Nucleus Accumbens Deep Brain Stimulation in Patients with Substance Use Disorders and Delay Discounting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
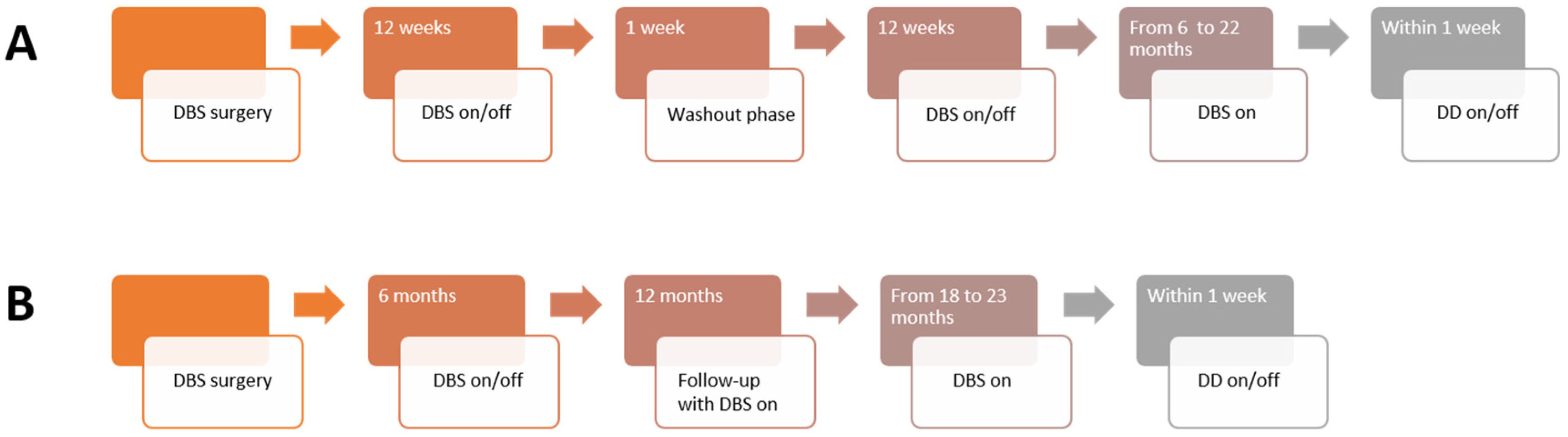
2.2. Clinical Trials
2.2.1. NASA
2.2.2. DeBraSTRA
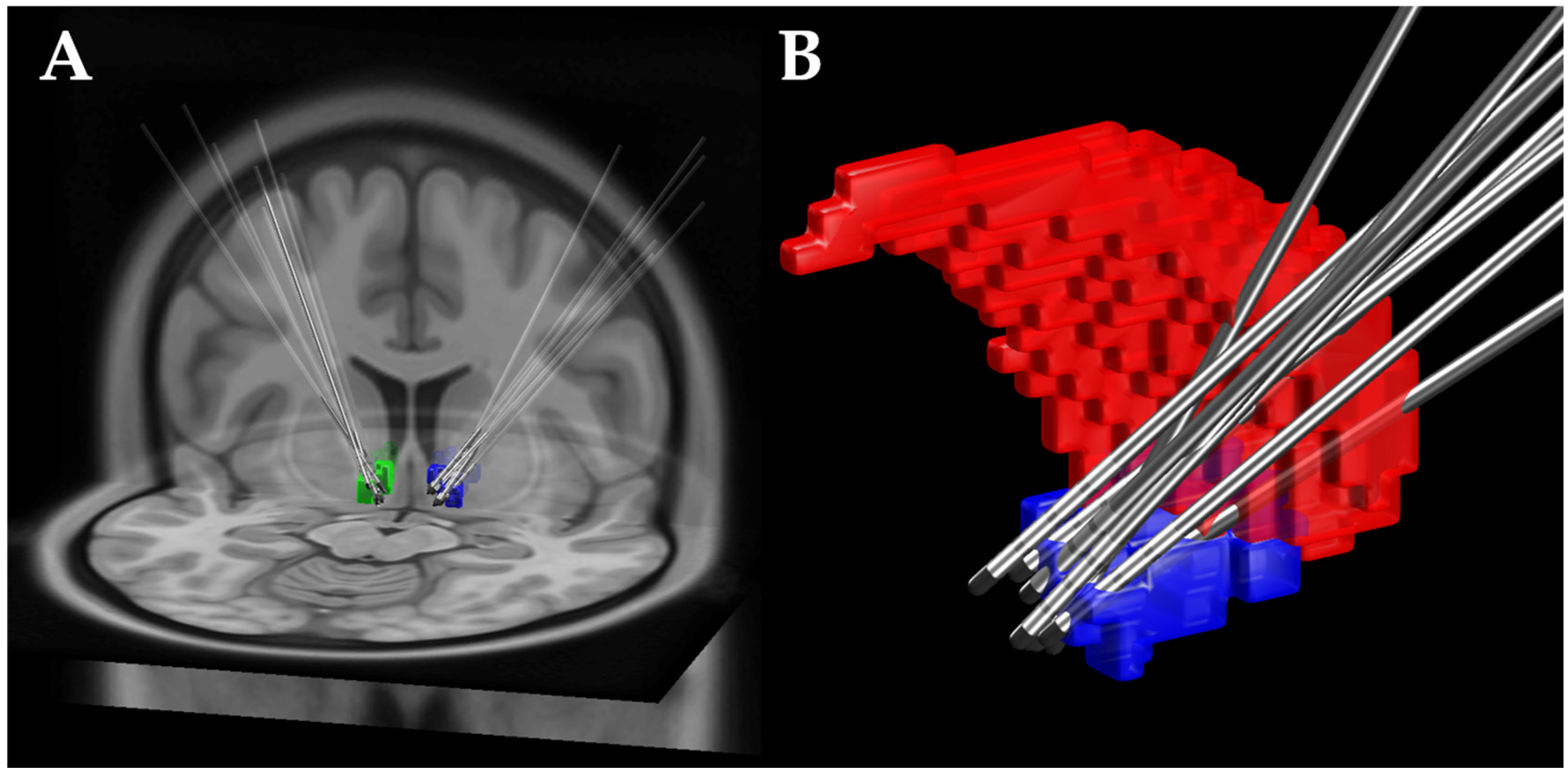
2.2.3. Deep Brain Stimulation
2.3. Questionnaires
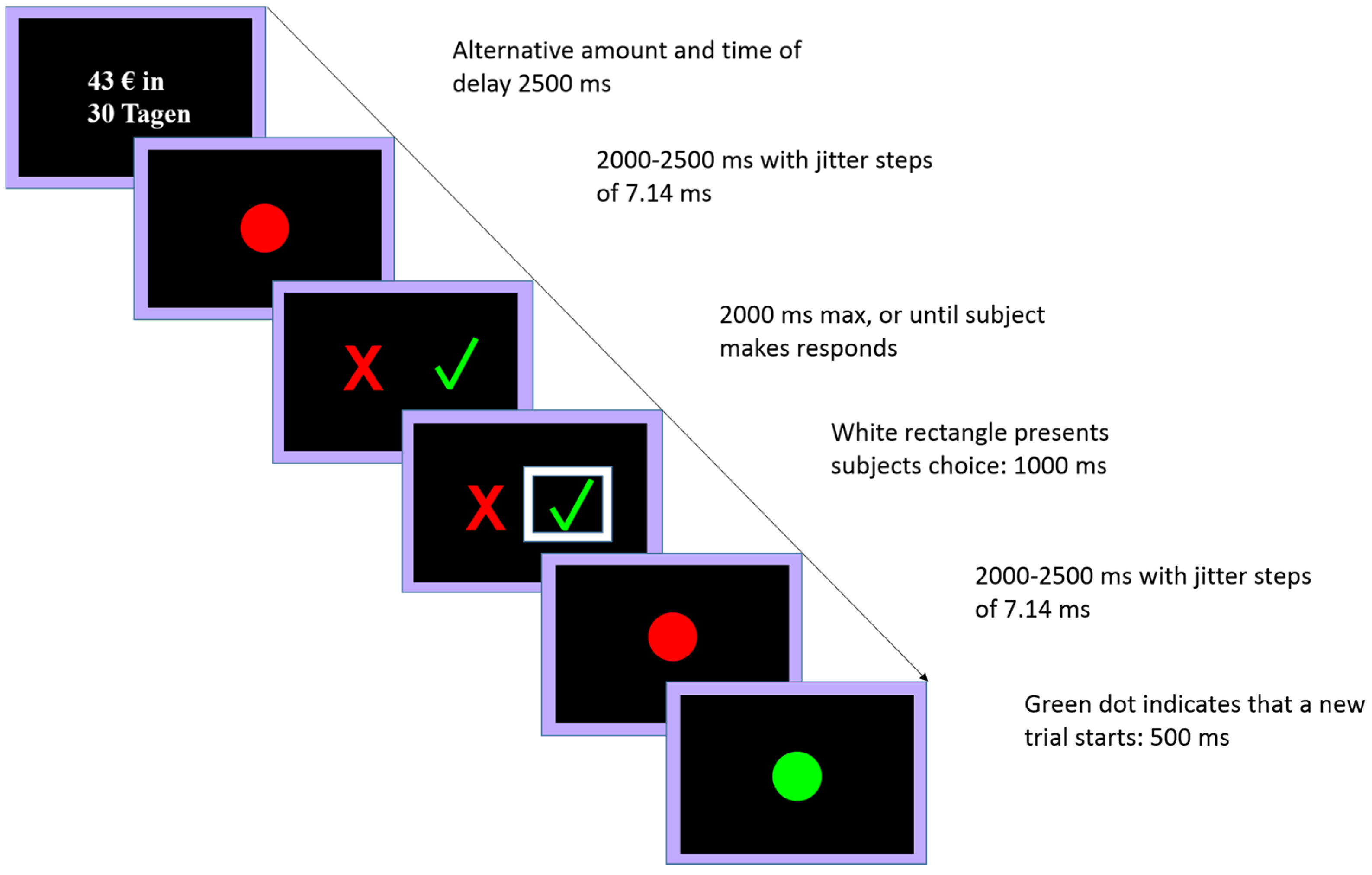
2.4. Delay Discounting Task
2.4.1. Behavioral Pretest
2.4.2. Experimental Session
2.5. Computational Modeling
2.6. Model-Free Analysis
3. Results
3.1. Demographic Characteristics
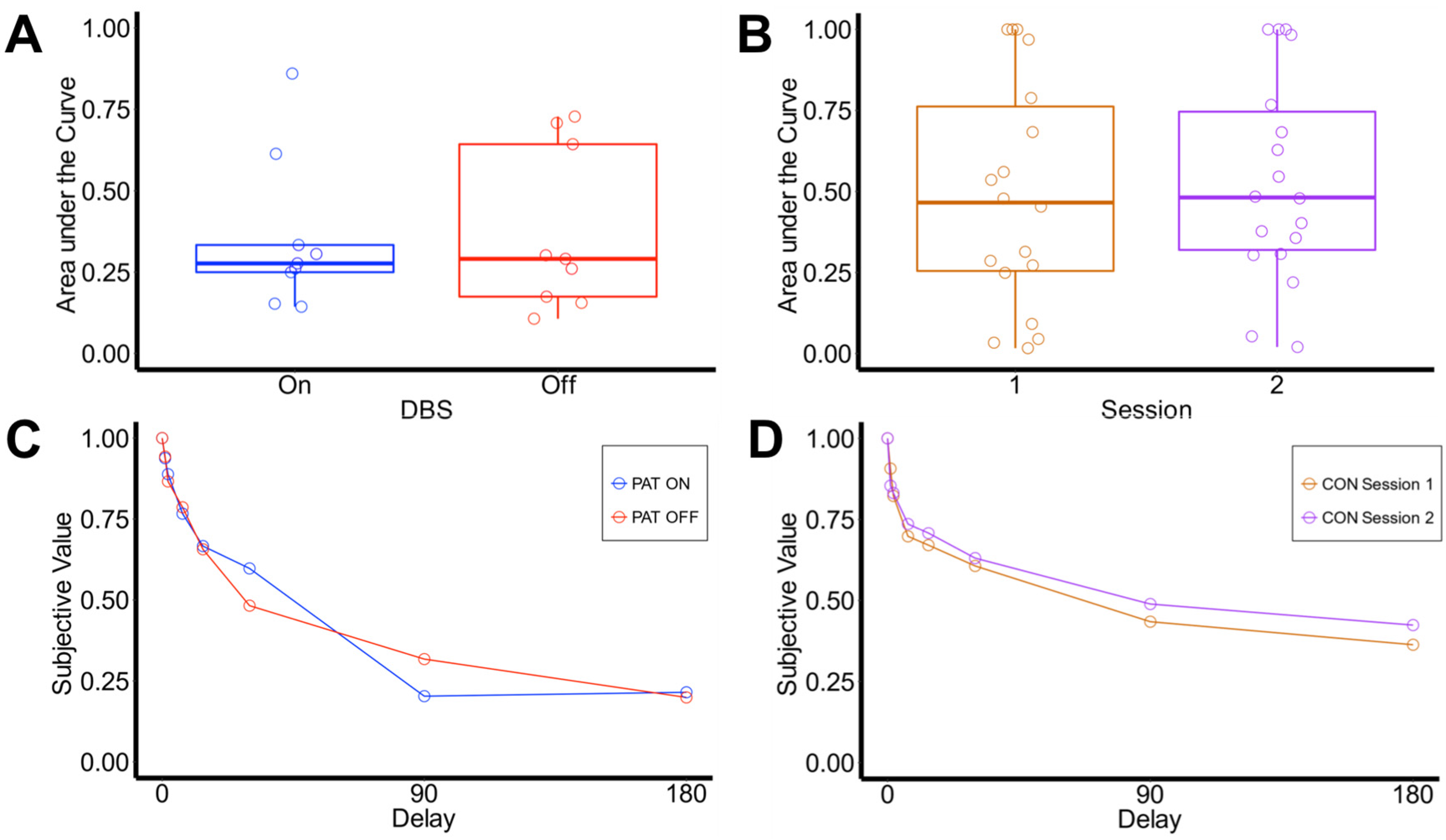
3.2. Delay Discounting
3.2.1. Area under the Curve
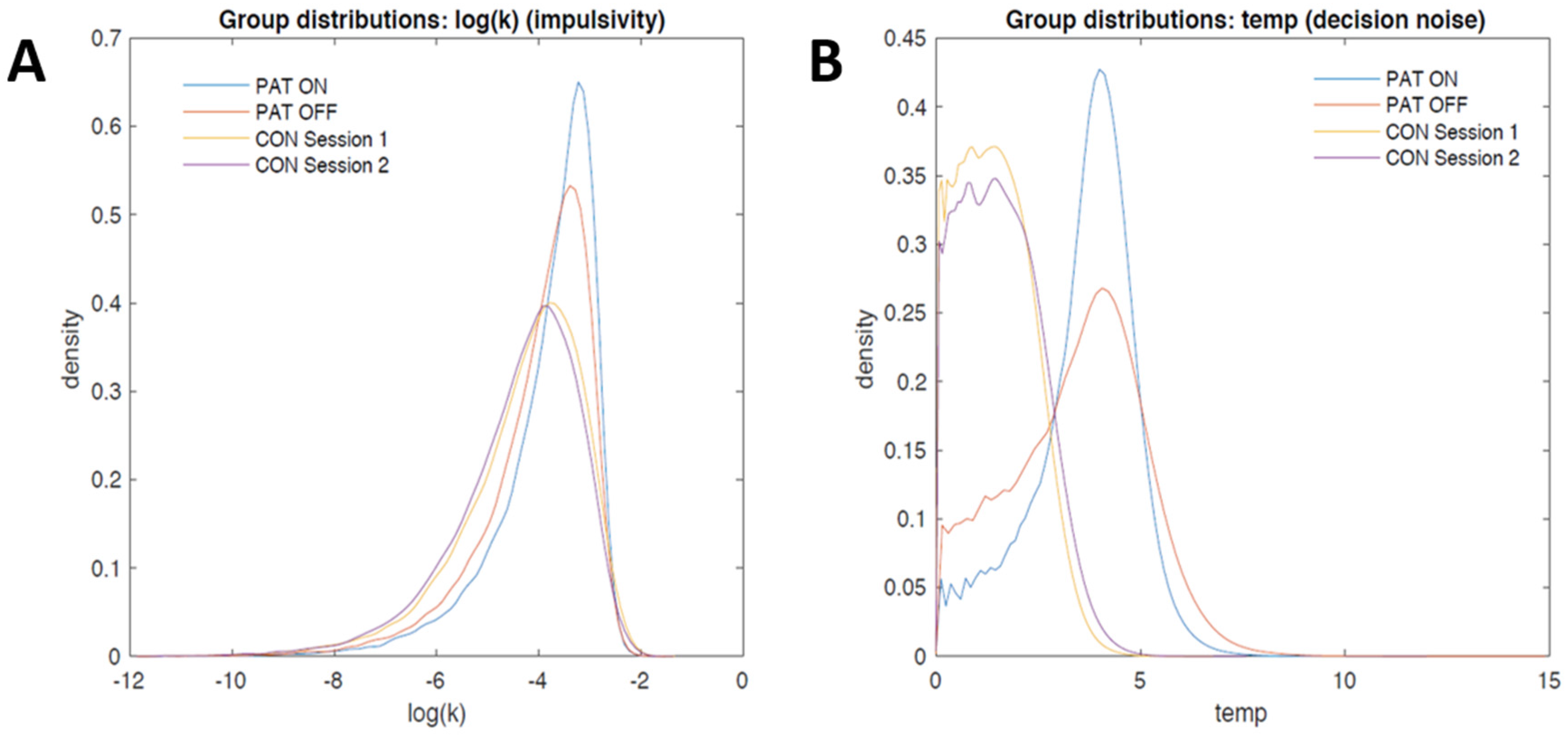
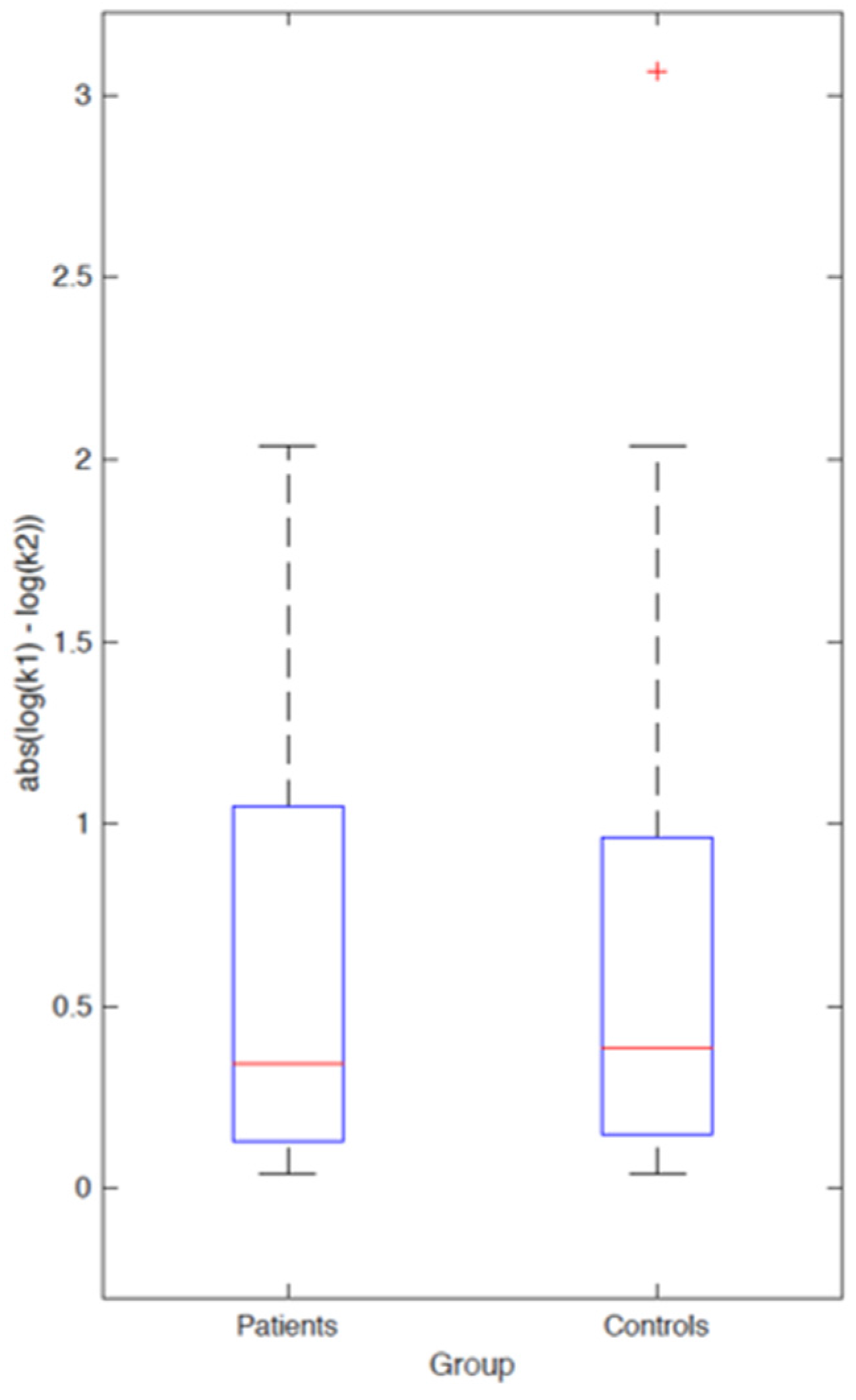
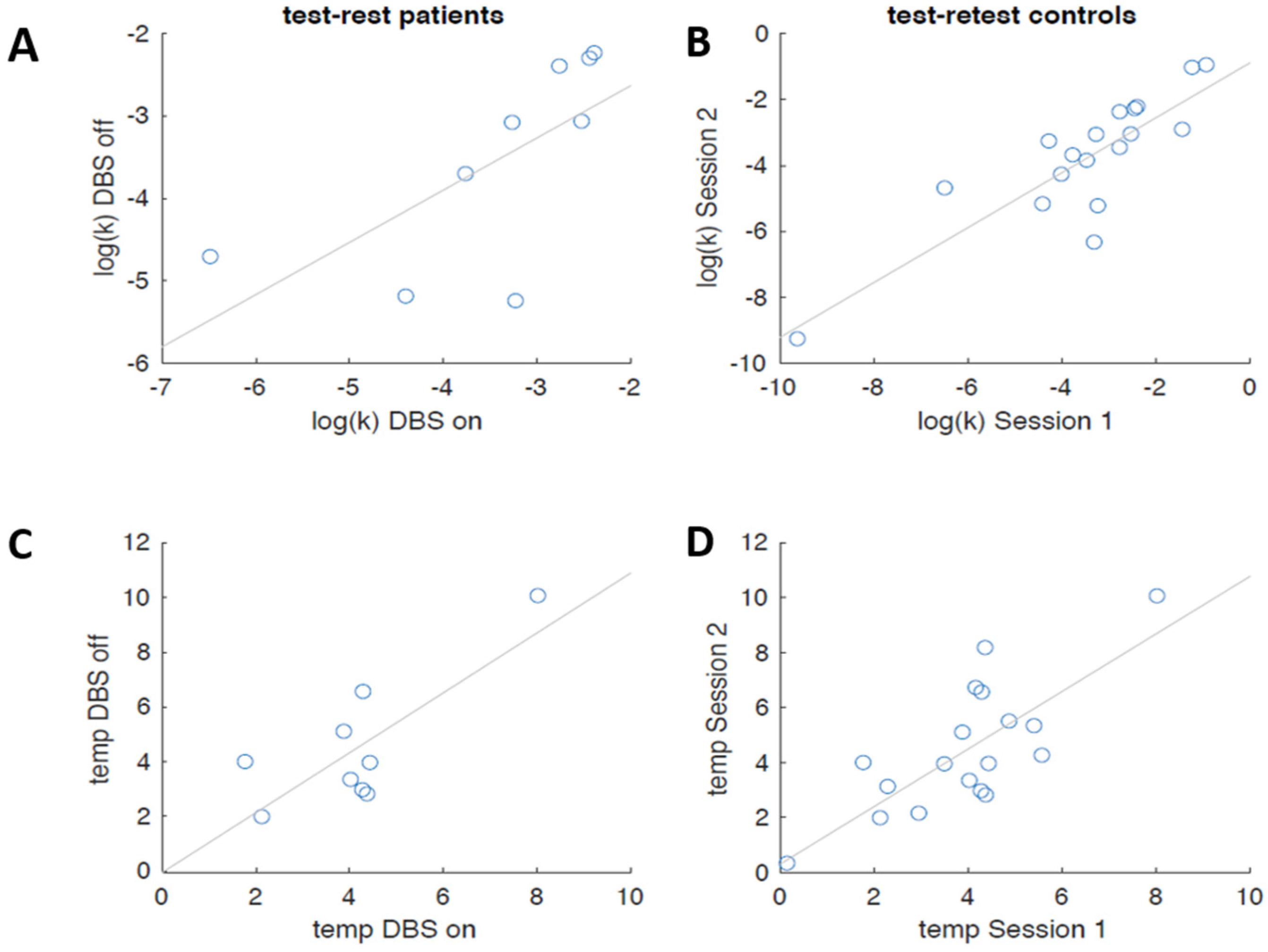
3.2.2. Computational Modeling
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Compton, W.M.; Thomas, Y.F.; Stinson, F.S.; Grant, B.F. Prevalence, Correlates, Disability, and Comorbidity of DSM-IV Drug Abuse and Dependence in the United States. Arch. Gen. Psychiatry 2007, 64, 566. [Google Scholar] [CrossRef] [PubMed]
- Hasin, D.S.; Stinson, F.S.; Ogburn, E.; Grant, B.F. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch. Gen. Psychiatry 2007, 64, 830–842. [Google Scholar] [CrossRef] [PubMed]
- McLellan, A.T.; Lewis, D.C.; O’Brien, C.P.; Kleber, H.D. Drug Dependence, a Chronic Medical Illness. JAMA 2000, 284, 1689. [Google Scholar] [CrossRef] [PubMed]
- Torregrossa, M.M.; Quinn, J.J.; Taylor, J.R. Impulsivity, compulsivity, and habit: The role of orbitofrontal cortex revisited. Biol. Psychiatry 2008, 63, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Baler, R.D.; Volkow, N.D. Drug addiction: The neurobiology of disrupted self-control. Trends Mol. Med. 2006, 12, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Bechara, A. Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nat. Neurosci. 2005, 8, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Fowler, J.S.; Wang, G.-J.; Telang, F.; Logan, J.; Jayne, M.; Ma, Y.; Pradhan, K.; Wong, C.; Swanson, J.M. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage 2010, 49, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Bickel, W.K.; Marsch, L.A. Toward a behavioral economic understanding of drug dependence: Delay discounting processes. Addiction 2001, 96, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Amlung, M.; MacKillop, J. Clarifying the relationship between impulsive delay discounting and nicotine dependence. Psychol. Addict. Behav. 2014. [Google Scholar] [CrossRef] [PubMed]
- Bickel, W.K.; Jarmolowicz, D.P.; Mueller, E.T.; Koffarnus, M.N.; Gatchalian, K.M. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: Emerging evidence. Pharmacol. Ther. 2012, 134, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Robles, E.; Huang, B.E.; Simpson, P.M.; McMillan, D.E. Delay discounting, impulsiveness, and addiction severity in opioid-dependent patients. J. Subst. Abuse Treat. 2011, 41, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Kirby, K.N.; Petry, N.M.; Bickel, W.K. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J. Exp. Psychol. Gen. 1999, 128, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Petry, N.M. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology 2001, 154, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.M.; Fields, H.L.; D’Esposito, M.; Boettiger, C.A. Impulsive Responding in Alcoholics. Alcohol. Clin. Exp. Res. 2005, 29, 2158–2169. [Google Scholar] [CrossRef] [PubMed]
- Bickel, W.K.; Odum, A.L.; Madden, G.J. Impulsivity and cigarette smoking: Delay discounting in current, never, and ex-smokers. Psychopharmacology 1999, 146, 447–454. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, O.; Secades-Villa, R.; Weidberg, S.; Yoon, J.H. A systematic assessment of delay discounting in relation to cocaine and nicotine dependence. Behav. Process. 2013, 99, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.W. An efficient operant choice procedure for assessing delay discounting in humans: Initial validation in cocaine-dependent and control individuals. Exp. Clin. Psychopharmacol. 2012, 20, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, W.F.; Moore, M.; Templin, R.; McFarland, B.; Hitzemann, R.J.; Mitchell, S.H. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology 2006, 188, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Monterosso, J.; Ainslle, G.; Xu, J.; Cordova, X.; Domier, C.P.; London, E.D. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum. Brain Mapp. 2007, 28, 383–393. [Google Scholar] [CrossRef] [PubMed]
- MacKillop, J.; Amlung, M.T.; Few, L.R.; Ray, L.A.; Sweet, L.H.; Munafo, M.R. Delayed reward discounting and addictive behavior: A meta-analysis. Psychopharmacology 2011, 216, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Sheffer, C.E.; Mennemeier, M.; Landes, R.D.; Bickel, W.K.; Brackman, S.; Dornhoffer, J.; Kimbrell, T.; Brown, G. Neuromodulation of delay discounting, the reflection effect, and cigarette consumption. J. Subst. Abuse Treat. 2013, 45, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.; Verdejo-García, A.; Goudriaan, A.E.; Roeyers, H.; Dom, G.; Vanderplasschen, W. Impulsivity as a vulnerability factor for poor addiction treatment outcomes: A review of neurocognitive findings among individuals with substance use disorders. J. Subst. Abuse Treat. 2014, 47, 58–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanger, C.; Ryan, S.R.; Fu, H.; Landes, R.D.; Jones, B.A.; Bickel, W.K.; Budney, A.J. Delay discounting predicts adolescent substance abuse treatment outcome. Exp. Clin. Psychopharmacol. 2012, 20, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Anokhin, A.P.; Golosheykin, S.; Grant, J.D.; Heath, A.C. Heritability of Delay Discounting in Adolescence: A Longitudinal Twin Study. Behav. Genet. 2011, 41, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, B. A review of delay-discounting research with humans: Relations to drug use and gambling. Behav. Pharmacol. 2006, 17, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Koob, G.F.; McLellan, A.T. Neurobiologic Advances from the Brain Disease Model of Addiction. N. Engl. J. Med. 2016, 374, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharmacology 2010, 35, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, R.N.; Parkinson, J.A.; Hall, J.; Everitt, B.J. Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 2002, 26, 321–352. [Google Scholar] [CrossRef]
- Pauli, W.M.; O’Reilly, R.C.; Yarkoni, T.; Wager, T.D. Regional specialization within the human striatum for diverse psychological functions. Proc. Natl. Acad. Sci. USA 2016, 113, 1907–1912. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, R.N. Impulsive Choice Induced in Rats by Lesions of the Nucleus Accumbens Core. Science 2001, 292, 2499–2501. [Google Scholar] [CrossRef] [PubMed]
- Acheson, A.; Farrar, A.M.; Patak, M.; Hausknecht, K.A.; Kieres, A.K.; Choi, S.; de Wit, H.; Richards, J.B. Nucleus accumbens lesions decrease sensitivity to rapid changes in the delay to reinforcement. Behav. Brain Res. 2006, 173, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Bezzina, G.; Cheung, T.H.C.; Asgari, K.; Hampson, C.L.; Body, S.; Bradshaw, C.M.; Szabadi, E.; Deakin, J.F.W.; Anderson, I.M. Effects of quinolinic acid-induced lesions of the nucleus accumbens core on inter-temporal choice: A quantitative analysis. Psychopharmacology 2007, 195, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Araújo, S.; Body, S.; Hampson, C.L.; Langley, R.W.; Deakin, J.F.W.; Anderson, I.M.; Bradshaw, C.M.; Szabadi, E. Effects of lesions of the nucleus accumbens core on inter-temporal choice: Further observations with an adjusting-delay procedure. Behav. Brain Res. 2009, 202, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Basar, K.; Sesia, T.; Groenewegen, H.; Steinbusch, H.W.M.; Visser-Vandewalle, V.; Temel, Y. Nucleus accumbens and impulsivity. Prog. Neurobiol. 2010, 92, 533–557. [Google Scholar] [CrossRef] [PubMed]
- McClure, S.M.; Laibson, D.I.; Loewenstein, G.; Cohen, J.D. Separate neural systems value immediate and delayed monetary rewards. Science 2004, 306, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Dell’Osso, B.; Altamura, A.C.; Allen, A.; Hollander, E. Brain stimulation techniques in the treatment of obsessive-compulsive disorder: Current and future directions. CNS Spectr. 2005, 10, 966–983. [Google Scholar] [CrossRef] [PubMed]
- Blomstedt, P.; Sjoberg, R.L.; Hansson, M.; Bodlund, O.; Hariz, M.I. Deep brain stimulation in the treatment of obsessive-compulsive disorder. World Neurosurg. 2013, 80, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.; Hardenacke, K.; Lenartz, D.; Gruendler, T.; Ullsperger, M.; Bartsch, C.; Mai, J.K.; Zilles, K.; Bauer, A.; Matusch, A.; et al. Deep brain stimulation of the nucleus basalis of Meynert in Alzheimer’s dementia. Mol. Psychiatry 2014, 20, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.; Lenartz, D.; Mai, J.K.; Huff, W.; Lee, S.H.; Koulousakis, A.; Klosterkoetter, J.; Sturm, V. Deep brain stimulation of the nucleus accumbens and the internal capsule in therapeutically refractory Tourette-syndrome. J. Neurol. 2007, 254, 963–965. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.; Lenartz, D.; Huff, W.; Lee, S.H.; Koulousakis, A.; Klosterkoetter, J.; Sturm, V. Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: Valuable therapeutic implications? J. Neurol. Neurosurg. Psychiatry 2007, 78, 1152–1153. [Google Scholar] [CrossRef] [PubMed]
- Müller, U.J.; Voges, J.; Steiner, J.; Galazky, I.; Heinze, H.-J.; Möller, M.; Pisapia, J.; Halpern, C.; Caplan, A.; Bogerts, B.; et al. Deep brain stimulation of the nucleus accumbens for the treatment of addiction. Ann. N. Y. Acad. Sci. 2013, 1282, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xu, J.; Jiang, J. Deep brain stimulation of nucleus accumbens on heroin-seeking behaviors: A case report. Biol. Psychiatry 2011, 69, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Alfonso, C.E.; Luigjes, J.; Smolders, R.; Cohen, M.X.; Levar, N.; Mazaheri, A.; Van Den Munckhof, P.; Schuurman, P.R.; Van Den Brink, W.; Denys, D. Effective deep brain stimulation in heroin addiction: A case report with complementary intracranial electroencephalogram. Biol. Psychiatry 2012, 71, e35–e37. [Google Scholar] [CrossRef] [PubMed]
- Schreckenberger, M.; Klega, A.; Grunder, G.; Buchholz, H.G.; Scheurich, A.; Schirrmacher, R.; Schirrmacher, E.; Muller, C.; Henriksen, G.; Bartenstein, P. Opioid receptor PET reveals the psychobiologic correlates of reward processing. J. Nucl. Med. 2008, 49, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Muller, U.J.; Sturm, V.; Voges, J.; Heinze, H.J.; Galazky, I.; Heldmann, M.; Scheich, H.; Bogerts, B. Successful treatment of chronic resistant alcoholism by deep brain stimulation of nucleus accumbens: First experience with three cases. Pharmacopsychiatry 2009, 42, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.; Gründler, T.O.J.; Bauer, R.; Huff, W.; Fischer, A.G.; Lenartz, D.; Maarouf, M.; Bührle, C.; Klosterkötter, J.; Ullsperger, M.; et al. Successful deep brain stimulation of the nucleus accumbens in severe alcohol dependence is associated with changed performance monitoring. Addict. Biol. 2011, 16, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Knapp, C.M.; Tozier, L.; Pak, A.; Ciraulo, D.A.; Kornetsky, C. Deep brain stimulation of the nucleus accumbens reduces ethanol consumption in rats. Pharmacol. Biochem. Behav. 2009, 92, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, N.; Wang, H.M.; Meng, F.G.; Zhang, J.G. Inhibition of the reinstatement of morphine-induced place preference in rats by high-frequency stimulation of the bilateral nucleus accumbens. Chin. Med. J. 2013, 126, 1939–1943. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Jin, J.; Tang, J.S.; Sun, W.X.; Jia, H.; Yang, X.P.; Cui, J.M.; Wang, C.G. Chronic deep brain stimulation in the rat nucleus accumbens and its effect on morphine reinforcement. Addict. Biol. 2008, 13, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhou, H.; Wang, R.; Xu, J.; Zhou, W.; Zhang, F.; Tang, S.; Liu, H.; Jiang, J. DBS of nucleus accumbens on heroin seeking behaviors in self-administering rats. Drug Alcohol Depend. 2013, 129, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.B.; Green, A.I.; Bradford, P.S.; Chau, D.T.; Roberts, D.W.; Leiter, J.C. Deep brain stimulation of the nucleus accumbens reduces alcohol intake in alcohol-preferring rats. Neurosurg. Focus 2010, 29, E12. [Google Scholar] [CrossRef] [PubMed]
- Vassoler, F.M.; Schmidt, H.D.; Gerard, M.E.; Famous, K.R.; Ciraulo, D.A.; Kornetsky, C.; Knapp, C.M.; Pierce, R.C. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats. J. Neurosci. 2008, 28, 8735–8739. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.C.; Hahn, P.J. Network perspectives on the mechanisms of deep brain stimulation. Neurobiol. Dis. 2010, 38, 329–337. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.C.; Anderson, R.W. Deep brain stimulation mechanisms: The control of network activity via neurochemistry modulation. J. Neurochem. 2016, 139, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Sesia, T.; Temel, Y.; Lim, L.W.; Blokland, A.; Steinbusch, H.W.; Visser-Vandewalle, V. Deep brain stimulation of the nucleus accumbens core and shell: Opposite effects on impulsive action. Exp. Neurol. 2008, 214, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Sesia, T.; Bulthuis, V.; Tan, S.; Lim, L.W.; Vlamings, R.; Blokland, A.; Steinbusch, H.W.M.; Sharp, T.; Visser-Vandewalle, V.; Temel, Y. Deep brain stimulation of the nucleus accumbens shell increases impulsive behavior and tissue levels of dopamine and serotonin. Exp. Neurol. 2010, 225, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Schippers, M.C.; Bruinsma, B.; Gaastra, M.; Mesman, T.I.; Denys, D.; De Vries, T.J.; Pattij, T. Deep Brain Stimulation of the Nucleus Accumbens Core Affects Trait Impulsivity in a Baseline-Dependent Manner. Front. Behav. Neurosci. 2017, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Horn, A.; Kühn, A.A. Lead-DBS: A toolbox for deep brain stimulation electrode localizations and visualizations. Neuroimage 2015, 107, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J.; Comrey, A.L.; Goodrich, D.W.; Guilford, J.P.; Guilford, J.P.; Hollingshead, A.B.; et al. An Inventory for Measuring Depression. Arch. Gen. Psychiatry 1961, 4, 561. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.; Büchel, C. Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. J. Neurosci. 2009, 29, 15727–15734. [Google Scholar] [CrossRef] [PubMed]
- McKerchar, T.L.; Green, L.; Myerson, J.; Pickford, T.S.; Hill, J.C.; Stout, S.C. A comparison of four models of delay discounting in humans. Behav. Process. 2009, 81, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.; Miedl, S.F.; Büchel, C. Formal Comparison of Dual-Parameter Temporal Discounting Models in Controls and Pathological Gamblers. PLoS ONE 2012, 7, e47225. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M. JAGS: A program for analysis of Bayesian graphical models using gibbs sampling JAGS: Just another gibbs sampler. In Proceedings of the 3rd International Workshop on Distributed Statistical Computing (DSC 2003), Vienna, Austria, 20–22 March 2003. [Google Scholar]
- Myerson, J.; Green, L.; Warusawitharana, M. Area under the curve as a measure of discounting. J. Exp. Anal. Behav. 2001, 76, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Kirby, K.N. One-year temporal stability of delay-discount rates. Psychon. Bull. Rev. 2009, 16, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Higgins, S.T.; Heil, S.H.; Sugarbaker, R.J.; Thomas, C.S.; Badger, G.J. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Exp. Clin. Psychopharmacol. 2007, 15, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Torta, D.M.E.; Vizzari, V.; Castelli, L.; Zibetti, M.; Lanotte, M.; Lopiano, L.; Geminiani, G. Impulsivities and Parkinson’s Disease: Delay Aversion Is Not Worsened by Deep Brain Stimulation of the Subthalamic Nucleus. PLoS ONE 2012, 7, e43261. [Google Scholar] [CrossRef] [PubMed]
- Seinstra, M.; Wojtecki, L.; Storzer, L.; Schnitzler, A.; Kalenscher, T. No effect of subthalamic deep brain stimulation on intertemporal decision making in Parkinson patients. eNeuro 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Denys, D.; Mantione, M.; Figee, M.; van den Munckhof, P.; Koerselman, F.; Westenberg, H.; Bosch, A.; Schuurman, R. Deep Brain Stimulation of the Nucleus Accumbens for Treatment-Refractory Obsessive-Compulsive Disorder. Arch. Gen. Psychiatry 2010, 67, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- De Koning, P.P.; Figee, M.; Endert, E.; van den Munckhof, P.; Schuurman, P.R.; Storosum, J.G.; Denys, D.; Fliers, E. Rapid effects of deep brain stimulation reactivation on symptoms and neuroendocrine parameters in obsessive-compulsive disorder. Transl. Psychiatry 2016, 6, e722. [Google Scholar] [CrossRef] [PubMed]
- Kohl, S.; Gruendler, T.O.J.; Huys, D.; Sildatke, E.; Dembek, T.A.; Hellmich, M.; Vorderwulbecke, M.; Timmermann, L.; Ahmari, S.E.; Klosterkoetter, J.; et al. Effects of deep brain stimulation on prepulse inhibition in obsessive-compulsive disorder. Transl. Psychiatry 2015, 5, e675. [Google Scholar] [CrossRef] [PubMed]
- Nachev, P.; Lopez-Sosa, F.; Gonzalez-Rosa, J.J.; Galarza, A.; Avecillas, J.; Pineda-Pardo, J.A.; Lopez-Ibor, J.J.; Reneses, B.; Barcia, J.A.; Strange, B. Dynamic risk control by human nucleus accumbens. Brain 2015, 138, 3496–3502. [Google Scholar] [CrossRef] [PubMed]
- Cury, R.G.; Fraix, V.; Castrioto, A.; Pérez Fernández, M.A.; Krack, P.; Chabardes, S.; Seigneuret, E.; Alho, E.J.L.; Benabid, A.-L.; Moro, E. Thalamic deep brain stimulation for tremor in Parkinson disease, essential tremor, and dystonia. Neurology 2017, 89, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Button, K.S.; Ioannidis, J.P.A.; Mokrysz, C.; Nosek, B.A.; Flint, J.; Robinson, E.S.J.; Munafò, M.R. Power failure: Why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013, 14, 365–376. [Google Scholar] [CrossRef] [PubMed]
- De Wit, H.; Flory, J.D.; Acheson, A.; McCloskey, M.; Manuck, S.B. IQ and nonplanning impulsivity are independently associated with delay discounting in middle-aged adults. Personal. Individ. Differ. 2007, 42, 111–121. [Google Scholar] [CrossRef]
- Vassileva, J.; Gonzalez, R.; Bechara, A.; Martin, E.M. Are all drug addicts impulsive? Effects of antisociality and extent of multidrug use on cognitive and motor impulsivity. Addict. Behav. 2007, 32, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Botvinick, M.M.; Braver, T.S.; Barch, D.M.; Carter, C.S.; Cohen, J.D. Conflict monitoring and cognitive control. Psychol. Rev. 2001, 108, 624–652. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.; Büchel, C. Episodic Future Thinking Reduces Reward Delay Discounting through an Enhancement of Prefrontal-Mediotemporal Interactions. Neuron 2010, 66, 138–148. [Google Scholar] [CrossRef] [PubMed]
| ID | Disorder | Sex | Age | Years of Addiction | Electrode Contacts | Frequency | Amplitude | Pulse-Width |
|---|---|---|---|---|---|---|---|---|
| (1) | Opioid | M | 53 | 35 | −0, −1 | 130 Hz | 3.5 V | 90 µs |
| (2) | Opioid | M | 58 | 40 | −0, −1 | 130 Hz | 3.5 V | 90 µs |
| (3) | Opioid | M | 47 | 24 | −0, −1 | 130 Hz | 3.5 V | 90 µs |
| (4) | Opioid | M | 24 | 4 | −0, −1 | 130 Hz | 2.7 V | 90 µs |
| (5) | Opioid | F | 34 | 7 | −0, −1, −2 | 130 Hz | 2.5 V | 90 µs |
| (6) | Alcohol | M | 47 | 31 | −0, −1 | 130 Hz | 4.5 V | 90 µs |
| (7) | Alcohol | M | 38 | 6 | −2, −3, | 130 Hz | L: 6 V R: 5.5 V | L: 450 µs R: 240 µs |
| (8) | Alcohol | M | 31 | 13 | −2, −3 | 130 Hz | 1.5 V | 90 µs |
| (9) | Alcohol | M | 51 | 30 | −3 | 130 Hz | 3.5 V | 210 µs |
| Patients with SUD (n = 9) | Healthy Controls (n = 18) | U/Χ2 | p | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age (Years) a | 42.6 | 11.3 | 43.4 | 10.6 | 77.6 | 0.860 |
| Years of education a | 10.6 | 1.1 | 12.3 | 1.2 | 28.5 | 0.001 |
| Male b | 88.9% | - | 61.1% | - | 2.2 | 0.149 |
| Right-handed b | 100% | - | 94.5% | - | 0.5 | 0.667 |
| Disease duration | 21.1 | 13.8 | - | - | - | - |
| BDI a | 15.0 | 15.7 | 5.1 | 6.2 | 49.5 | 0.106 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peisker, C.B.; Schüller, T.; Peters, J.; Wagner, B.J.; Schilbach, L.; Müller, U.J.; Visser-Vandewalle, V.; Kuhn, J. Nucleus Accumbens Deep Brain Stimulation in Patients with Substance Use Disorders and Delay Discounting. Brain Sci. 2018, 8, 21. https://doi.org/10.3390/brainsci8020021
Peisker CB, Schüller T, Peters J, Wagner BJ, Schilbach L, Müller UJ, Visser-Vandewalle V, Kuhn J. Nucleus Accumbens Deep Brain Stimulation in Patients with Substance Use Disorders and Delay Discounting. Brain Sciences. 2018; 8(2):21. https://doi.org/10.3390/brainsci8020021
Chicago/Turabian StylePeisker, Canan B., Thomas Schüller, Jan Peters, Ben J. Wagner, Leonhard Schilbach, Ulf J. Müller, Veerle Visser-Vandewalle, and Jens Kuhn. 2018. "Nucleus Accumbens Deep Brain Stimulation in Patients with Substance Use Disorders and Delay Discounting" Brain Sciences 8, no. 2: 21. https://doi.org/10.3390/brainsci8020021





