Selective Serotonin Reuptake Inhibitors for Treating Neurocognitive and Neuropsychiatric Disorders Following Traumatic Brain Injury: An Evaluation of Current Evidence
Abstract
:1. Introduction
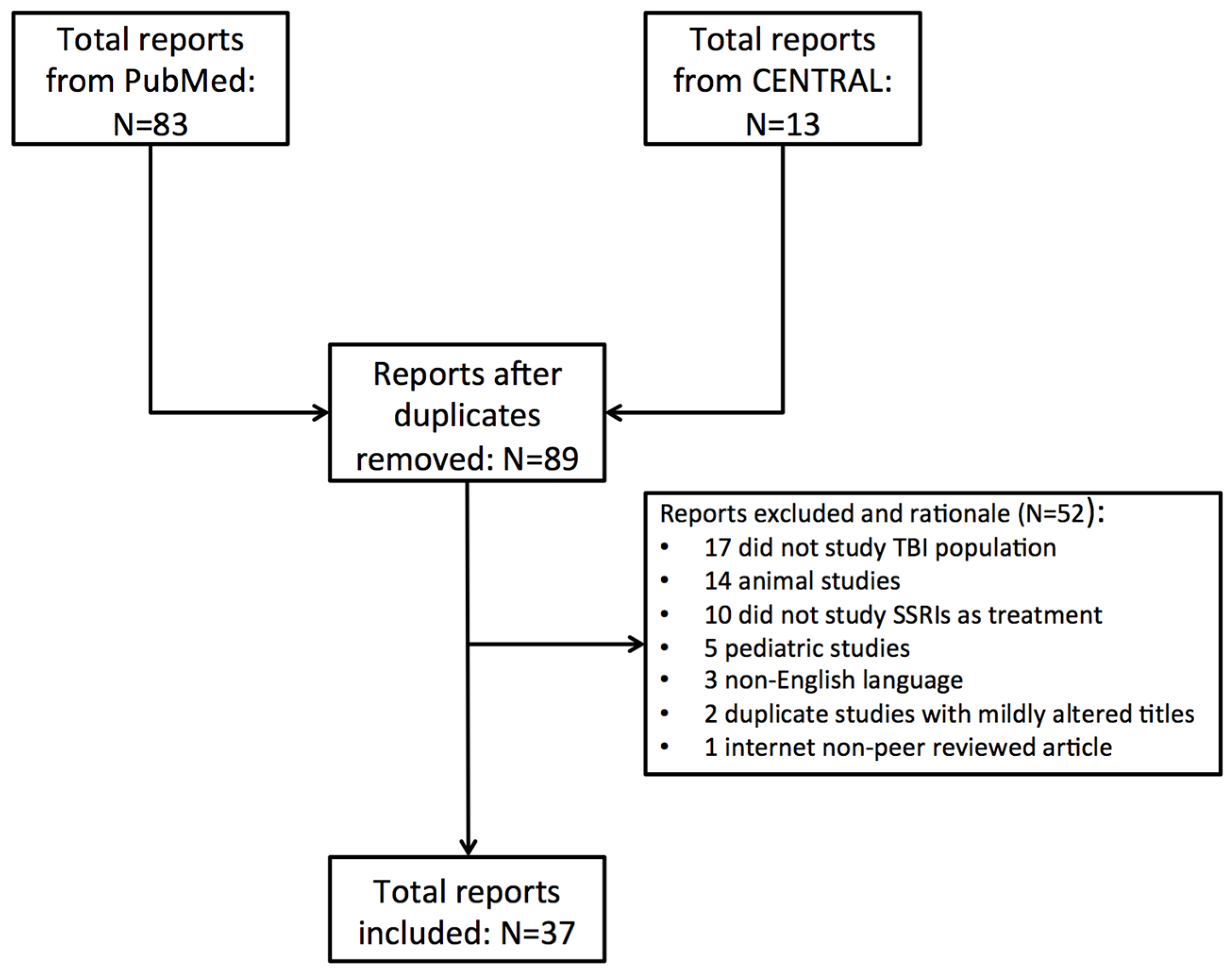
2. Methods
2.1. Study Selection
2.2. Statistical Analysis
3. Results
3.1. Depression
3.1.1. Background and Pathophysiology
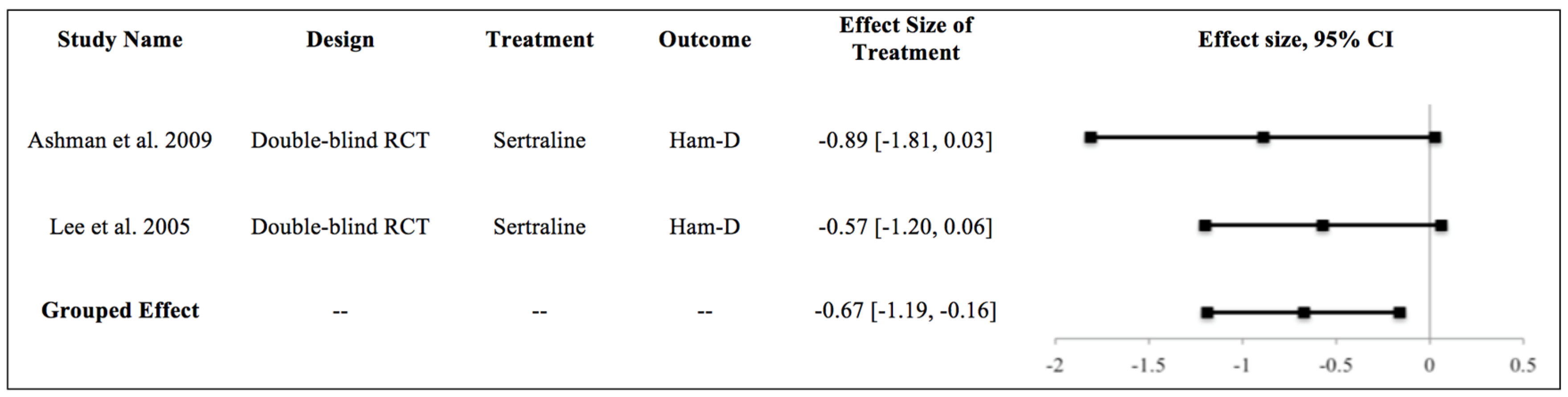
3.1.2. Evidence Synthesis: Sertraline
3.1.3. Meta-Analysis
3.1.4. Evidence Synthesis: Citalopram
3.1.5. Evidence Synthesis: Fluoxetine
3.1.6. Commentary
3.2. Cognition
3.2.1. Background and Pathophysiology
3.2.2. Evidence Synthesis
3.2.3. Commentary
3.3. Post-Concussive Symptoms/Syndrome
3.3.1. Background and Pathophysiology
3.3.2. Evidence Synthesis
3.3.3. Commentary
3.4. Sleep Disturbance
3.4.1. Background and Pathophysiology
3.4.2. Evidence Synthesis
3.4.3. Commentary
3.5. Anxiety Spectrum Disorders
3.5.1. Background and Pathophysiology
3.5.2. Evidence Synthesis
3.5.3. Obsessive-Compulsive Disorder (OCD)
3.5.4. Panic Disorder
3.5.5. Emotional Incontinence
3.5.6. Commentary
3.6. Side Effects of SSRI Administration
3.6.1. Depression-Indicated Studies
3.6.2. Akathisia
3.6.3. Sexual Dysfunction
3.7. Post-Traumatic Stress Disorder (PTSD): Commentary and Implications
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Hyder, A.A.; Wunderlich, C.A.; Puvanachandra, P.; Gururaj, G.; Kobusingye, O.C. The impact of traumatic brain injuries: A global perspective. NeuroRehabilitation 2007, 22, 341–353. [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention. Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation; National Center for Injury Prevention and Control, Division of Unintentional Injury Prevention: Atlanta, GA, USA, 2015.
- Corrigan, J.D.; Selassie, A.W.; Orman, J.A.L. The Epidemiology of Traumatic Brain Injury. J. Head Trauma Rehabil. 2010, 25, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Bruns, J., Jr.; Hauser, W.A. The epidemiology of traumatic brain injury: A review. Epilepsia 2003, 44 (Suppl. S10), 2–10. [Google Scholar] [CrossRef] [PubMed]
- Kay, T.; Harrington, D.E.; Adams, R.; Anderson, T.; Berrol, S.; Cicerone, K.; Dahlberg, C.; Gerber, D.; Goka, R.; Harley, P.; Hilt, J.; et al. Definition of mild traumatic brain injury. J. Head Trauma Rehabil. 1993, 8, 86–87. [Google Scholar] [CrossRef]
- Elgmark Andersson, E.; Emanuelson, I.; Björklund, R.; Stålhammar, D.A. Mild traumatic brain injuries: The impact of early intervention on late sequelae. A randomized controlled trial. Acta Neurochir. 2007, 149, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, W.H.; Merrett, J.D.; McDonald, J.R. Symptoms at one year following concussion from minor head injuries. Injury 1979, 10, 225–230. [Google Scholar] [CrossRef]
- Zaloshnja, E.; Miller, T.; Langlois, J.A.; Selassie, A.W. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J. Head Trauma Rehabil. 2008, 23, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Koponen, S.; Taiminen, T.; Portin, R.; Himanen, L.; Isoniemi, H.; Heinonen, H.; Hinkka, S.; Tenovuo, O. Axis I and II psychiatric disorders after traumatic brain injury: A 30-year follow-up study. Am. J. Psychiatry 2002, 159, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Koponen, S.; Taiminen, T.; Hiekkanen, H.; Tenovuo, O. Axis I and II psychiatric disorders in patients with traumatic brain injury: A 12-month follow-up study. Brain Inj. 2011, 25, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.L.; Fornal, C.A. Activity of Brain Serotonergic Neurons in Relation to Physiology and Behavior. In Handbook of Behavioral Neuroscience; Academic Press: Cambridge, MA, USA, 2010; pp. 153–162. [Google Scholar]
- Sukonick, D.L.; Pollock, B.G.; Sweet, R.A.; Mulsant, B.H.; Rosen, J.; Klunk, W.E.; Kastango, K.B.; DeKosky, S.T.; Ferrell, R.E. The 5-HTTPR*S/*L polymorphism and aggressive behavior in Alzheimer disease. Arch. Neurol. 2001, 58, 1425–1428. [Google Scholar] [CrossRef] [PubMed]
- Zanardi, R.; Serretti, A.; Rossini, D.; Franchini, L.; Cusin, C.; Lattuada, E.; Dotoli, D.; Smeraldi, E. Factors affecting fluvoxamine antidepressant activity: Influence of pindolol and 5-HTTLPR in delusional and nondelusional depression. Biol. Psychiatry 2001, 50, 323–330. [Google Scholar] [CrossRef]
- Markianos, M.; Seretis, A.; Kotsou, A.; Christopoulos, M. CSF neurotransmitter metabolites in comatose head injury patients during changes in their clinical state. Acta Neurochir. 1996, 138, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Tsuiki, K.; Yamamoto, Y.L.; Diksic, M. Effect of acute fluoxetine treatment on the brain serotonin synthesis as measured by the alpha-methyl-L-tryptophan autoradiographic method. J. Neurochem. 1995, 65, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Tsuiki, K.; Takada, A.; Nagahiro, S.; Grdiša, M.; Diksic, M.; Pappius, H.M. Synthesis of Serotonin in Traumatized Rat Brain. J. Neurochem. 1995, 64, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Kline, A.E.; Yu, J.; Horváth, E.; Marion, D.W.; Dixon, C.E. The selective 5-HT(1A) receptor agonist repinotan HCl attenuates histopathology and spatial learning deficits following traumatic brain injury in rats. Neuroscience 2001, 106, 547–555. [Google Scholar] [CrossRef]
- Tiraboschi, E.; Tardito, D.; Kasahara, J.; Moraschi, S.; Pruneri, P.; Gennarelli, M.; Racagni, G.; Popoli, M. Selective phosphorylation of nuclear CREB by fluoxetine is linked to activation of CaM kinase IV and MAP kinase cascades. Neuropsychopharmacology 2004, 29, 1831–1840. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Maudsley, S.; Martin, B. BDNF and 5-HT: A dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004, 27, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.K.; Peng, L.; Chen, Y.; Yu, A.C.; Hertz, L. Up-regulation of 5-HT2B receptor density and receptor-mediated glycogenolysis in mouse astrocytes by long-term fluoxetine administration. Neurochem. Res. 2002, 27, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Nahon, E.; Israelson, A.; Abu-Hamad, S.; Varda, S.B. Fluoxetine (Prozac) interaction with the mitochondrial voltage-dependent anion channel and protection against apoptotic cell death. FEBS Lett. 2005, 579, 5105–5110. [Google Scholar] [CrossRef] [PubMed]
- Deák, F.; Lasztóczi, B.; Pacher, P.; Petheö, G.L.; Kecskeméti, V.; Spät, A. Inhibition of voltage-gated calcium channels by fluoxetine in rat hippocampal pyramidal cells. Neuropharmacology 2000, 39, 1029–1036. [Google Scholar] [CrossRef]
- Zafonte, R.D.; Cullen, N.; Lexell, J. Serotonin agents in the treatment of acquired brain injury. J. Head Trauma Rehabil. 2002, 17, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Mostert, J.P.; Koch, M.W.; Heerings, M.; Heersema, D.J.; De Keyser, J. Therapeutic potential of fluoxetine in neurological disorders. CNS Neurosci. Ther. 2008, 14, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.; Jain, A.; Sharma, A.; Mittal, R.S.; Gupta, I.D. Role of sertraline in posttraumatic brain injury depression and quality-of-life in TBI. Asian J. Neurosurg. 2014, 9, 182–188. [Google Scholar] [PubMed]
- Ashman, T.A.; Cantor, J.B.; Gordon, W.A.; Spielman, L.; Flanagan, S.; Ginsberg, A.; Engmann, C.; Egan, M.; Ambrose, F.; Greenwald, B. A randomized controlled trial of sertraline for the treatment of depression in persons with traumatic brain injury. Arch. Phys. Med. Rehabil. 2009, 90, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Baños, J.H.; Novack, T.A.; Brunner, R.; Renfroe, S.; Lin, H.-Y.; Meythaler, J. Impact of early administration of sertraline on cognitive and behavioral recovery in the first year after moderate to severe traumatic brain injury. J. Head Trauma Rehabil. 2010, 25, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Jorge, R.E.; Acion, L.; Burin, D.I.; Robinson, R.G. Sertraline for Preventing Mood Disorders Following Traumatic Brain Injury: A Randomized Clinical Trial. JAMA Psychiatry 2016, 73, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, S.-W.; Kim, J.-M.; Shin, I.-S.; Yang, S.-J.; Yoon, J.-S. Comparing effects of methylphenidate, sertraline and placebo on neuropsychiatric sequelae in patients with traumatic brain injury. Hum. Psychopharmacol. 2005, 20, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Meythaler, J.M.; Depalma, L.; Devivo, M.J.; Guin-Renfroe, S.; Novack, T.A. Sertraline to improve arousal and alertness in severe traumatic brain injury secondary to motor vehicle crashes. Brain Inj. 2001, 15, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Novack, T.A.; Baños, J.H.; Brunner, R.; Renfroe, S.; Meythaler, J.M. Impact of early administration of sertraline on depressive symptoms in the first year after traumatic brain injury. J. Neurotrauma 2009, 26, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, M.J.; Mitchell, R.A.; McCullagh, S.; Herrmann, N.; Chan, F.; Kiss, A.; Feinstein, A.; Lanctôt, K.L. A randomized controlled trial of antidepressant continuation for major depression following traumatic brain injury. J. Clin. Psychiatry 2010, 71, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Dolberg, O.T.; Klag, E.; Gross, Y.; Schreiber, S. Relief of serotonin selective reuptake inhibitor induced sexual dysfunction with low-dose mianserin in patients with traumatic brain injury. Psychopharmacology 2002, 161, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Fann, J.R.; Uomoto, J.M.; Katon, W.J. Sertraline in the treatment of major depression following mild traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Horsfield, S.A.; Rosse, R.B.; Tomasino, V.; Schwartz, B.L.; Mastropaolo, J.; Deutsch, S.I. Fluoxetine’s effects on cognitive performance in patients with traumatic brain injury. Int. J. Psychiatry Med. 2002, 32, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Lanctôt, K.L.; Rapoport, M.J.; Chan, F.; Rajaram, R.D.; Strauss, J.; Sicard, T.; McCullagh, S.; Feinstein, A.; Kiss, A.; Kennedy, J.L.; et al. Genetic predictors of response to treatment with citalopram in depression secondary to traumatic brain injury. Brain Inj. 2010, 24, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Chai, Y.; Jiang, R.; Chen, X.; Yan, T. Cortisol Supplement Combined with Psychotherapy and Citalopram Improves Depression Outcomes in Patients with Hypocortisolism after Traumatic Brain Injury. Aging Dis. 2015, 6, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Muller, U.; Murai, T.; Bauer-Wittmund, T.; von Cramon, D.Y. Paroxetine versus citalopram treatment for pathological crying after brain injury. Brain Inj. 1999, 13, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Perino, C.; Rago, R.; Cicolini, A.; Torta, R.; Monaco, F. Mood and behavioural disorders following traumatic brain injury: Clinical evaluation and pharmacological management. Brain Inj. 2001, 15, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, M.J.; Chan, F.; Lanctot, K.; Herrmann, N.; McCullagh, S.; Feinstein, A. An open-label study of citalopram for major depression following traumatic brain injury. J. Psychopharmacol. 2008, 22, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Turner-Stokes, L.; Hassan, N.; Pierce, K.; Clegg, F. Managing depression in brain injury rehabilitation: The use of an integrated care pathway and preliminary report of response to sertraline. Clin. Rehabil. 2002, 16, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Hensley, P.L.; Reeve, A. A case of antidepressant-induced akathisia in a patient with traumatic brain injury. J. Head Trauma Rehabil. 2001, 16, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Nahas, Z.; Arlinghaus, K.A.; Kotrla, K.J.; Clearman, R.R.; George, M.S. Rapid response of emotional incontinence to selective serotonin reuptake inhibitors. J. Neuropsychiatry Clin. Neurosci. 1998, 10, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D.E.; Braverman, S.E.; Belandres, P.V. Speech dysfunction due to trazodone--fluoxetine combination in traumatic brain injury. Brain Inj. 1997, 11, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Scheutzow, M.H.; Wiercisiewski, D.R. Panic disorder in a patient with traumatic brain injury: A case report and discussion. Brain Inj. 1999, 13, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, J.; Bobo, W.; Childers, M.K. Selective serotonin reuptake inhibitor treatment of post-traumatic Klüver-Bucy syndrome. Brain Inj. 1999, 13, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Sloan, R.L.; Brown, K.W.; Pentland, B. Fluoxetine as a treatment for emotional lability after brain injury. Brain Inj. 1992, 6, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Spinella, M.; Eaton, L.A. Hypomania induced by herbal and pharmaceutical psychotropic medicines following mild traumatic brain injury. Brain Inj. 2002, 16, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Stanislav, S.W.; Childs, N.L. Dystonia associated with sertraline. J. Clin. Psychopharmacol. 1999, 19, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Stengler-Wenzke, K.; Müller, U. Fluoxetine for OCD after brain injury. Am. J. Psychiatry 2002, 159, 872. [Google Scholar] [CrossRef] [PubMed]
- Workman, E.A.; Harrington, D.P. Sertraline-augmented lithium therapy of organic mood syndrome. Psychosomatics 1992, 33, 472–473. [Google Scholar] [CrossRef]
- Wroblewski, B.A.; Guidos, A.; Leary, J.; Joseph, A.B. Control of depression with fluoxetine and antiseizure medication in a brain-injured patient. Am. J. Psychiatry 1992, 149, 273. [Google Scholar] [PubMed]
- Fann, J.R.; Hart, T.; Schomer, K.G. Treatment for depression after traumatic brain injury: A systematic review. J. Neurotrauma 2009, 26, 2383–2402. [Google Scholar] [CrossRef] [PubMed]
- Fleminger, S.; Oliver, D.L.; Williams, W.H.; Evans, J. The neuropsychiatry of depression after brain injury. Neuropsychol. Rehabil. 2003, 13, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Jorge, R.; Robinson, R.G. Mood disorders following traumatic brain injury. Int. Rev. Psychiatry 2003, 15, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.B.; Lyketsos, C.G.; Rao, V. Pharmacological management of the psychiatric aspects of traumatic brain injury. Int. Rev. Psychiatry 2003, 15, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, F. Pharmacological treatment of neurobehavioural sequelae of traumatic brain injury. Eur. J. Anaesthesiol. Suppl. 2008, 42, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.M.; McAllister, T.W.; Arciniegas, D.B. Depression and Cognitive Complaints Following Mild Traumatic Brain Injury. Am. J. Psychiatry 2009, 166, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Tenovuo, O. Pharmacological enhancement of cognitive and behavioral deficits after traumatic brain injury. Curr. Opin. Neurol. 2006, 19, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Wheaton, P.; Mathias, J.L.; Vink, R. Impact of pharmacological treatments on cognitive and behavioral outcome in the postacute stages of adult traumatic brain injury: A meta-analysis. J. Clin. Psychopharmacol. 2011, 31, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.K.; Aydin, B.; Mizumoto, A. MAVIS: Meta Analysis via Shiny; v1.1.1; Comprehensive R Archive Network (CRAN): Merced, CA, USA, September 2015. [Google Scholar]
- Cooper, H.; Hedges, L.V.; Valentine, J.C. The Handbook of Research Synthesis and Meta-Analysis; Russell Sage Foundation: New York City, NY, USA, 2009. [Google Scholar]
- Albert, P.R.; Benkelfat, C. The neurobiology of depression—Revisiting the serotonin hypothesis. II. Genetic, epigenetic and clinical studies. Philos. Trans. R. Soc. Lond B Biol. Sci. 2013. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.W. Neurobiological consequences of traumatic brain injury. Dialogues Clin. Neurosci. 2011, 13, 287–300. [Google Scholar] [PubMed]
- Bombardier, C.H.; Fann, J.R.; Temkin, N.R.; Esselman, P.C.; Barber, J.; Dikmen, S.S. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA 2010, 303, 1938–1945. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.B.; Kocsis, J.H.; Thase, M.E.; Gelenberg, A.J.; Rush, A.J.; Koran, L.; Schatzberg, A.; Russell, J.; Hirschfeld, R.; Klein, D.; et al. Maintenance phase efficacy of sertraline for chronic depression: A randomized controlled trial. JAMA 1998, 280, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, J.H.; Schatzberg, A.; Rush, A.J.; Klein, D.N.; Howland, R.; Gniwesch, L.; Davis, S.M.; Harrison, W. Psychosocial outcomes following long-term, double-blind treatment of chronic depression with sertraline vs. placebo. Arch. Gen. Psychiatry 2002, 59, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.F.; Neale, M.C.; Kendler, K.S. Genetic epidemiology of major depression: Review and meta-analysis. Am. J. Psychiatry 2000, 157, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Capruso, D.X.; Levin, H.S. Cognitive impairment following closed head injury. Neurol. Clin. 1992, 10, 879–893. [Google Scholar] [PubMed]
- Elder, G.A. Update on TBI and Cognitive Impairment in Military Veterans. Curr. Neurol. Neurosci. Rep. 2015, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Cullen, B.; O’Neill, B.; Evans, J.J.; Coen, R.F.; Lawlor, B.A. A review of screening tests for cognitive impairment. J. Neurol. Neurosurg. Psychiatry 2007, 78, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Hibbard, M.R.; Ashman, T.A.; Spielman, L.A.; Chun, D.; Charatz, H.J.; Melvin, S. Relationship between depression and psychosocial functioning after traumatic brain injury. Arch. Phys. Med. Rehabil. 2004, 85, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Resch, J.A.; Villarreal, V.; Johnson, C.L.; Elliott, T.R.; Kwok, O.-M.; Berry, J.W.; Underhill, A.T. Trajectories of life satisfaction in the first 5 years following traumatic brain injury. Rehabil. Psychol. 2009, 54, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Fann, J.R.; Uomoto, J.M.; Katon, W.J. Cognitive improvement with treatment of depression following mild traumatic brain injury. Psychosomatics 2001, 42, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003, 301, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Malberg, J.E.; Eisch, A.J.; Nestler, E.J.; Duman, R.S. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000, 20, 9104–9110. [Google Scholar] [PubMed]
- Wang, J.W.; David, D.J.; Monckton, J.E.; Battaglia, F.; Hen, R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J. Neurosci. 2008, 28, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Naudon, L.; Hotte, M.; Jay, T.M. Effects of acute and chronic antidepressant treatments on memory performance: A comparison between paroxetine and imipramine. Psychopharmacology 2007, 191, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Vythilingam, M.; Vermetten, E.; Anderson, G.M.; Luckenbaugh, D.; Anderson, E.R.; Snow, J.; Staib, L.H.; Charney, D.S.; Bremner, J.D. Hippocampal volume, memory, and cortisol status in major depressive disorder: Effects of treatment. Biol. Psychiatry 2004, 56, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Gasquione, P.G. Postconcussion symptoms. Neuropsychol. Rev. 1997, 7, 77–85. [Google Scholar] [CrossRef]
- Evans, R.W. The postconcussion syndrome and the sequelae of mild head injury. Neurol. Clin. 1992, 10, 815–847. [Google Scholar] [PubMed]
- Ryan, L.M.; Warden, D.L. Post concussion syndrome. Int. Rev. Psychiatry 2003, 15, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Arciniegas, D.B.; Anderson, C.A.; Topkoff, J.; McAllister, T.W. Mild traumatic brain injury: A neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatr. Dis. Treat. 2005, 1, 311–327. [Google Scholar] [PubMed]
- Scher, L.M.; Loomis, E.; McCarron, R.M. Traumatic brain injury: Pharmacotherapy options for cognitive deficits. Curr. Psychiatry 2011, 10, 21–23. [Google Scholar]
- King, N.S.; Crawford, S.; Wenden, F.J.; Moss, N.E.; Wade, D.T. The Rivermead Post Concussion Symptoms Questionnaire: A measure of symptoms commonly experienced after head injury and its reliability. J. Neurol. 1995, 242, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Babcock, L.; Byczkowski, T.; Wade, S.L.; Ho, M.; Mookerjee, S.; Bazarian, J.J. Predicting postconcussion syndrome after mild traumatic brain injury in children and adolescents who present to the emergency department. JAMA Pediatr. 2013, 167, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Ingebrigtsen, T.; Waterloo, K.; Marup-Jensen, S.; Attner, E.; Romner, B. Quantification of post-concussion symptoms 3 months after minor head injury in 100 consecutive patients. J. Neurol. 1998, 245, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.; Leigh, E.; Wade, D.; Fleminger, S. The Rivermead Post Concussion Symptoms Questionnaire: A confirmatory factor analysis. J. Neurol. 2006, 253, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Eyres, S.; Carey, A.; Gilworth, G.; Neumann, V.; Tennant, A. Construct validity and reliability of the Rivermead Post-Concussion Symptoms Questionnaire. Clin. Rehabil. 2005, 19, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S.; Remington, G. Serotonin-dopamine interaction and its relevance to schizophrenia. Am. J. Psychiatry 1996, 153, 466–476. [Google Scholar] [PubMed]
- Ouellet, M.-C.; Savard, J.; Morin, C.M. Book Review: Insomnia following Traumatic Brain Injury: A Review. Neurorehabil. Neural Repair 2004, 18, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Viola-Saltzman, M.; Watson, N.F. Traumatic brain injury and sleep disorders. Neurol. Clin. 2012, 30, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Pandi-Perumal, S.R.; Trahkt, I.; Spence, D.W.; Poeggeler, B.; Hardeland, R.; Cardinali, D.P. Melatonin and melatonergic drugs on sleep: Possible mechanisms of action. Int. J. Neurosci. 2009, 119, 821–846. [Google Scholar] [CrossRef] [PubMed]
- Beasley, C.M., Jr.; Koke, S.C.; Nilsson, M.E.; Gonzales, J.S. Adverse events and treatment discontinuations in clinical trials of fluoxetine in major depressive disorder: An updated meta-analysis. Clin. Ther. 2000, 22, 1319–1330. [Google Scholar] [CrossRef]
- Yıldız, A.; Gönül, A.S.; Tamam, L. Mechanism of actions of antidepressants: Beyond the receptors. Bull. Clin. Psychopharmacol. 2002, 12, 194–200. [Google Scholar]
- Portas, C.M.; Bjorvatn, B.; Ursin, R. Serotonin and the sleep/wake cycle: Special emphasis on microdialysis studies. Prog. Neurobiol. 2000, 60, 13–35. [Google Scholar] [CrossRef]
- Scahill, L.; Carroll, D.; Burke, K. Methylphenidate: Mechanism of action and clinical update. J. Child Adolesc. Psychiatr. Nurs. 2004, 17, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Sobanski, E.; Schredl, M.; Kettler, N.; Alm, B. Sleep in adults with attention deficit hyperactivity disorder (ADHD) before and during treatment with methylphenidate: A controlled polysomnographic study. Sleep 2008, 31, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Ashman, T.A.; Spielman, L.A.; Hibbard, M.R.; Silver, J.M.; Chandna, T.; Gordon, W.A. Psychiatric challenges in the first 6 years after traumatic brain injury: Cross-sequential analyses of Axis I disorders. Arch. Phys. Med. Rehabil. 2004, 85, S36–S42. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Lyons, I.; Koutzoukis, C.; Ali, I.; McCarthy, G. Rate of psychiatric illness 1 year after traumatic brain injury. Am. J. Psychiatry 1999, 156, 374–378. [Google Scholar] [PubMed]
- Bryant, R.A.; O’Donnell, M.L.; Creamer, M.; McFarlane, A.C.; Clark, C.R.; Silove, D. The psychiatric sequelae of traumatic injury. Am. J. Psychiatry 2010, 167, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Soomro, G.M.; Altman, D.; Rajagopal, S.; Oakley-Browne, M. Selective serotonin re-uptake inhibitors (SSRIs) versus placebo for obsessive compulsive disorder (OCD). Cochrane Database Syst. Rev. 2008, CD001765. [Google Scholar] [CrossRef]
- Thomsen, P.H. Obsessive-compulsive disorder: Pharmacological treatment. Eur. Child Adolesc. Psychiatry 9 2000, 9, S76–S84. [Google Scholar] [CrossRef]
- Van Reekum, R.; Cohen, T.; Wong, J. Can traumatic brain injury cause psychiatric disorders? J. Neuropsychiatry Clin. Neurosci. 2000, 12, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Kanetani, K.; Kimura, M.; Endo, S. Therapeutic effects of milnacipran (serotonin noradrenalin reuptake inhibitor) on depression following mild and moderate traumatic brain injury. J. Nippon Med. Sch. 2003, 70, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.M. SSRI-induced extrapyramidal side-effects and akathisia: Implications for treatment. J. Psychopharmacol. 1998, 12, 192–214. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, D.; Balon, R. How do SSRIs cause sexual dysfunction? Understanding key mechanisms can help improve patient adherence, prognosis. Curr. Psychiatry 2010, 9, 30–34. [Google Scholar]
- Howlett, J.R.; Stein, M.B. Post-Traumatic Stress Disorder: Relationship to Traumatic Brain Injury and Approach to Treatment. In Translational Research in Traumatic Brain Injury; Laskowitz, D., Grant, G., Eds.; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2015. [Google Scholar]
- Stein, M.B.; McAllister, T.W. Exploring the Convergence of Posttraumatic Stress Disorder and Mild Traumatic Brain Injury. Am. J. Psychiatry 2009, 166, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Lew, H.L.; Vanderploeg, R.D.; Moore, D.F.; Schwab, K.; Friedman, L.; Yesavage, J.; Keane, T.M.; Warden, D.L.; Sigford, B.J. Overlap of mild TBI and mental health conditions in returning OIF/OEF service members and veterans. J. Rehabil. Res. Dev. 2008, 45, xi–xvi. [Google Scholar] [PubMed]
- Marshall, R.D.; Beebe, K.L.; Oldham, M.; Zaninelli, R. Efficacy and safety of paroxetine treatment for chronic PTSD: A fixed-dose, placebo-controlled study. Am. J. Psychiatry 2001, 158, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Brady, K.; Pearlstein, T.; Asnis, G.M.; Baker, D.; Rothbaum, B.; Sikes, C.R.; Farfel, G.M. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: A randomized controlled trial. JAMA 2000, 283, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Connor, K.M.; Sutherland, S.M.; Tupler, L.A.; Malik, M.L.; Davidson, J.R. Fluoxetine in post-traumatic stress disorder. Randomised, double-blind study. Br. J. Psychiatry 1999, 175, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Raskind, M.A.; Peskind, E.R.; Hoff, D.J.; Hart, K.L.; Holmes, H.A.; Warren, D.; Shofer, J.; O’Connell, J.; Taylor, F.; Gross, C.; et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol. Psychiatry 2007, 61, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Whyte, J.; Vaccaro, M.; Grieb-Neff, P.; Hart, T. Psychostimulant use in the rehabilitation of individuals with traumatic brain injury. J. Head Trauma Rehabil. 2002, 17, 284–299. [Google Scholar] [CrossRef] [PubMed]
- Meythaler, J.M.; Brunner, R.C.; Johnson, A.; Novack, T.A. Amantadine to improve neurorecovery in traumatic brain injury-associated diffuse axonal injury: A pilot double-blind randomized trial. J. Head Trauma Rehabil. 2002, 17, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, D.L.; Whyte, J.; Sandel, M.E. Improved arousal and initiation following tricyclic antidepressant use in severe brain injury. Arch. Phys. Med. Rehabil. 1996, 77, 80–83. [Google Scholar] [CrossRef]
- Van Waes, V.; Beverley, J.; Marinelli, M.; Steiner, H. Selective serotonin reuptake inhibitor antidepressants potentiate methylphenidate (Ritalin)-induced gene regulation in the adolescent striatum. Eur. J. Neurosci. 2010, 32, 435–447. [Google Scholar] [CrossRef] [PubMed]
| Randomized-Controlled Trials | ||||||
| Author | Treatment | Description | N | Endpoints | Results | Post-TBI Disorder |
| Ansari et al., 2014 [26] | Sertraline | 80 adult male patients with post-TBI depression. 40 patients given sertraline 50 mg/day, 40 patients given placebo. | 80 | PHQ-9 | Sertraline group showed significant improvement in mood and QOL domains (PHQ-9: 14.88 ± 3.60 vs. 5.33 ± 2.98, p = 0.040). | Depression |
| Ashman et al., 2009 [27] | Sertraline | 10-week program studying 52 patients with TBI and MDD treated with sertraline or placebo. | 52 | Ham-D | Both groups significantly improved (59% treatment group, 32% placebo group) with Ham-D reduction by 50%. | Depression |
| Banos et al., 2010 [28] | Sertraline | Three-month study of 99 subjects with moderate/severe TBI randomized to sertraline 50 mg (n = 49) or placebo (n = 50). | 99 | WMS, TMT, NFI | No sertraline treatment effect was observed for cognitive performance. | Cognition |
| Jorge et al., 2016 [29] | Sertraline | 94 patients administered sertraline vs. placebo at 100 mg/day for 24-weeks. | 94 | MINI | Number needed to treat to prevent depression after TBI at 24-weeks is 5.9 for sertraline vs. placebo (p = 0.03). Sertraline effects were well-tolerated. | Depression |
| Lee et al., 2005 [30] | Sertraline | Four-week study of 30 patients with MDD treated with sertraline 25–100 mg/day (n = 10), methylphenidate 5–20 mg/day (n = 10), or placebo (n = 10). | 30 | Ham-D, ESS, RPQ | Methylphenidate and sertraline showed improvement in depressive symptomatology. Methylphenidate and placebo showed improvement in cognitive function vs. sertraline. | Cognition, Depression, PCS |
| Meythaler et al., 2001 [31] | Sertraline | Two-week study of 11 patients with severe TBI post MVA. Patients received sertraline 100 mg/day or placebo. | 11 | OL, ABS, GOAT | No effect of sertraline treatment was identified. | Cognition |
| Novack et al., 2009 [32] | Sertraline | One-year study of 99 non-depressed TBI subjects received either sertraline 50 mg/day (n = 49) or placebo (n = 50). | 99 | Ham-D, NFI | Placebo group developed more depressive symptoms (p = 0.023). Sertraline associated with decreased neurobehavioral functioning. | Depression |
| Rapoport et al., 2010 [33] | Citalopram | 21 patients in remission from depression after TBI were randomized to same-dose citalopram (n = 10) or placebo (n = 11) and followed for 40 weeks. | 21 | Ham-D | Relapse occurred in 11 subjects (52.4%). Treatment groups did not differ in relapse rates (citalopram: 50% vs. placebo: 54.5%; p = 0.835). This trial suggested limitations of pharmacotherapy in the prevention of MDD relapse following TBI. | Depression |
| Open-Label Studies | ||||||
| Author | Treatment | Description | N | Endpoints | Results | Post-TBI Disorder |
| Dolberg et al., 2002 [34] | Fluoxetine, Citalopram, Paroxetine, Sertraline | 17 TBI patients were given SSRIs. All complained of sexual dysfunction which was resolved with mianserin (tetracyclic anti-depressant). | 17 | Occurrence of sexual dysfunction | SSRI use associated with sexual dysfunction. 15 patients (88%) reported improvement of symptoms with mianserin. | Sexual dysfunction |
| Fann et al., 2000 [35] | Sertraline | 15 patients with mild TBI within the past 3–24 months. Placebo in-run design where all subjects received 1-week placebo followed by 8-week single-blind course of sertraline. | 15 | Ham-D | Sertraline significantly improved depressive symptoms (Ham-D 25.0 ± 4.4 to 7.2 ± 5.3 at Week 8 (p < 0.001). There was improvement of cognitive functions in psychomotor speed, cognitive efficiency, flexible thinking, and recent memory ability. | Cognition, Depression |
| Horsfield et al., 2002 [36] | Fluoxetine | 5 TBI patients with no to moderate depressive symptoms followed for 8 months. | 5 | TMT, AMT, WAIS-III, USCREMT, MMSE, Ham-D | Fluoxetine improved mood and performance on some but not all cognitive measures. More studies needed. | Cognition |
| Lanctot et al., 2010 [37] | Citalopram | 90 patients with major depressive episode following TBI in a six week study also examining six serotonergic SNPs. | 90 | Ham-D | MTHFR and BDNF SNPs predicted greater treatment response (r2 = 0.098, F = 4.65, p = 0.013). The 5HTTLPR SNP predicted greater occurrence of adverse events (r2 = 0.069, F = 5.72, p = 0.020). Serotonergic SNPs may associate with tolerability and efficacy of SSRIs. | Depression |
| Luo et al., 2015 [38] | Citalopram, prednisone | 68 patients with depression following TBI. | 68 | Glasgow Coma Scale, Ham-D | Over 60% of patients who did not respond to psychotherapy alone (60/68) responded to citalopram treatment. Patients with hypocortisolism also were treated with prednisone | Depression |
| Muller et al., 1999 [39] | Paroxetine, Citalopram | 26 patients with brain damage and pathological crying. Only 2 TBI related. | 2 | Clinical interviews related to pathological crying | Both paroxetine and citalopram improved symptoms for 24/26 (92.3%) patients within 3 days. | Emotional incontinence |
| Perino et al., 2001 [40] | Citalopram, Carbamazepine | 20 patients with MDD following TBI were divided into two groups: group A with recent TBI (<6 months), and group B with long-term TBI (24–36 months). | 20 | BPRS | BPRS and CGI scores of the total sample showed significant improvement between baseline and 12 weeks (BPRS baseline: 62.3 ± 17.6 vs. 12 weeks: 51.7 ± 12.8; p < 0.05), (CGI severity-scale baseline: 4.4 ± 1.1 vs. 12 weeks: 3.4 ± 0.8; p < 0.005). No group effects were observed. | Depression |
| Rapoport et al., 2008 [41] | Citalopram | 54 patients with mild to moderate depression post-TBI. 29 patients underwent 6 week fixed dose treatment; 36 underwent 10-week flexible dose treatment. | 54 | Ham-D | The mean Ham-D at baseline and 6 weeks were 23.66 (SD 6.8) and 16.30 (SD 9.3), respectively (p < 0.0001). The mean Ham-D at 10 weeks was 12.96 (SD 7.9) (p < 0.0001). Treatment showed significant reduction in depressive symptoms. | Depression, PCS |
| Turner-Stokes et al., 2002 [42] | Sertraline | 27 patients with depression due to brain injury - 5 due to TBI. | 27 | BDI-II | The BDI-II was assessable in 17/21 patients, showing a mean improvement of 14.5 ± 9.7 (p < 0.001). | Depression |
| Case Reports | ||||||
| Author | Treatment | Description | N | Endpoints | Results | Post-TBI Disorder |
| Hensley et al., 2010 [43] | Sertraline, Paroxetine | 22-year-old female with MVA-related TBI. | 1 | -- | Treatment of alcohol withdrawal related anxiety with SSRIs led to akathisia that resolved with TCA treatment. | Akathisia, Anxiety |
| Nahas et al., 1998 [44] | Fluoxetine | 21-year-old male with MVA-related TBI leading to pathological crying. | 1 | -- | Fluoxetine treatment led to complete resolution of pathological crying within 1 week. | Emotional incontinence |
| Patterson et al., 1997 [45] | Sertraline, Trazodone | 43-year-old male with TBI following fall prescribed trazodone for chronic pain and sleep disturbance, and fluoxetine for treatment of depression. | 1 | -- | Fluoxetine addition led to dysarthria that resolved with fluoxetine discontinuation. | Depression |
| Scheutzow et al., 1999 [46] | Sertraline | 60-year-old male with TBI following MVA experiencing panic attacks. | 1 | -- | Sertraline improved mood and appetite but did not resolve all panic and anxiety symptoms. | Anxiety, Panic |
| Slaughter et al., 1999 [47] | Sertraline | 2 male patients with Kluver-Bucy syndrome from MVA related TBI. | 2 | -- | Resolution of symptoms (hyperorality, hyper-sexuality) with high dose SSRI similar to OCD. | Emotional incontinence, OCD |
| Sloan et al., 1992 [48] | Fluoxetine | 28-year-old assault victim with TBI related pathological laughter, dysarthria and hemiataxia. | 1 | -- | Fluoxetine plus speech therapy helped with emotional lability grading and was well tolerated. | Emotional incontinence |
| Spinella et al., 2002 [49] | Fluoxetine, Buspirone, Ginkgo Biloba | 42-year-old female with mild TBI following MVA. | 1 | -- | Herbal supplements plus SSRI led to hypomania, highlighting the need to study SSRI interactions with other medications following TBI. | Depression |
| Stanislav et al., 1999 [50] | Sertraline | 24-year-old with severe TBI following MVA presenting with PTSD. | 1 | -- | Clinical case of dystonia following SSRI treatment. | Depression, PTSD |
| Stengler-Wenzke et al., 2002 [51] | Fluoxetine | 18-year-old male with severe TBI following MVA. | 1 | -- | Fluoxetine treatment drastically reduced OCD symptoms and increased quality of life. | OCD |
| Workman et al., 1992 [52] | Sertraline, Lithium | 56-year-old female with severe TBI from fall requiring bilateral frontal lobectomies. | 1 | -- | Sertraline plus lithium treatment reduced patients Ham-D score from 15 to 4. Symptoms of mood lability and conceptual disorganization resolved. | Emotional incontinence |
| Wroblewski et al., 1992 [53] | Fluoxetine, Phenytoin | 23-year-old TBI patient with seizures following TCA treatment of depression. | 1 | -- | Resolution of seizures and improvement of mood with fluoxetine treatment with phenytoin. | Depression |
| Review Articles | ||||||
| Author | Treatment | Description | N | Endpoints | Results | Post-TBI Disorder |
| Fann et al., 2009 [54] | Review | -- | -- | -- | Serotonergic system modulation through antidepressants has high tolerability in treatment of TBI patients with depression. | General |
| Fleminger et al., 2003 [55] | Review | -- | -- | -- | Three- to four-fold increase in suicide rates following TBI, suggested heightened surveillance for TBI subpopulations. Little conclusive evidence for SSRI use highlights need for longitudinal care. | Depression |
| Jorge et al., 2003 [56] | Review | -- | -- | -- | Mood disorders are frequent complications of TBI and are often overlooked. Further research is needed for the neuropsychiatric sequelae of these disorders. | Anxiety, OCD, Panic |
| Lee et al., 2003 [57] | Review | -- | -- | -- | Up to 60% of TBI patients are affected by neuropsychiatric sequelae. Drugs exist to treat specific conditions but RCTs are needed to delineate true treatment effects following TBI. | Anxiety, Cognition, Depression |
| Lombardi et al., 2008 [58] | Review | -- | -- | -- | Symptomatic treatment studies that understand and address underlying neurobiological recovery processes are needed. | Cognition, PCS |
| Silver et al., 2009 [59] | Review | -- | -- | -- | Depression and cognitive impairment are common neuropsychiatric symptoms after TBI. Several small studies suggest that SSRIs and tricyclic antidepressants may improve depression in this population. | Cognition, Depression |
| Tenovuo et al., 2006 [60] | Review | -- | -- | -- | Lack of large scale RCTs place burden on clinician for pharmacologic treatment of TBI-related depression. | Cognition |
| Wheaton et al., 2011 [61] | Review | -- | -- | -- | Pharmacological treatments that are administered to adults in the postacute stage (≥4 weeks) after TBI have the potential to reduce persistent cognitive and behavioral problems. Sertraline can possibly impair cognition and psychomotor speed. | Cognition |
| Zafonte et al., 2002 [23] | Review | -- | -- | -- | Limited studies exist among patients with TBI, but serotonin agents including SSRIs seem to be effective for a variety of behavioral disorders. Care should be used when combining agents, and rapid withdrawal should be avoided. | Anxiety, Cognition, Depression |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, J.K.; Burke, J.F.; Upadhyayula, P.S.; Winkler, E.A.; Deng, H.; Robinson, C.K.; Pirracchio, R.; Suen, C.G.; Sharma, S.; Ferguson, A.R.; et al. Selective Serotonin Reuptake Inhibitors for Treating Neurocognitive and Neuropsychiatric Disorders Following Traumatic Brain Injury: An Evaluation of Current Evidence. Brain Sci. 2017, 7, 93. https://doi.org/10.3390/brainsci7080093
Yue JK, Burke JF, Upadhyayula PS, Winkler EA, Deng H, Robinson CK, Pirracchio R, Suen CG, Sharma S, Ferguson AR, et al. Selective Serotonin Reuptake Inhibitors for Treating Neurocognitive and Neuropsychiatric Disorders Following Traumatic Brain Injury: An Evaluation of Current Evidence. Brain Sciences. 2017; 7(8):93. https://doi.org/10.3390/brainsci7080093
Chicago/Turabian StyleYue, John K., John F. Burke, Pavan S. Upadhyayula, Ethan A. Winkler, Hansen Deng, Caitlin K. Robinson, Romain Pirracchio, Catherine G. Suen, Sourabh Sharma, Adam R. Ferguson, and et al. 2017. "Selective Serotonin Reuptake Inhibitors for Treating Neurocognitive and Neuropsychiatric Disorders Following Traumatic Brain Injury: An Evaluation of Current Evidence" Brain Sciences 7, no. 8: 93. https://doi.org/10.3390/brainsci7080093






