P300 Source Localization Contrasts in Body-Focused Repetitive Behaviors and Tic Disorders
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Experimental and Recording Settings
2.2.1. Experimental Task: The Motor Oddball Paradigm
2.2.2. Electrophysiological Recordings
2.2.3. ERP Extraction
2.3. Statistical Analyses
2.4. P300 Source Localization
3. Results
3.1. Socio-Demographic and Clinical Description
3.2. Reaction Times and Performance during the Oddball Task
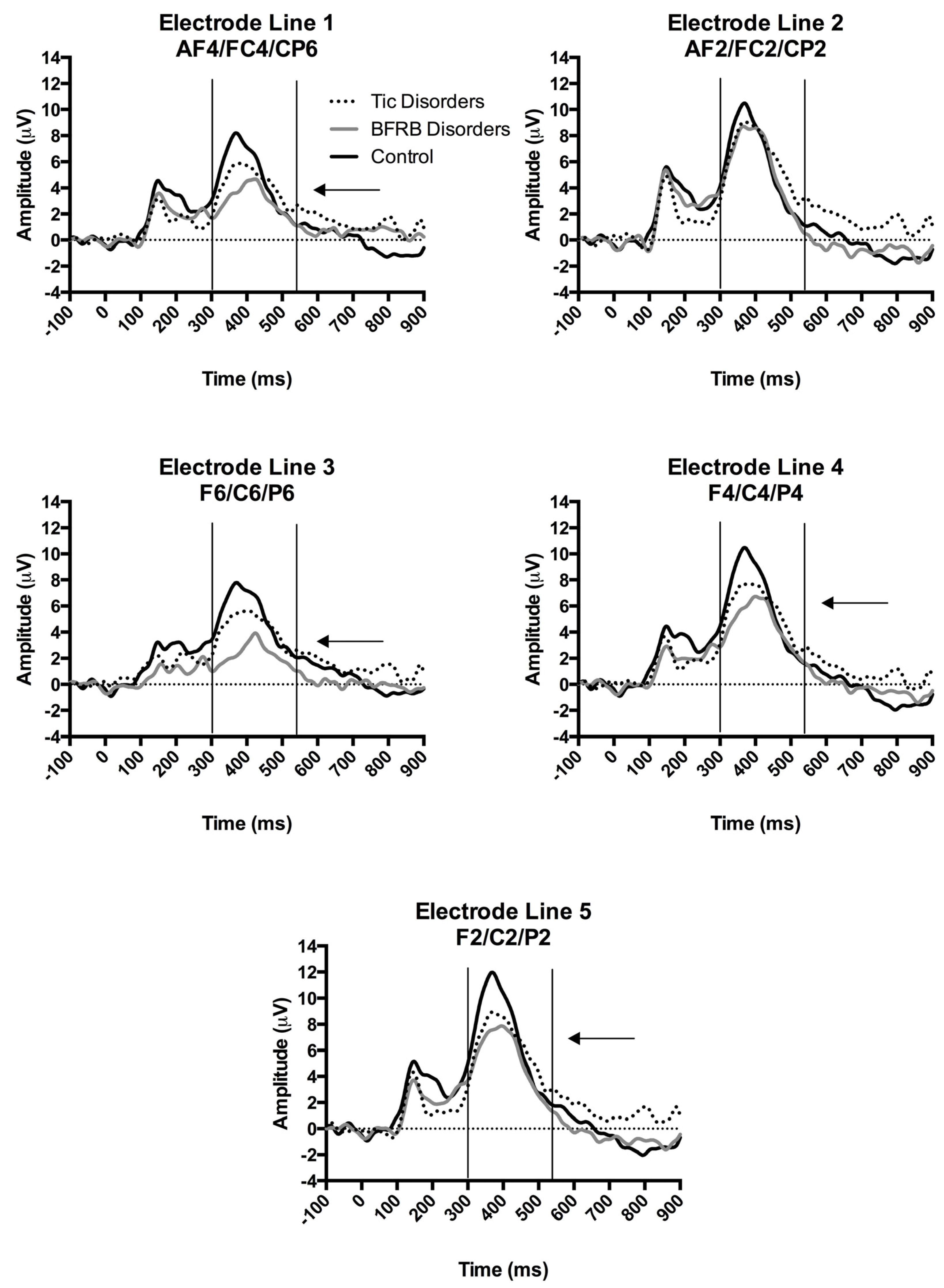
3.3. Amplitude of the P300 Component
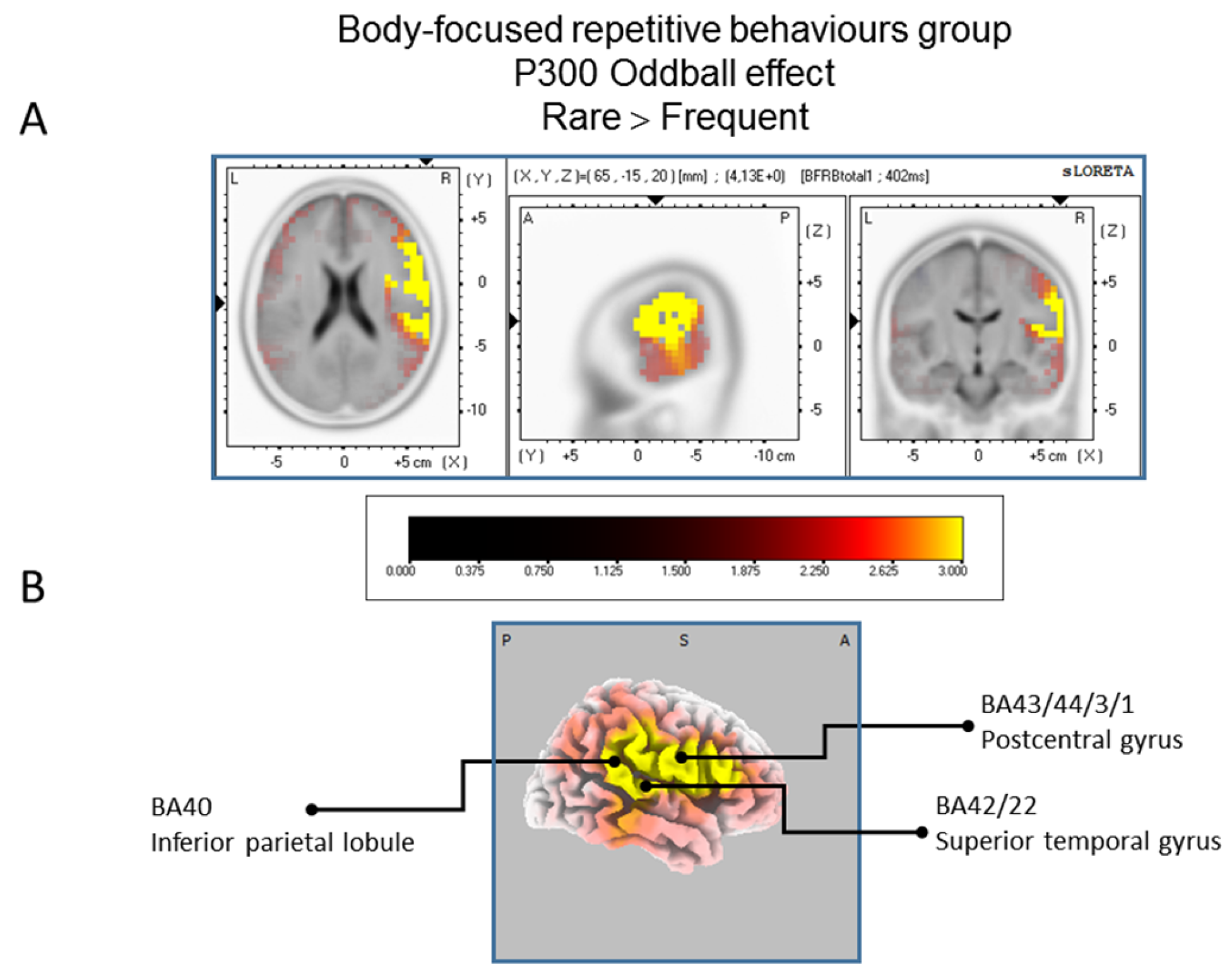
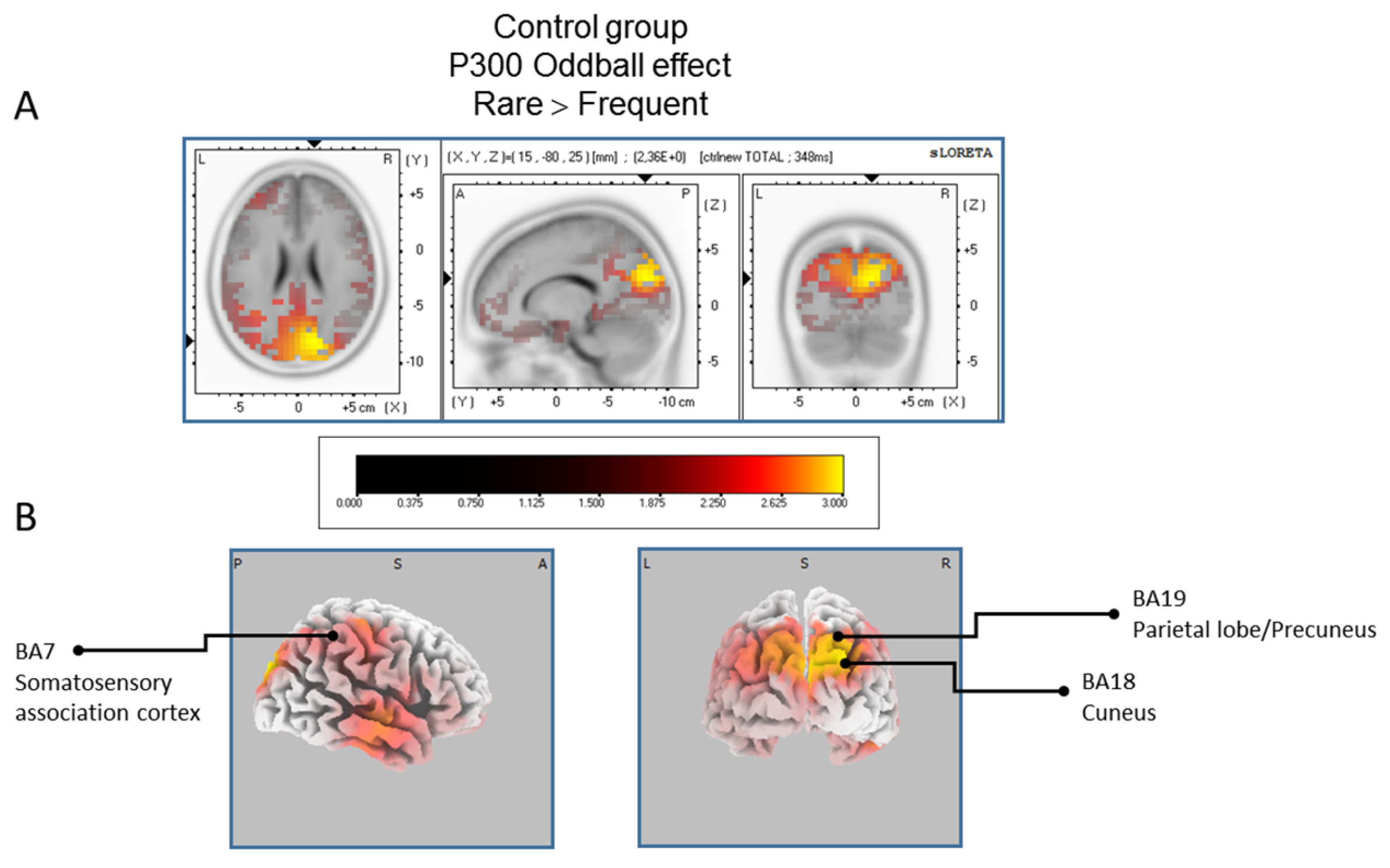
3.3.1. sLORETA Source Localization—The Rare–Frequent Oddball Contrast
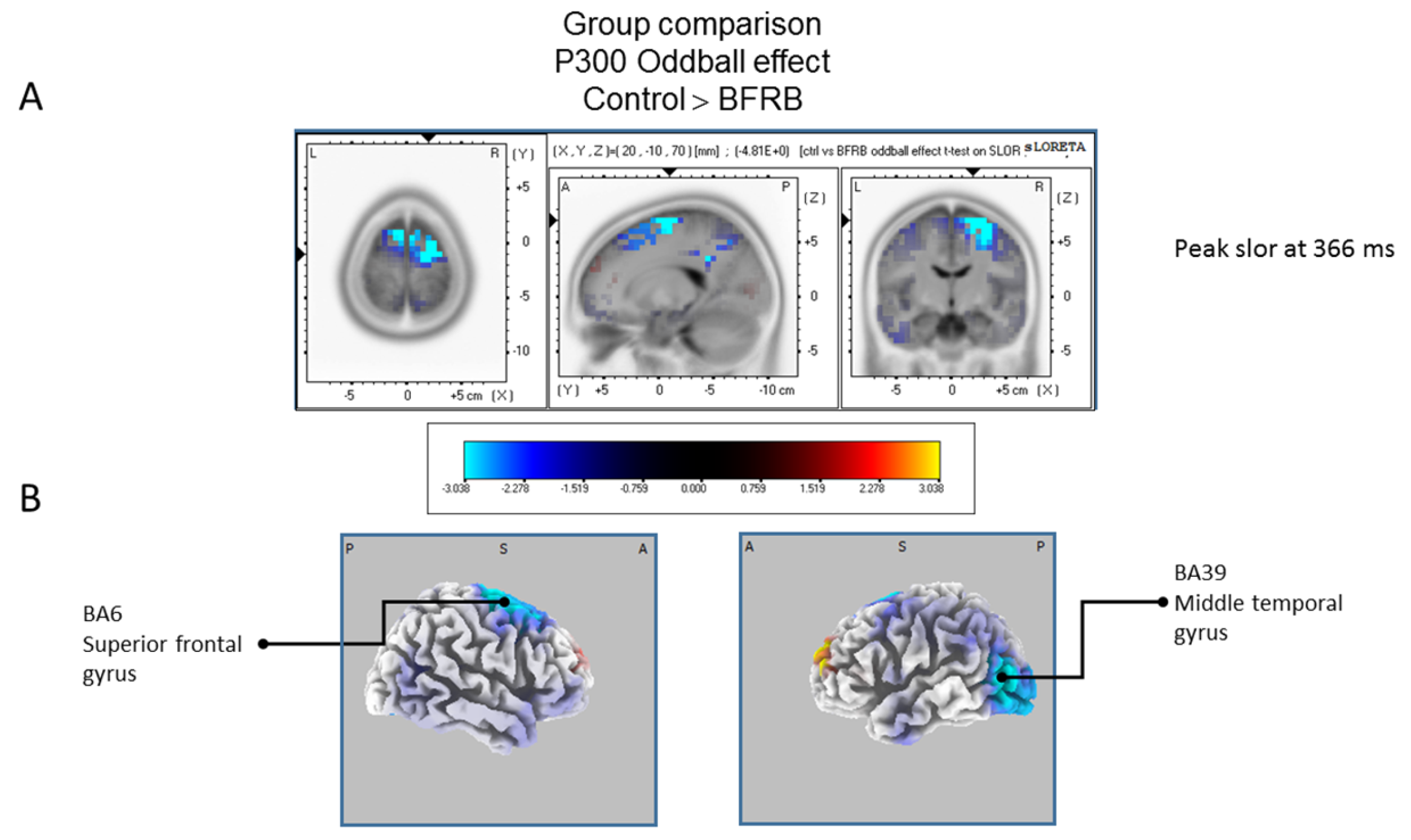
3.3.2. The Group Comparison Contrast
4. Discussion
5. Conclusions
6. Clinical Implications
7. Limitations
Acknowledgments
Author Contributions
Conflicts of Interest
References
- O’Connor, K.; St-Pierre-Delorme, M.E.; Leclerc, J.; Lavoie, M.; Blais, M.T. Meta-cognitions in tourette syndrome, tic disorders, and body-focused repetitive disorder. Can. J. Psychiatry 2014, 59, 417–425. [Google Scholar] [PubMed]
- Morand-Beaulieu, S.; O’Connor, K.P.; Richard, M.; Sauve, G.; Leclerc, J.B.; Blanchet, P.J.; Lavoie, M.E. The Impact of a Cognitive-Behavioral Therapy on Event-Related Potentials in Patients with Tic Disorders or Body-Focused Repetitive Behaviors. Front. Psychiatry 2016, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Christenson, G.A.; Mackenzie, T.B.; Mitchell, J.E. Characteristics of 60 adult chronic hair pullers. Am. J. Psychiatry 1991, 148, 365–370. [Google Scholar] [PubMed]
- Freeman, R.D. Tic disorders and ADHD: Answers from a world-wide clinical dataset on Tourette syndrome. Eur. Child Adolesc. Psychiatry 2007, 16, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.W.; Flessner, C.A.; Franklin, M.E.; Keuthen, N.J.; Goodwin, R.D.; Stein, D.J.; Walther, M.R. Trichotillomania Learning Center-Scientific Advisory, B. The Trichotillomania Impact Project (TIP): Exploring phenomenology, functional impairment, and treatment utilization. J. Clin. Psychiatry 2006, 67, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
- Snorrason, I.; Ricketts, E.J.; Flessner, C.A.; Franklin, M.E.; Stein, D.J.; Woods, D.W. Skin picking disorder is associated with other body-focused repetitive behaviors: Findings from an internet study. Ann. Clin. Psychiatry Off. J. Am. Acad. Clin. Psychiatr. 2012, 24, 292–299. [Google Scholar]
- Leclerc, J.; O’Connor, K.; Forget, J.; Lavoie, M. Behavioural program for managing explosive outbursts in children with Tourette syndrome. J. Dev. Phys. Disabil. 2011, 23, 33–47. [Google Scholar] [CrossRef]
- Roberts, S.; O’Connor, K.; Belanger, C. Emotion regulation and other psychological models for body-focused repetitive behaviors. Clin. Psychol. Rev. 2013, 33, 745–762. [Google Scholar] [CrossRef] [PubMed]
- Reese, H.E.; Scahill, L.; Peterson, A.L.; Crowe, K.; Woods, D.W.; Piacentini, J.; Walkup, J.T.; Wilhelm, S. The premonitory urge to tic: Measurement, characteristics, and correlates in older adolescents and adults. Behav. Ther. 2014, 45, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; O’Connor, K.; Aardema, F.; Belanger, C. The impact of emotions on body-Focused repetitive behaviors: Evidence from a non-treatment-seeking sample. J. Behav. Ther. Exp. Psychiatry 2015, 46, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Snorrason, I.; Smari, J.; Olafsson, R.P. Emotion regulation in pathological skin picking: Findings from a non-treatment seeking sample. J. Behav. Ther. Exp. Psychiatry 2010, 41, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Tucker, B.T.; Woods, D.W.; Flessner, C.A.; Franklin, S.A.; Franklin, M.E. The Skin Picking Impact Project: Phenomenology, interference, and treatment utilization of pathological skin picking in a population-based sample. J. Anxiety Disord. 2011, 25, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, S.; Seri, A.S.; Cavanna, A.E. Premonitory urges and sensorimotor processing in Tourette syndrome. Behav. Neurol. 2013, 27, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Snorrason, I.; Smari, J.; Olafsson, R.P. Motor inhibition, reflection impulsivity, and trait impulsivity in pathological skin picking. Behav. Ther. 2011, 42, 521–532. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, K.P.; Gareau, D.; Blowers, G.H. Changes in construals of tic-producing situations following cognitive and behavioral therapy. Percept. Mot. Sk. 1993, 77, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.D.; Fast, D.K.; Burd, L.; Kerbeshian, J.; Robertson, M.M.; Sandor, P. An international perspective on Tourette syndrome: Selected findings from 3500 individuals in 22 countries. Dev. Med. Child Neurol. 2000, 42, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Ferrão, Y.A.; Miguel, E.; Stein, D.J. Tourette’s syndrome, trichotillomania, and obsessive—Compulsive disorder: How closely are they related? Psychiatry Res. 2009, 170, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Ghanizadeh, A.; Mosallaei, S. Psychiatric disorders and behavioral problems in children and adolescents with Tourette syndrome. Brain Dev. 2009, 31, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Albin, R.L.; Koeppe, R.A.; Bohnen, N.I.; Nichols, T.E.; Meyer, P.; Wernette, K.; Minoshima, S.; Kilbourn, M.R.; Frey, K.A. Increased ventral striatal monoaminergic innervation in Tourette syndrome. Neurology 2003, 61, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Bloch, M.H.; Leckman, J.F.; Zhu, H.; Peterson, B.S. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology 2005, 65, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.R.; Menzies, L.A.; Fineberg, N.A.; Del Campo, N.; Suckling, J.; Craig, K.; Muller, U.; Robbins, T.W.; Bullmore, E.T.; Sahakian, B.J. Grey matter abnormalities in trichotillomania: Morphometric magnetic resonance imaging study. Br. J. Psychiatry J. Ment. Sci. 2008, 193, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y.; Kalanithi, P.S.; Grantz, H.; Schwartz, M.L.; Saper, C.; Leckman, J.F.; Vaccarino, F.M. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J. Comp. Neurol. 2010, 518, 277–291. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, R.L.; Rauch, S.L.; Breiter, H.C.; Grachev, I.D.; Baer, L.; Kennedy, D.N.; Keuthen, N.J.; Savage, C.R.; Manzo, P.A.; Caviness, V.S.; et al. Reduced basal ganglia volumes in trichotillomania measured via morphometric magnetic resonance imaging. Biol. Psychiatry 1997, 42, 39–45. [Google Scholar] [CrossRef]
- Wang, Z.; Maia, T.V.; Marsh, R.; Colibazzi, T.; Gerber, A.; Peterson, B.S. The neural circuits that generate tics in Tourette’s syndrome. Am. J. Psychiatry 2011, 168, 1326–1337. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.J. Basal Ganglia. In The Synaptic Organization of the Brain; Shepherd, G.M., Ed.; Oxford University Press: New York, NY, USA, 2004. [Google Scholar]
- Grachev, I.D. MRI-based morphometric topographic parcellation of human neocortex in trichotillomania. Psychiatry Clin. Neurosci. 1997, 51, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, K.J.; Schooler, C.; Schoenbach, C.; Herscovitch, P.; Chase, T.N.; Braun, A.R. The functional neuroanatomy of Tourette’s syndrome: An FDG PET study III: Functional coupling of regional cerebral metabolic rates. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2002, 27, 92–104. [Google Scholar] [CrossRef]
- Roessner, V.; Overlack, S.; Schmidt-Samoa, C.; Baudewig, J.; Dechent, P.; Rothenberger, A.; Helms, G. Increased putamen and callosal motor subregion in treatment-naive boys with Tourette syndrome indicates changes in the bihemispheric motor network. J. Child Psychol. Psychiatry Allied Discip. 2011, 52, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, U.; Paulus, W.; Rothenberger, A. Decreased motor inhibition in Tourette’s disorder: Evidence from transcranial magnetic stimulation. Am. J. Psychiatry 1997, 154, 1277–1284. [Google Scholar] [PubMed]
- Chang, S.W.; McCracken, J.T.; Piacentini, J.C. Neurocognitive correlates of child obsessive compulsive disorder and Tourette syndrome. J. Clin. Exp. Neuropsychol. 2007, 29, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Schuerholz, L.J.; Baumgardner, T.L.; Singer, H.S.; Reiss, A.L.; Denckla, M.B. Neuropsychological status of children with Tourette’s syndrome with and without attention deficit hyperactivity disorder. Neurology 1996, 46, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Staley, D.; Wand, R.; Shady, G. Tourette disorder: A cross-cultural review. Compr. Psychiatry 1997, 38, 6–16. [Google Scholar] [CrossRef]
- Bohne, A.; Savage, C.R.; Deckersbach, T.; Keuthen, N.J.; Wilhelm, S. Motor inhibition in trichotillomania and obsessive-compulsive disorder. J. Psychiatr. Res. 2008, 42, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.R.; Fineberg, N.A.; Blackwell, A.D.; Robbins, T.W.; Sahakian, B.J. Motor inhibition and cognitive flexibility in obsessive-compulsive disorder and trichotillomania. Am. J. Psychiatry 2006, 163, 1282–1284. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.E.; Odlaug, B.L.; Chamberlain, S.R. A cognitive comparison of pathological skin picking and trichotillomania. J. Psychiatr. Res. 2011, 45, 1634–1638. [Google Scholar] [CrossRef] [PubMed]
- Odlaug, B.L.; Chamberlain, S.R.; Grant, J.E. Motor inhibition and cognitive flexibility in pathologic skin picking. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Morand-Beaulieu, S.; Grot, S.; Lavoie, J.; Leclerc, J.B.; Luck, D.; Lavoie, M.E. The puzzling question of inhibitory control in Tourette syndrome: A meta-analysis. Neurosci. Biobehav. Rev. 2017, 80, 240–262. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.R.; Fineberg, N.A.; Blackwell, A.D.; Clark, L.; Robbins, T.W.; Sahakian, B.J. A neuropsychological comparison of obsessive-compulsive disorder and trichotillomania. Neuropsychologia 2007, 45, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Channon, S.; Flynn, D.; Robertson, M.M. Attention deficits in Gilles de la Tourette syndrome. Neuropsychiatry Neuropsychol. Behav. Neurol. 1992, 5, 170–177. [Google Scholar]
- Stebbins, G.T.; Singh, J.; Weiner, J.; Wilson, R.S.; Goetz, C.G.; Gabrieli, J.D.E. Selective impairments of memory functioning in unmedicated adults with Gilles de la Tourette’s syndrome. Neuropsychology 1995, 9, 329–337. [Google Scholar] [CrossRef]
- Verte, S.; Geurts, H.M.; Roeyers, H.; Oosterlaan, J.; Sergeant, J.A. Executive functioning in children with autism and Tourette syndrome. Dev. Psychopathol. 2005, 17, 415–445. [Google Scholar] [CrossRef] [PubMed]
- Channon, S.; Gunning, A.; Frankl, J.; Robertson, M.M. Tourette’s syndrome (TS): Cognitive performance in adults with uncomplicated TS. Neuropsychology 2006, 20, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.; Channon, S.; Robertson, M.M. Tourette’s syndrome: Performance on tests of behavioural inhibition, working memory and gambling. J. Child Psychol. Psychiatry Allied Discip. 2005, 46, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Eddy, C.M.; Rickards, H.E.; Cavanna, A.E. Executive functions in uncomplicated Tourette syndrome. Psychiatry Res. 2012, 200, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Goudriaan, A.E.; Oosterlaan, J.; de Beurs, E.; van den Brink, W. Neurocognitive functions in pathological gambling: A comparison with alcohol dependence, Tourette syndrome and normal controls. Addiction 2006, 101, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Hershey, T.; Black, K.J.; Hartlein, J.M.; Barch, D.M.; Braver, T.S.; Carl, J.L.; Perlmutter, J.S. Cognitive-pharmacologic functional magnetic resonance imaging in tourette syndrome: A pilot study. Biol. Psychiatry 2004, 55, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Serrien, D.J.; Orth, M.; Evans, A.H.; Lees, A.J.; Brown, P. Motor inhibition in patients with Gilles de la Tourette syndrome: Functional activation patterns as revealed by EEG coherence. Brain J. Neurol. 2005, 128, 116–125. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, K.P.; Lavoie, M.E.; Robert, M.; Stip, E.; Borgeat, F. Brain-behavior relations during motor processing in chronic tic and habit disorder. Cognit. Behav. Neurol. Off. J. Soc. Behav. Cognit. Neurol. 2005, 18, 79–88. [Google Scholar] [CrossRef]
- Roberts, K.; Stanley, E.M.; Franklin, M.E.; Simons, R.F. Decreased response monitoring in individuals with symptoms of trichotillomania. Psychophysiology 2014, 51, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Johannes, S.; Wieringa, B.M.; Nager, W.; Muller-Vahl, K.R.; Dengler, R.; Munte, T.F. Excessive action monitoring in Tourette syndrome. J. Neurol. 2002, 249, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Johannes, S.; Weber, A.; Muller-Vahl, K.R.; Kolbe, H.; Dengler, R.; Munte, T.F. Event-related brain potentials show changed attentional mechanisms in Gilles de la Tourette Syndrome. Eur. J. Neurol. Off. J. Eur. Fed. Neurol. Soc. 1997, 4, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Johannes, S.; Wieringa, B.M.; Mantey, M.; Nager, W.; Rada, D.; Muller-Vahl, K.R.; Emrich, H.M.; Dengler, R.; Munte, T.F.; Dietrich, D. Altered inhibition of motor responses in Tourette Syndrome and Obsessive-Compulsive Disorder. Acta Neurol. Scand. 2001, 104, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Johannes, S.; Wieringa, B.M.; Nager, W.; Muller-Vahl, K.R.; Dengler, R.; Munte, T.F. Electrophysiological measures and dual-task performance in Tourette syndrome indicate deficient divided attention mechanisms. Eur. J. Neurol. Off. J. Eur. Fed. Neurol. Soc. 2001, 8, 253–260. [Google Scholar] [CrossRef]
- Johannes, S.; Wieringa, B.M.; Nager, W.; Rada, D.; Muller-Vahl, K.R.; Emrich, H.M.; Dengler, R.; Munte, T.F.; Dietrich, D. Tourette syndrome and obsessive-compulsive disorder: Event-related brain potentials show similar mechanisms (correction of mechansims) of frontal inhibition but dissimilar target evaluation processes. Behav. Neurol. 2003, 14, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Eichele, H.; Eichele, T.; Bjelland, I.; Hovik, M.F.; Sorensen, L.; van Wageningen, H.; Worren, M.K.; Hugdahl, K.; Plessen, K.J. Performance Monitoring in Medication-Naive Children with Tourette Syndrome. Front. Neurosci. 2016, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Shephard, E.; Jackson, G.M.; Groom, M.J. Electrophysiological correlates of reinforcement learning in young people with Tourette syndrome with and without co-occurring ADHD symptoms. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2016, 51, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Shephard, E.; Jackson, G.M.; Groom, M.J. The effects of co-occurring ADHD symptoms on electrophysiological correlates of cognitive control in young people with Tourette syndrome. J. Neuropsychol. 2016, 10, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R., Jr.; Donchin, E. On how P300 amplitude varies with the utility of the eliciting stimuli. Electroencephalogr. Clin. Neurophysiol. 1978, 44, 424–437. [Google Scholar] [CrossRef]
- Huettel, S.A.; McCarthy, G. What is odd in the oddball task? Prefrontal cortex is activated by dynamic changes in response strategy. Neuropsychologia 2004, 42, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Eddy, C.M.; Rizzo, R.; Cavanna, A.E. Neuropsychological aspects of Tourette syndrome: A review. J. Psychosom. Res. 2009, 67, 503–513. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, K.P.; Lavoie, M.E.; Desaulniers, B.; Audet, J.-S. Cognitive Psychophysiological Treatment of Bodily-Focused Repetitive Behaviours in Adults: An Open Trial. J. Clin. Psychol. 2017, in press. [Google Scholar]
- O’Connor, K.P.; Lavoie, M.; Blanchet, P.; St-Pierre-Delorme, M.E. Evaluation of a cognitive psychophysiological model for management of tic disorders: An open trial. Br. J. Psychiatry 2015, 209, 76–83. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, K.P. Cognitive Behavioral Management of Tic Disorders; John Wiley & Sons: Chichester, UK, 2005. [Google Scholar]
- O’Connor, K.P.; Brault, M.; Robillard, S.; Loiselle, J.; Borgeat, F.; Stip, E. Evaluation of a cognitive-behavioural program for the management of chronic tic and habit disorders. Behav. Res. Ther. 2001, 39, 667–681. [Google Scholar] [CrossRef]
- Hyler, S.E. The Personality Diagnostic Questionnaire 4+; New York State Psychiatric Institute: New York, NY, USA, 1994. [Google Scholar]
- Raven, J.C. Progressive Matrices: A Perceptual Test of Intelligence; H.K. Lewis & Co.: London, UK, 1938. [Google Scholar]
- Harcherik, D.F.; Leckman, J.F.; Detlor, J.; Cohen, D.J. A new instrument for clinical studies of Tourette’s syndrome. J. Am. Acad. Child Psychiatry 1984, 23, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Leckman, J.F.; Riddle, M.A.; Hardin, M.T.; Ort, S.I.; Swartz, K.L.; Stevenson, J.; Cohen, D.J. The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. J. Am. Acad. Child Adoles. Psychiatry 1989, 28, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Storch, E.A.; Murphy, T.K.; Geffken, G.R.; Sajid, M.; Allen, P.; Roberti, J.W.; Goodman, W.K. Reliability and validity of the Yale Global Tic Severity Scale. Psychol. Assess. 2005, 17, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Walkup, J.T.; Rosenberg, L.A.; Brown, J.; Singer, H.S. The validity of instruments measuring tic severity in Tourette’s syndrome. J. Am. Acad. Child Adolesc. Psychiatry 1992, 31, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Keuthen, N.J.; O’Sullivan, R.L.; Ricciardi, J.N.; Shera, D.; Savage, C.R.; Borgmann, A.S.; Jenike, M.A.; Baer, L. The Massachusetts General Hospital (MGH) Hairpulling Scale: 1. development and factor analyses. Psychother. Psychosom. 1995, 64, 141–145. [Google Scholar] [CrossRef] [PubMed]
- First, M.B.; Spitzer, R.L.; Gibbon, M.; Williams, J.B.W. Structured Clinical Interview for DSM-IV-TR Axis I Disorders; Research Version, Patient Edition; Biometrics Research: New York, NY, USA, 2002. [Google Scholar]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Thordarson, D.S.; Radomsky, A.S.; Rachman, S.; Shafran, R.; Sawchuk, C.N.; Ralph Hakstian, A. The Vancouver Obsessional Compulsive Inventory (VOCI). Behav. Res. Ther. 2004, 42, 1289–1314. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- American Electroencephalography Society. Guideline thirteen: Guidelines for standard electrode position nomenclature. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 1994, 11, 111–113. [Google Scholar]
- Gratton, G.; Coles, M.G.; Donchin, E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983, 55, 468–484. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): Technical details. Methods Find. Exp. Clin. Pharmacol. 2002, 24, 5–12. [Google Scholar] [PubMed]
- Pascual-Marqui, R.D.; Lehmann, D.; Koenig, T.; Kochi, K.; Merlo, M.C.; Hell, D.; Koukkou, M. Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic-naive, first-episode, productive schizophrenia. Psychiatry Res. 1999, 90, 169–179. [Google Scholar] [CrossRef]
- Mazziotta, J.; Toga, A.; Evans, A.; Fox, P.; Lancaster, J.; Zilles, K.; Woods, R.; Paus, T.; Simpson, G.; Pike, B.; et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos. Trans. Royal Soc. Lond. Ser. B 2001, 356, 1293–1322. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, J.L.; Woldorff, M.G.; Parsons, L.M.; Liotti, M.; Freitas, C.S.; Rainey, L.; Kochunov, P.V.; Nickerson, D.; Mikiten, S.A.; Fox, P.T. Automated Talairach atlas labels for functional brain mapping. Hum. Brain Mapp. 2000, 10, 120–131. [Google Scholar] [CrossRef]
- Talairach, J.; Tournoux, P. Co-planar Stereotaxic Atlas of the Human Brain; Thieme: New York, NY, USA, 1988. [Google Scholar]
- Nichols, T.E.; Holmes, A.P. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum. Brain Mapp. 2002, 15, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.; Amitai, Y.; Connors, B.W. Intrinsic oscillations of neocortex generated by layer 5 pyramidal neurons. Science 1991, 251, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Haalman, I.; Vaadia, E. Dynamics of neuronal interactions: Relation to behavior, firing rates, and distance between neurons. Hum. Brain Mapp. 1997, 5, 249–253. [Google Scholar] [CrossRef]
- Friston, K.J. Statistical parametric mapping and other analysis of functional imaging data. In Brain Mapping; Toga, A.W., Mazziotta, J.C., Eds.; Academic Press: Amsterdam, The Netherlands, 2002; pp. 363–385. [Google Scholar]
- Brodmann, K.; Gary, L.J. Brodmann’s Localisation in the Cerebral Cortex: The Principles of Comparative Localisation in the Cerebral Cortex Based on Cytoarchitectonics; Springer: New York, NY, USA, 2006. [Google Scholar]
- Holmes, C.J.; Hoge, R.; Collins, L.; Woods, R.; Toga, A.W.; Evans, A.C. Enhancement of MR images using registration for signal averaging. J. Comput. Assist. Tomogr. 1998, 22, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Donchin, E.; Coles, E.M. Is the P300 component a manifestation of context updating? Behav. Brain Sci. 1988, 11, 357–374. [Google Scholar] [CrossRef]
- Kutas, M.; McCarthy, G.; Donchin, E. Augmenting mental chronometry: The P300 as a measure of stimulus evaluation time. Science 1977, 197, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Verleger, R. On the utility of P3 latency as an index of mental chronometry. Psychophysiology 1997, 34, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.F.; Wickens, C.D.; Donchin, E. An analysis of the processing requirements of a complex perceptual-motor task. Hum. Factors 1983, 25, 597–621. [Google Scholar] [PubMed]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Neuropsychology of P300. In Handbook of Event-Related Potential Components; Luck, S.J., Kappenman, E.S., Eds.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Li, Y.; Wang, L.Q.; Hu, Y. Localizing P300 generators in high-density event- related potential with fMRI. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2009, 15, MT47–MT53. [Google Scholar]
- Warbrick, T.; Reske, M.; Shah, N.J. Do EEG paradigms work in fMRI? Varying task demands in the visual oddball paradigm: Implications for task design and results interpretation. NeuroImage 2013, 77, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Mink, J.W. Neurobiology of basal ganglia and Tourette syndrome: Basal ganglia circuits and thalamocortical outputs. Adv. Neurol. 2006, 99, 89–98. [Google Scholar] [PubMed]
- O’Connor, K.P. A cognitive-behavioral/psychophysiological model of tic disorders. Behav. Res. Ther. 2002, 40, 1113–1142. [Google Scholar] [CrossRef]
- Filipowicz, A.; Anderson, B.; Danckert, J. Adapting to change: The role of the right hemisphere in mental model building and updating. Can. J. Exp. Psychol. Rev. Can. Psychol. Exp. 2016, 70, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Andreou, C.; Leicht, G.; Popescu, V.; Pogarell, O.; Mavrogiorgou, P.; Rujescu, D.; Giegling, I.; Zaudig, M.; Juckel, G.; Hegerl, U.; et al. P300 in obsessive-compulsive disorder: Source localization and the effects of treatment. J. Psychiatr. Res. 2013, 47, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhong, M.; Zhu, X.; Lei, H.; Dong, J.; Zhou, C.; Liu, W. An attentional inhibitory deficit for irrelevant information in obsessive-compulsive disorder: Evidence from ERPs. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2014, 94, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Ischebeck, M.; Endrass, T.; Simon, D.; Kathmann, N. Auditory novelty processing is enhanced in obsessive-compulsive disorder. Depression Anxiety 2011, 28, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Kivircik, B.B.; Yener, G.G.; Alptekin, K.; Aydin, H. Event-related potentials and neuropsychological tests in obsessive-compulsive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 601–606. [Google Scholar] [CrossRef]
- Mavrogiorgou, P.; Juckel, G.; Frodl, T.; Gallinat, J.; Hauke, W.; Zaudig, M.; Dammann, G.; Moller, H.J.; Hegerl, U. P300 subcomponents in obsessive-compulsive disorder. J. Psychiatr. Res. 2002, 36, 399–406. [Google Scholar] [CrossRef]
- Miyata, A.; Matsunaga, H.; Kiriike, N.; Iwasaki, Y.; Takei, Y.; Yamagami, S. Event-related potentials in patients with obsessive-compulsive disorder. Psychiatry Clin. Neurosci. 1998, 52, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Towey, J.; Bruder, G.; Hollander, E.; Friedman, D.; Erhan, H.; Liebowitz, M.; Sutton, S. Endogenous event-related potentials in obsessive-compulsive disorder. Biol. Psychiatry 1990, 28, 92–98. [Google Scholar] [CrossRef]
- Van Woerkom, T.C.; Roos, R.A.; van Dijk, J.G. Altered attentional processing of background stimuli in Gilles de la Tourette syndrome: A study in auditory event-related potentials evoked in an oddball paradigm. Acta Neurol. Scand. 1994, 90, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Comings, D.E. Clinical and molecular genetics of ADHD and Tourette syndrome. Two related polygenic disorders. Ann. N. Y. Acad. Sci. 2001, 931, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Pringsheim, T.; Doja, A.; Gorman, D.; McKinlay, D.; Day, L.; Billinghurst, L.; Carroll, A.; Dion, Y.; Luscombe, S.; Steeves, T.; et al. Canadian guidelines for the evidence-based treatment of tic disorders: Pharmacotherapy. Can. J. Psychiatry 2012, 57, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.M.; Tanaka, J.W.; Holroyd, C.B. What can topology changes in the oddball N2 reveal about underlying processes? Neuroreport 2011, 22, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Kok, A. Overlap between P300 and movement-related-potentials: A response to Verleger. Biol. Psychol. 1988, 27, 51–58. [Google Scholar] [CrossRef]
- Morand-Beaulieu, S.; O’Connor, K.P.; Sauvé, G.; Blanchet, P.J.; Lavoie, M.E. Cognitive-behavioral therapy induces sensorimotor and specific electrocortical changes in chronic tic and Tourette’s disorder. Neuropsychologia 2015, 79 Pt B, 310–321. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistic Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]

| Tic Disorders (A) (n = 12) | BFRB Disorders (B) (n = 12) | Control (C) (n = 15) | ANOVA 1 | |
|---|---|---|---|---|
| Age (years old) | 33 (9) | 30 (8) | 31 (9) | ns (p = 0.71; ŋ2 < 0.001) |
| Sex (M/F) | 7/5 | 1/11 | 5/10 | A vs. B (p = 0.04; ŋ2 = 0.28) |
| Schooling (years) | 14 (2) | 15 (2) | 14 (2) | ns (p = 0.37; ŋ2 < 0.07) |
| Non-verbal intelligence (Raven Progressive Matrices) | 90 (9) | 74 (18) | 81 (18) | ns (p = 0.06; ŋ2 = 0.15) |
| Anxiety (BAI) | 6 (6) | 11 (8) | 5 (5) | B vs. C (p = 0.03; ŋ2 = 0.18) |
| Depression (BDI) | 11 (12) | 10 (5) | 4 (4) | ns (p = 0.08; ŋ2 = 0.13) |
| OCS (VOCI) | 25 (21) | 24 (15) | - | ns (p = 0.90; Cohen’s d = 0.06) |
| Tic severity (YGTSS) | 35 (16) | 25 (7) | - | ns (p = 0.06; Cohen’s d = 0.82) |
| BFRB severity (MGH-HPS) | - | 14 (3) | - | - |
| BFRB severity (MGH-adapted for trichotillomania) | - | 13 (6) | - | - |
| BFRB severity (MGH-adapted for onycophagia) | - | 12 (5) | - | - |
| BFRB severity (MGH-adapted for skin-picking) | - | 12 (5) | - | - |
| BFRB severity (MGH-adapted for skin-scratching) | - | 15 (5) | - | - |
| Brain Regions | BA | Hemisphere | Lobe | Coordinates (X, Y, Z) | t-Values | Effect Sizes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI | Talairach MNI | Max Voxel Value | No of Activated Voxels | |||||||||
| Precentral/Medial/Middle Frontal/Sub gyral | 6 | L/R | Frontal | −20 | −5 | 70 | −20 | −2 | 65 | 3.72 * | 291 | 0.75 |
| Cingulate Gyrus | 24 | L | Limbic | −15 | −5 | 50 | −15 | −3 | 46 | 3.03 * | 91 | 0.68 |
| Paracentral Lobule | 31 | R | Limbic | 10 | −15 | 50 | 10 | −12 | 47 | 2.90 * | 35 | 0.66 |
| Superior Frontal Gyrus | 10 | L | Frontal | −30 | 50 | 30 | −30 | 50 | 25 | 2.63 * | 53 | 0.62 |
| Superior and medial Frontal Gyrus | 9 | L | Frontal | −10 | 50 | 35 | −10 | 50 | 30 | 2.58 * | 73 | 0.62 |
| Medial Frontal Gyrus/Cingulate Gyrus | 32 | L | Frontal | −5 | 5 | 50 | −5 | 7 | 46 | 2.57 * | 19 | 0.61 |
| Superior Occipital Gyrus/Cuneus/Precuneus | 19 | R | Occipital | 35 | −85 | 30 | 35 | −81 | 32 | 2.41 * | 105 | 0.59 |
| Precentral Gyrus | 4 | R | Frontal | 45 | −15 | 55 | 45 | −12 | 51 | 2.41 * | 24 | 0.59 |
| Postcentral Gyrus | 3 | R | Parietal | 50 | −15 | 55 | 50 | −12 | 51 | 2.37 * | 11 | 0.58 |
| Angular Gyrus | 39 | R | Temporal | 35 | −80 | 30 | 35 | −76 | 31 | 2.33 * | 3 | 0.58 |
| Paracentral Lobule | 5 | M | Frontal | 0 | −30 | 55 | 0 | −27 | 52 | 2.32 * | 7 | 0.57 |
| Cingulate Gyrus | 23 | M | Limbic | 0 | −15 | 35 | 0 | −13 | 33 | 2.32 * | 11 | 0.57 |
| Superior Frontal Gyrus | 8 | L | Frontal | −20 | 45 | 45 | −20 | 46 | 39 | 2.25 * | 7 | 0.56 |
| Cuneus | 18 | R | Occipital | 20 | −85 | 25 | 20 | −81 | 27 | 2.25 * | 20 | 0.56 |
| Somatosensory association cortex | 7 | R | Occipital | 15 | −80 | 30 | 15 | −76 | 31 | 2.19 * | 31 | 0.55 |
| Brain Regions | BA | Hemisphere | Lobe | Coordinates (X, Y, Z) | t-Values | Effect Sizes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI | Talairach MNI | Max Voxel Values | No of Activated Voxels | |||||||||
| Postcentral Gyrus/Precentral Gyrus | 43 | R | Parietal | 65 | −15 | 20 | 64 | −14 | 19 | 4.13 ** | 12 | 0.78 |
| Inferior Parietal/Postcentral Gyrus | 40 | R | Parietal | 65 | −25 | 25 | 64 | −23 | 24 | 4.11 ** | 121 | 0.78 |
| Postcentral Gyrus | 3 | R | Parietal | 65 | −15 | 25 | 64 | −13 | 24 | 4.05 ** | 25 | 0.77 |
| Transverse Temporal/Superior Temporal | 42 | R | Temporal | 60 | −10 | 15 | 59 | −9 | 14 | 4.02 ** | 20 | 0.77 |
| Postcentral Gyrus | 1 | R | Parietal | 65 | −20 | 30 | 64 | −18 | 29 | 3.97 ** | 7 | 0.77 |
| Precentral Gyrus | 4 | R | Frontal | 60 | −10 | 25 | 59 | −9 | 23 | 3.97 ** | 21 | 0.77 |
| Postcentral Gyrus | 2 | R | Parietal | 60 | −20 | 30 | 59 | −18 | 29 | 3.92 ** | 40 | 0.76 |
| Precentral Gyrus | 6 | R | Frontal | 50 | −5 | 20 | 50 | −4 | 19 | 3.88 ** | 42 | 0.76 |
| Superior Temporal | 22 | R | Temporal | 65 | −5 | 15 | 64 | −4 | 14 | 3.85 ** | 55 | 0.76 |
| Inferior Frontal Gyrus | 44 | R | Frontal | 50 | 0 | 20 | 50 | 1 | 18 | 3.84 ** | 29 | 0.76 |
| Inferior Frontal Gyrus | 9 | R | Frontal | 50 | 0 | 25 | 50 | 1 | 23 | 3.76 ** | 51 | 0.75 |
| Transverse Temporal Gyrus | 41 | R | Temporal | 55 | −20 | 10 | 54 | −19 | 10 | 3.55 ** | 15 | 0.73 |
| Inferior Frontal Gyrus | 45 | R | Frontal | 60 | 10 | 20 | 59 | 11 | 18 | 3.54 ** | 33 | 0.73 |
| Insula | 13 | R | Sub-lobar | 40 | 0 | 20 | 40 | 1 | 18 | 3.53 ** | 66 | 0.73 |
| Brain Regions | BA | Hemisphere | Lobe | Coordinates (X, Y, Z) | t-Values | Effect Sizes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI | Talairach MNI | Max Voxel Values | No of Activated Voxels | |||||||||
| Cuneus | 18 | R | Occipital | 15 | −80 | 25 | 15 | −76 | 27 | 2.36 ** | 40 | 0.53 |
| Somatosensory association cortex | 7 | R | Occipital/Parietal | 15 | −80 | 30 | 15 | −76 | 31 | 2.34 ** | 44 | 0.53 |
| Precuneus | 19 | R | Occipital | 25 | −90 | 30 | 25 | −86 | 32 | 2.33 ** | 76 | 0.53 |
| Precuneus | 31 | R | Occipital/Parietal | 10 | −75 | 25 | 10 | −72 | 27 | 2.32 ** | 20 | 0.53 |
| Postcentral Gyrus | 3 | R | Parietal | 45 | −20 | 50 | 45 | −17 | 47 | 2.09 ** | 4 | 0.49 |
| Precentral Gyrus | 4 | R | Parietal | 45 | −20 | 45 | 45 | −17 | 42 | 2.05 ** | 2 | 0.48 |
| Middle Temporal Gyrus | 21 | R | Temporal | 60 | −15 | −10 | 59 | −15 | -8 | 2.06 ** | 5 | 0.47 |
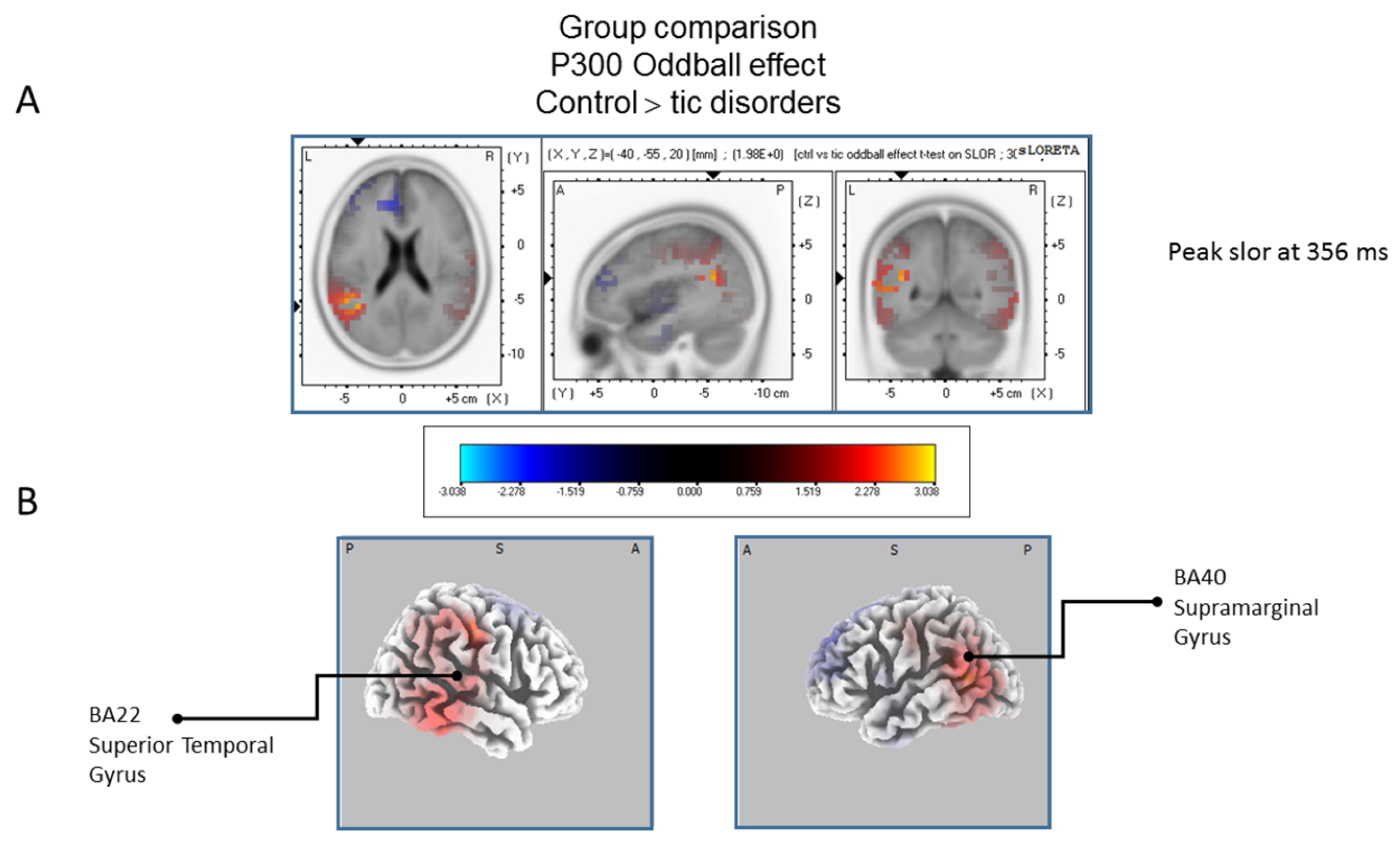
| Brain Regions | BA | Hemisphere | Lobe | Coordinates (X, Y, Z) | t-Values | Effect Sizes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI | Talairach MNI | Max Voxel Value | No of Activated Voxels | |||||||||
| Superior Frontal Gyrus | 6 | R | Frontal | 20 | −10 | 70 | 20 | −6 | 65 | −4.81 ** | 128 | 0.69 |
| Middle Temporal Gyrus | 39 | L | Temporal | −50 | −75 | 20 | −50 | −72 | 22 | −3.53 * | 27 | 0.57 |
| Middle Frontal Gyrus | 8 | R | Frontal | 30 | 20 | 45 | 30 | 21 | 40 | −3.28 * | 32 | 0.54 |
| Middle Occipital Gyrus | 19 | L | Occipital | −55 | −75 | 5 | −54 | −72 | 8 | −3.27 * | 42 | 0.54 |
| Middle Temporal Gyrus | 22 | L | Temporal | −40 | −60 | 15 | −40 | −57 | 17 | −3.27 * | 2 | 0.54 |
| Middle Occipital Gyrus | 37 | L | Occipital | −55 | −75 | 0 | −54 | −73 | 4 | −3.16 * | 15 | 0.53 |
| Superior Frontal Gyrus | 10 | L | Frontal | −10 | 60 | 30 | −10 | 60 | 25 | 3.13 * | 8 | 0.52 |
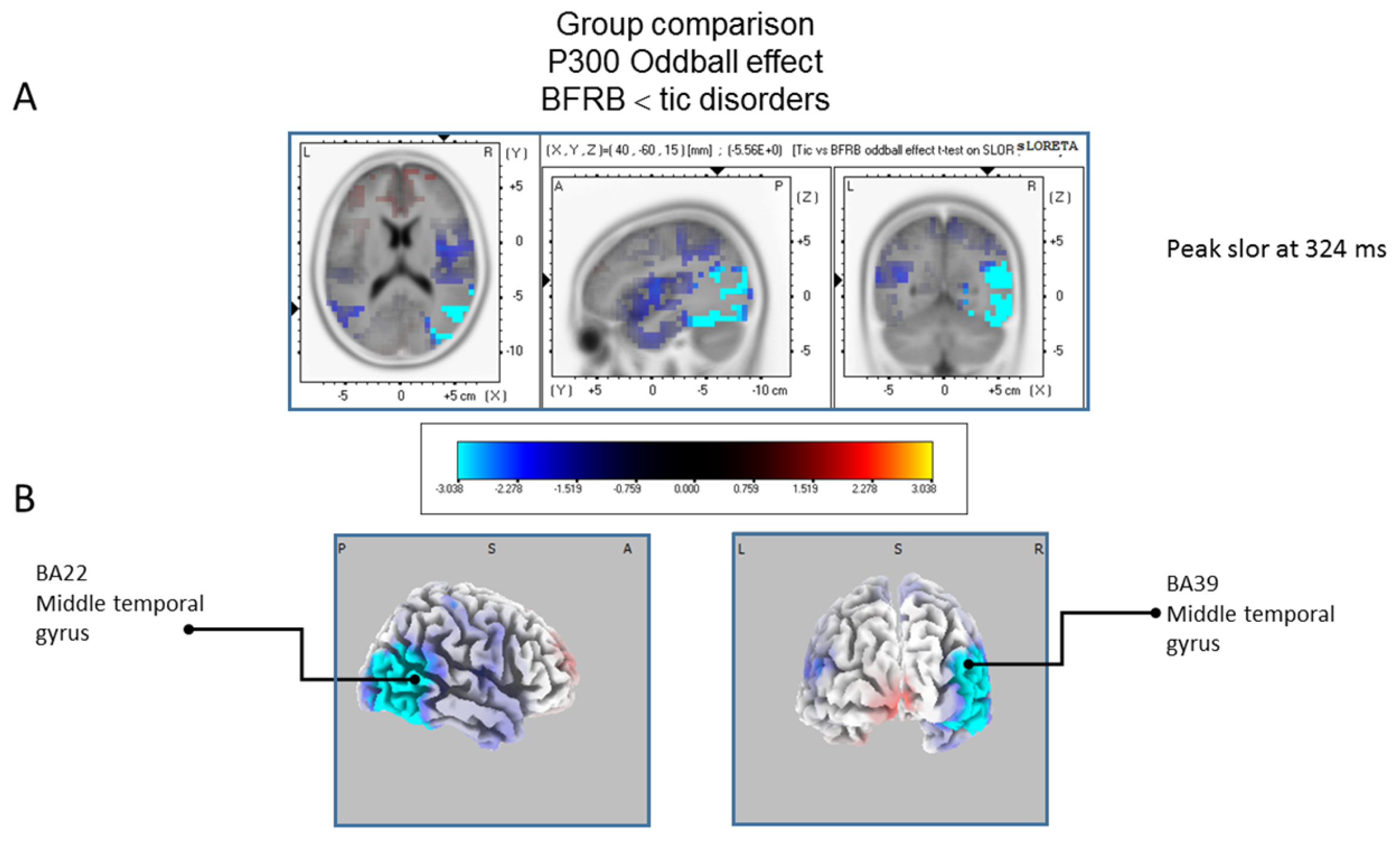
| Brain Regions | BA | Hemisphere | Lobe | Coordinates X, Y, Z | t-Values | Effect Sizes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI | Talairach | Max Voxel Value | No of Activated Voxels | |||||||||
| Middle Temporal Gyrus | 22 | R | Temporal | 40 | −60 | 15 | 40 | −57 | 17 | −5.56 ** | 19 | 0.75 |
| Middle Temporal Gyrus | 39 | R | Temporal | 45 | −65 | 20 | 45 | −62 | 22 | −5.34 ** | 36 | 0.74 |
| Fusiform Gyrus | 37 | R | Temporal | 35 | −50 | −15 | 35 | −49 | -10 | −4.86 ** | 85 | 0.70 |
| Parahippocampal Gyrus | 19 | R | Limbic | 30 | −50 | −5 | 30 | −49 | −2 | −4.81 ** | 59 | 0.70 |
| Inferior Temporal Gyrus | 20 | R | Temporal | 50 | −50 | −20 | 50 | −49 | −14 | −4.39 ** | 13 | 0.67 |
| Supramarginal Gyrus | 40 | R | Temporal | 60 | −55 | 20 | 59 | −52 | 21 | −4.37 ** | 7 | 0.67 |
| Parahippocampal Gyrus | 30 | R | Limbic | 25 | −55 | 0 | 25 | −53 | 3 | −4.29 ** | 3 | 0.66 |
| Middle Temporal Gyrus | 21 | R | Temporal | 60 | −60 | 0 | 59 | −58 | 3 | −4.10 ** | 10 | 0.64 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sauvé, G.; Morand-Beaulieu, S.; O’Connor, K.P.; Blanchet, P.J.; Lavoie, M.E. P300 Source Localization Contrasts in Body-Focused Repetitive Behaviors and Tic Disorders. Brain Sci. 2017, 7, 76. https://doi.org/10.3390/brainsci7070076
Sauvé G, Morand-Beaulieu S, O’Connor KP, Blanchet PJ, Lavoie ME. P300 Source Localization Contrasts in Body-Focused Repetitive Behaviors and Tic Disorders. Brain Sciences. 2017; 7(7):76. https://doi.org/10.3390/brainsci7070076
Chicago/Turabian StyleSauvé, Geneviève, Simon Morand-Beaulieu, Kieron P. O’Connor, Pierre J. Blanchet, and Marc E. Lavoie. 2017. "P300 Source Localization Contrasts in Body-Focused Repetitive Behaviors and Tic Disorders" Brain Sciences 7, no. 7: 76. https://doi.org/10.3390/brainsci7070076







