar, U.; McNeill, J.H.; Krassioukov, A.V. Cardiac consequences of autonomic dysreflexia in spinal cord injury. Hypertension 2016, 68, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Hyam, J.A.; Kringelbach, M.L.; Silburn, P.A.; Aziz, T.Z.; Green, A.L. The autonomic effects of deep brain stimulation—A therapeutic opportunity. Nat. Rev. Neurol. 2012, 8, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Herzog, J.; Weiss, P.H.; Assmus, A.; Wefer, B.; Seif, C.; Braun, P.M.; Herzog, H.; Volkmann, J.; Deuschl, G.; Fink, G.R. Subthalamic stimulation modulates cortical control of urinary bladder in Parkinson’s disease. Brain 2006, 129 Pt. 12, 3366–3375. [Google Scholar] [CrossRef] [PubMed]
- Herzog, J.; Weiss, P.H.; Assmus, A.; Wefer, B.; Seif, C.; Braun, P.M.; Pinsker, M.O.; Herzog, H.; Volkmann, J.; Deuschl, G.; et al. Improved sensory gating of urinary bladder afferents in Parkinson’s disease following subthalamic stimulation. Brain 2008, 131 Pt. 1, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Kessler, T.M.; Burkhard, F.C.; Z’Brun, S.; Stibal, A.; Studer, U.E.; Hess, C.W.; Kaelin-Lang, A. Effect of thalamic deep brain stimulation on lower urinary tract function. Eur. Urol. 2008, 53, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Mordasini, L.; Kessler, T.M.; Kiss, B.; Schupbach, M.; Pollo, C.; Kaelin-Lang, A. Bladder function in patients with dystonia undergoing deep brain stimulation. Parkinsonism Relat. Disord. 2014, 20, 1015–1017. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Baumgartner, L.; Binder, D.K. Switching off micturition using deep brain stimulation at midbrain sites. Ann. Neurol. 2012, 72, 144–147. [Google Scholar]
- Halim, A.; Baumgartner, L.; Binder, D.K. Effect of deep brain stimulation on autonomic dysfunction in patients with Parkinson’s disease. J. Clin. Neurosci. 2011, 18, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Green, A.L.; Paterson, D.J. Identification of neurocircuitry controlling cardiovascular function in humans using functional neurosurgery: Implications for exercise control. Exp. Physiol. 2008, 93, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.; Green, A. Autonomic neurosurgery: From microvascular decompression to image guided stimulation. Biomed. Imaging Interv. J. 2007, 3, e14. [Google Scholar] [CrossRef] [PubMed]
- Green, A.L.; Hyam, J.A.; Williams, C.; Wang, S.; Shlugman, D.; Stein, J.F.; Paterson, D.J.; Aziz, T.Z. Intra-operative deep brain stimulation of the periaqueductal grey matter modulates blood pressure and heart rate variability in humans. Neuromodulation 2010, 13, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Green, A.L.; Wang, S.; Bittar, R.G.; Owen, S.L.; Paterson, D.J.; Stein, J.F.; Bain, P.G.; Shlugman, D.; Aziz, T.Z. Deep brain stimulation: A new treatment for hypertension? J. Clin. Neurosci. 2007, 14, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Green, A.L.; Wang, S.; Owen, S.L.; Paterson, D.J.; Stein, J.F.; Aziz, T.Z. Controlling the heart via the brain: A potential new therapy for orthostatic hypotension. Neurosurgery 2006, 58, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.A.; Wang, S.; Paterson, D.J.; Stein, J.F.; Aziz, T.Z.; Green, A.L. Sustained reduction of hypertension by deep brain stimulation. J. Clin. Neurosci. 2010, 17, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.A.; Lu, G.; Wang, S.; Schweder, P.M.; Hyam, J.A.; Stein, J.F.; Paterson, D.J.; Aziz, T.Z.; Green, A.L. Ventral periaqueductal grey stimulation alters heart rate variability in humans with chronic pain. Exp. Neurol. 2010, 223, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Brindley, G.S. The first 500 patients with sacral anterior root stimulator implants: General description. Paraplegia 1994, 32, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Sievert, K.D.; Amend, B.; Gakis, G.; Toomey, P.; Badke, A.; Kaps, H.P.; Stenzl, A. Early sacral neuromodulation prevents urinary incontinence after complete spinal cord injury. Ann. Neurol. 2010, 67, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, A.P.; Knight, S.L.; Craggs, M.D.; Casey, A.T.; Shah, P.J. Neuromodulation through sacral nerve roots 2 to 4 with a Finetech-Brindley sacral posterior and anterior root stimulator. Spinal Cord 2002, 40, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Andrade, D.C.; Borges, I.; Bravo, G.L.; Bolognini, N.; Fregni, F. Therapeutic time window of noninvasive brain stimulation for pain treatment: Inhibition of maladaptive plasticity with early intervention. Expert Rev. Med. Devices 2013, 10, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Little, S.; Beudel, M.; Zrinzo, L.; Foltynie, T.; Limousin, P.; Hariz, M.; Neal, S.; Cheeran, B.; Cagnan, H.; Gratwicke, J.; et al. Bilateral adaptive deep brain stimulation is effective in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2016, 87, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Little, S.; Pogosyan, A.; Neal, S.; Zavala, B.; Zrinzo, L.; Hariz, M.; Foltynie, T.; Limousin, P.; Ashkan, K.; FitzGerald, J.; et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann. Neurol. 2013, 74, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Keifer, O.P., Jr.; Riley, J.P.; Boulis, N.M. Deep brain stimulation for chronic pain: Intracranial targets, clinical outcomes, and trial design considerations. Neurosurg. Clin. N. Am. 2014, 25, 671–692. [Google Scholar] [CrossRef] [PubMed]
- Hosobuchi, Y.; Rossier, J.; Bloom, F.E.; Guillemin, R. Stimulation of human periaqueductal gray for pain relief increases immunoreactive beta-endorphin in ventricular fluid. Science 1979, 203, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Hosobuchi, Y.; Adams, J.E.; Linchitz, R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science 1977, 197, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Fessler, R.G.; Brown, F.D.; Rachlin, J.R.; Mullan, S.; Fang, V.S. Elevated beta-endorphin in cerebrospinal fluid after electrical brain stimulation: Artifact of contrast infusion? Science 1984, 224, 1017–1019. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Mehdizadeh, A.R. Deep brain stimulation and gene expression alterations in Parkinson’s disease. J. Biomed. Phys. Eng. 2016, 6, 47–50. [Google Scholar]
- Tilley, D.M.; Cedeno, D.L.; Kelley, C.A.; Benyamin, R.; Vallejo, R. Spinal cord stimulation modulates gene expression in the spinal cord of an animal model of peripheral nerve injury. Reg. Anesth. Pain Med. 2016, 41, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.J. Neuroprotection at the nanolevel—Part II: Nanodevices for neuromodulation—Deep brain stimulation and spinal cord injury. Ann. N. Y. Acad. Sci. 2007, 1122, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Lammertse, D.P. Clinical trials in spinal cord injury: Lessons learned on the path to translation. The 2011 International Spinal Cord Society Sir Ludwig Guttmann Lecture. Spinal Cord 2013, 51, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Green, A.L.; Shad, A.; Watson, R.; Nandi, D.; Yianni, J.; Aziz, T.Z. N-of-1 Trials for assessing the efficacy of deep brain stimulation in neuropathic pain. Neuromodulation 2004, 7, 76–81. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, P.; Altman, D.G.; Campbell, W.B.; Flum, D.R.; Glasziou, P.; Marshall, J.C.; Nicholl, J.; Aronson, J.K.; Barkun, J.S.; Blazeby, J.M.; et al. No surgical innovation without evaluation: The IDEAL recommendations. Lancet 2009, 374, 1105–1112. [Google Scholar] [CrossRef]
- Williamson, P.R.; Altman, D.G.; Blazeby, J.M.; Clarke, M.; Devane, D.; Gargon, E.; Tugwell, P. Developing core outcome sets for clinical trials: Issues to consider. Trials 2012, 13, 132. [Google Scholar] [CrossRef] [PubMed]
| ASIA Impairment Scale | Definition | Explanation |
|---|---|---|
| A | Complete | No motor or sensory function is preserved in the sacral segments S4–S5. |
| B | Incomplete | Sensory but not motor function is preserved below the neurological level and includes the sacral segments S4–S5. |
| C | Incomplete | Motor function is preserved below the neurological level, and more than half of the key muscles below the neurological level have a muscle grade less than 3. |
| D | Incomplete | Motor function is preserved below the neurological level, and more than half of the key muscles below the neurological level have a muscle grade of 3 or more. |
| E | Normal | Motor and sensory function are normal. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chari, A.; Hentall, I.D.; Papadopoulos, M.C.; Pereira, E.A.C. Surgical Neurostimulation for Spinal Cord Injury. Brain Sci. 2017, 7, 18. https://doi.org/10.3390/brainsci7020018
Chari A, Hentall ID, Papadopoulos MC, Pereira EAC. Surgical Neurostimulation for Spinal Cord Injury. Brain Sciences. 2017; 7(2):18. https://doi.org/10.3390/brainsci7020018
Chicago/Turabian StyleChari, Aswin, Ian D. Hentall, Marios C. Papadopoulos, and Erlick A. C. Pereira. 2017. "Surgical Neurostimulation for Spinal Cord Injury" Brain Sciences 7, no. 2: 18. https://doi.org/10.3390/brainsci7020018






