How Hyperarousal and Sleep Reactivity Are Represented in Different Adult Age Groups: Results from a Large Cohort Study on Insomnia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.3. Analyses
3. Results
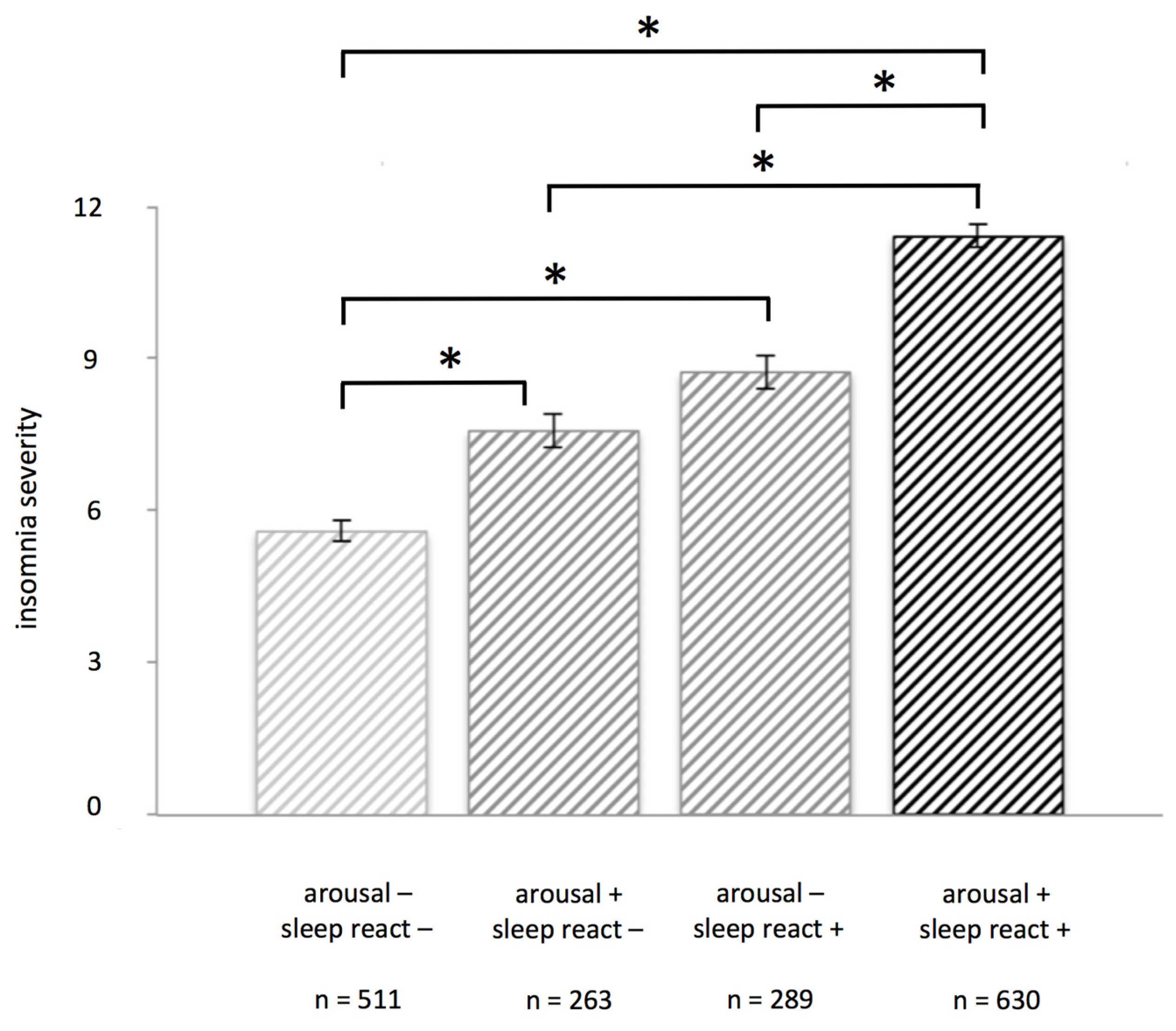
3.1. Insomnia Severity in High and Low Vulnerability Groups
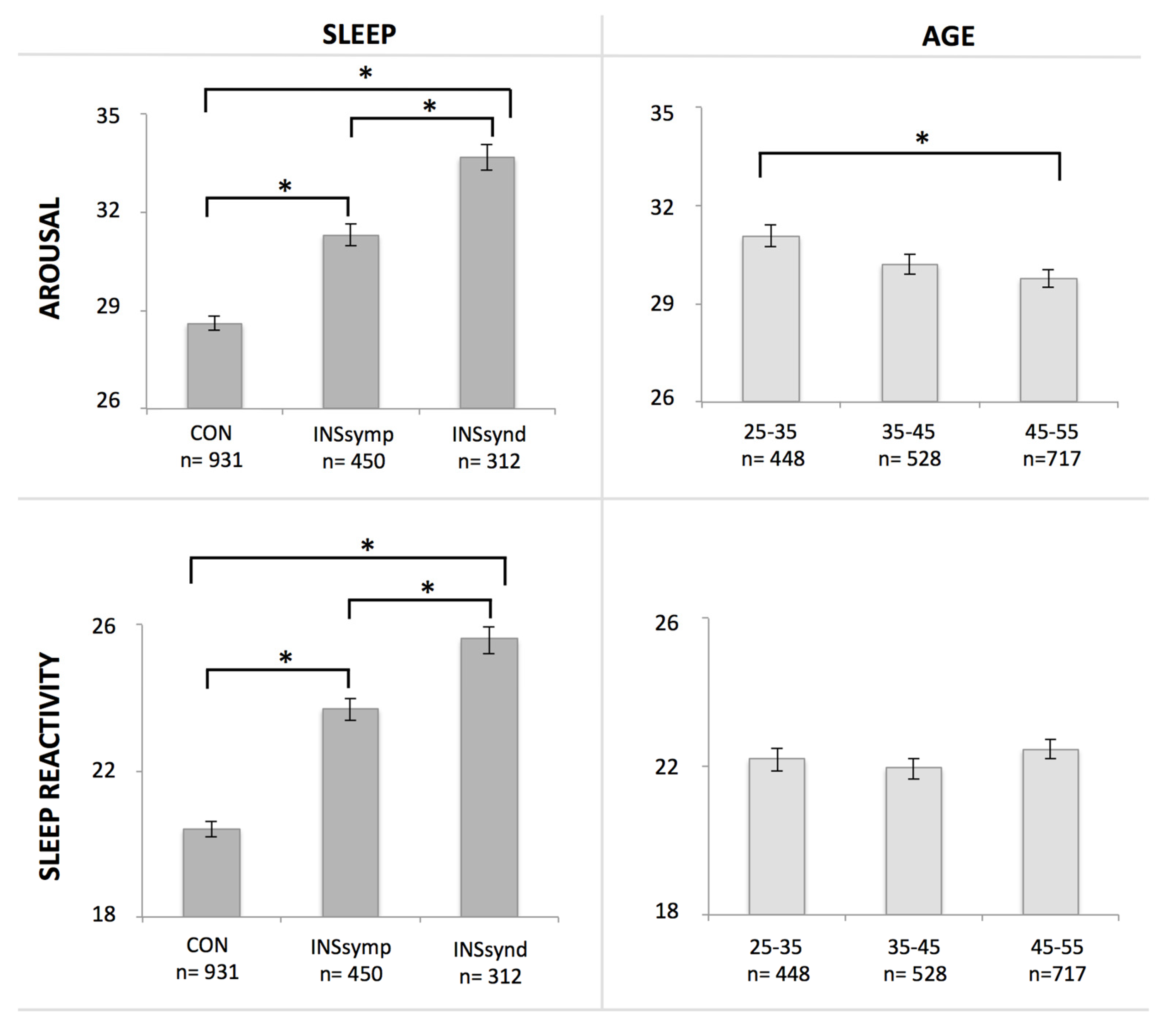
3.2. Hyperarousal and Sleep Reactivity as a Function of Age and Insomnia Symptoms
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ancoli-Israel, S.; Cooke, J.R. Prevalence and comorbidity of insomnia and effect on functioning in elderly populations. J. Am. Geriatr. Soc. 2005, 53, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.H.; Arand, D.L. Hyperarousal and insomnia: State of the science. Sleep Med. Rev. 2010, 14, 9–15. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.; Merette, C.; Savard, J.; Ivers, H.; Baillargeon, L.; Morin, C.M. Incidence and risk factors of insomnia in a population-based sample. Sleep 2009, 32, 1027–1037. [Google Scholar] [PubMed]
- Coren, S.; Mah, K.B. Prediction of physiological arousability: A validation of the arousal predisposition scale. Behav. Res. Ther. 1993, 31, 215–219. [Google Scholar] [CrossRef]
- Morin, C.M.; Rodrigue, S.; Ivers, H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom. Med. 2003, 65, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.; Richardson, G.; Roehrs, T.; Scofield, H.; Roth, T. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep 2004, 27, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.L.; Pillai, V.; Roth, T. Stress and sleep reactivity: A prospective investigation of the stress-diathesis model of insomnia. Sleep 2014, 37, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Mendoza, J.; Vela-Bueno, A.; Vgontzas, A.N.; Ramos-Platon, M.J.; Olavarrieta-Bernardino, S.; Bixler, E.O.; De la Cruz-Troca, J.J. Cognitive-emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomnia. Psychosom. Med. 2010, 72, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T.; Nishikido, N.; Kobayashi, T.; Kurokawa, Y.; Kaneko, T.; Kabuto, M. Self-reported sleep quality, job stress, and daytime autonomic activities assessed in terms of short-term heart rate variability among male white-collar workers. Ind. Health 1998, 36, 263–272. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.; Beaulieu-Bonneau, S.; Merette, C.; Savard, J.; Ivers, H.; Morin, C.M. Psychological and health-related quality of life factors associated with insomnia in a population-based sample. J. Psychosom. Res. 2007, 63, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Stawski, R.S.; Sliwinski, M.J.; Almeida, D.M.; Smyth, J.M. Reported exposure and emotional reactivity to daily stressors: The roles of adult age and global perceived stress. Psychol. Aging 2008, 23, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Folkman, S.; Lazarus, R.S.; Pimley, S.; Novacek, J. Age differences in stress and coping processes. Psychol. Aging 1987, 2, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.B.; Sliwinski, M.J.; Blanchard-Fields, F. Age differences in emotional responses to daily stress: The role of timing, severity, and global perceived stress. Psychol. Aging 2013, 28, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Altena, E.; Micoulaud-Franchi, J.A.; Geoffroy, P.A.; Sanz-Arigita, E.; Bioulac, S.; Philip, P. The bidirectional relation between emotional reactivity and sleep: From disruption to recovery. Behav. Neurosci. 2016, 130, 336–350. [Google Scholar] [CrossRef] [PubMed]
- Kish, L. Survey Sampling; Wiley: New York, NY, USA, 1965. [Google Scholar]
- Morin, C.M.; LeBlanc, M.; Daley, M.; Gregoire, J.P.; Merette, C. Epidemiology of insomnia: Prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006, 7, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Bastien, C.H.; Vallieres, A.; Morin, C.M. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Morin, C.M.; Belleville, G.; Belanger, L.; Ivers, H. The insomnia severity index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011, 34, 601–608. [Google Scholar] [PubMed]
- Coren, S. Prediction of insomnia from arousability predisposition scores: Scale development and cross-validation. Behav. Res. Ther. 1988, 26, 415–420. [Google Scholar] [CrossRef]
- Daley, M.; Morin, C.M.; LeBlanc, M.; Gregoire, J.P.; Savard, J. The economic burden of insomnia: Direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep 2009, 32, 55–64. [Google Scholar] [PubMed]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, WA, USA, 1994. [Google Scholar]
- Classification of Diseases. Available online: http://www.who.int/classifications/icd/en/ (accessed on 4 November 2016).
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Jarrin, D.C.; Chen, I.Y.; Ivers, H.; Drake, C.L.; Morin, C.M. Temporal stability of the ford insomnia response to stress test (first). J. Clin. Sleep Med. 2016, 12, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.; Roth, T.; Mullins, H.M.; Drake, C.L. Moderators and mediators of the relationship between stress and insomnia: Stressor chronicity, cognitive intrusion, and coping. Sleep 2014, 37, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Okajima, I.; Nakajima, S.; Ochi, M.; Inoue, Y. Association among changes in sleep-related beliefs, sleep reactivity, and improvement of insomnia following cognitive behavioral therapy. Sleep Med. 2017, 29, 96–97. [Google Scholar] [CrossRef] [PubMed]
- Palagini, L.; Bruno, R.M.; Paolo, T.; Caccavale, L.; Gronchi, A.; Mauri, M.; Riemann, D.; Drake, C.L. Association between stress-related sleep reactivity and metacognitive beliefs about sleep in insomnia disorder: Preliminary results. Behav. Sleep Med. 2016, 14, 636–649. [Google Scholar] [CrossRef] [PubMed]
- Leger, D.; Guilleminault, C.; Dreyfus, J.P.; Delahaye, C.; Paillard, M. Prevalence of insomnia in a survey of 12,778 adults in france. J. Sleep Res. 2000, 9, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Phillips, B.A. Why is the prevalence of insomnia skyrocketing? And what can be done about it? Sleep Med. 2015, 16, 555–556. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altena, E.; Chen, I.Y.; Daviaux, Y.; Ivers, H.; Philip, P.; Morin, C.M. How Hyperarousal and Sleep Reactivity Are Represented in Different Adult Age Groups: Results from a Large Cohort Study on Insomnia. Brain Sci. 2017, 7, 41. https://doi.org/10.3390/brainsci7040041
Altena E, Chen IY, Daviaux Y, Ivers H, Philip P, Morin CM. How Hyperarousal and Sleep Reactivity Are Represented in Different Adult Age Groups: Results from a Large Cohort Study on Insomnia. Brain Sciences. 2017; 7(4):41. https://doi.org/10.3390/brainsci7040041
Chicago/Turabian StyleAltena, Ellemarije, Ivy Y. Chen, Yannick Daviaux, Hans Ivers, Pierre Philip, and Charles M. Morin. 2017. "How Hyperarousal and Sleep Reactivity Are Represented in Different Adult Age Groups: Results from a Large Cohort Study on Insomnia" Brain Sciences 7, no. 4: 41. https://doi.org/10.3390/brainsci7040041





