Neurobiology of Propofol Addiction and Supportive Evidence: What Is the New Development?
Abstract
:1. Introduction
2. Clinical Properties of Propofol
3. Abuse Potential of Propofol
4. Status of Propofol as a Controlled Drug
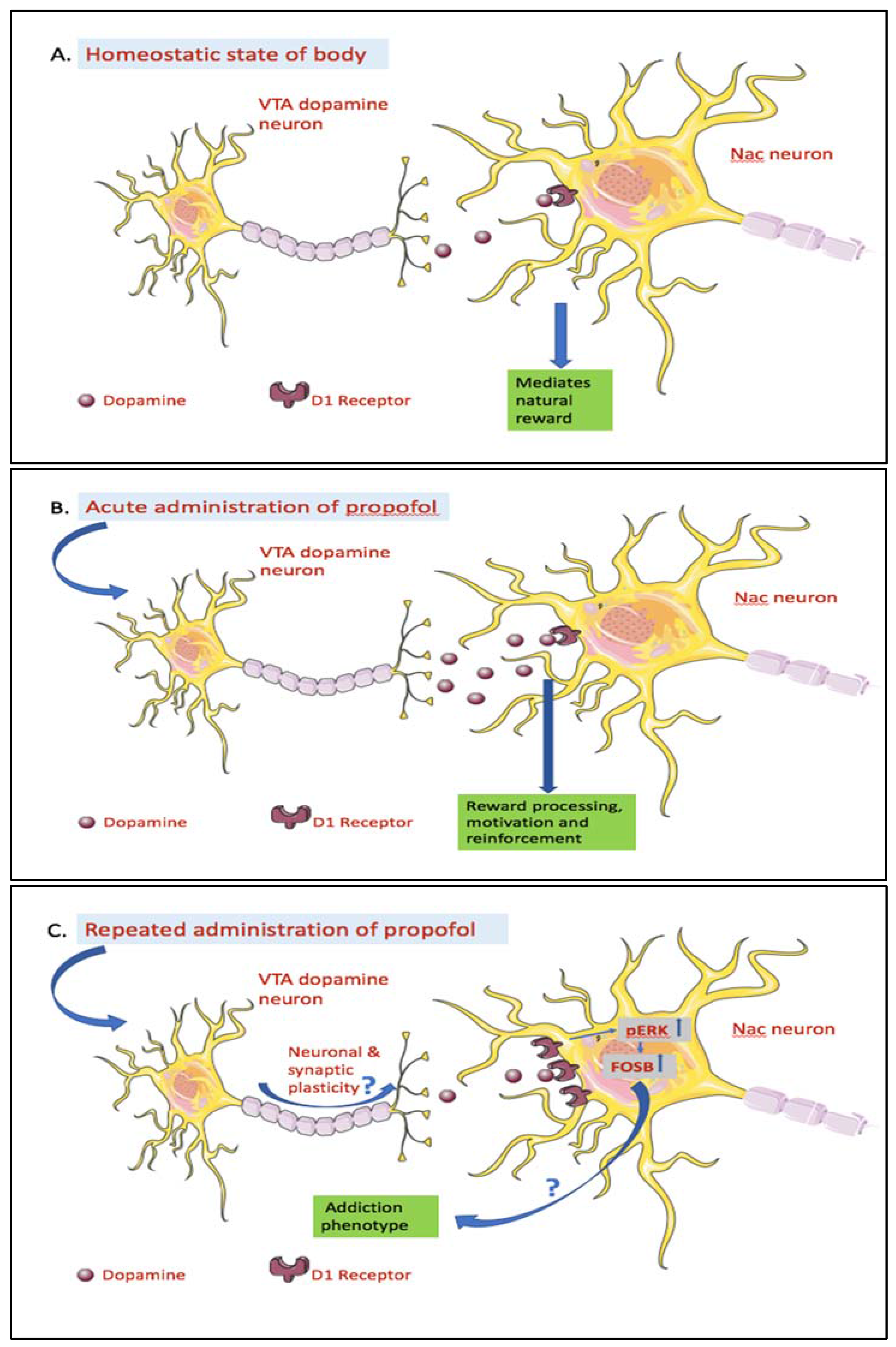
5. Molecular and Cellular Mechanisms of Propofol Addiction
6. Conclusions
7. Future Directions
Acknowledgments
Conflicts of Interest
References
- Lundström, S.; Twycross, R.; Mihalyo, M.; Wilcock, A. Propofol. J. Pain Symptom Manag. 2010, 40, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Trapani, G.; Altomare, C.; Liso, G.; Sanna, E.; Biggio, G. Propofol in anesthesia. Mechanism of action, structure-activity relationships, and drug delivery. Curr. Med. Chem. 2000, 7, 249–271. [Google Scholar] [CrossRef] [PubMed]
- Hales, T.G.; Lambert, J.J. The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurones. Br. J. Pharmacol. 1991, 104, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Welliver, M.D.; Bertrand, A.; Garza, J.; Baker, K. Two New Case Reports of Propofol Abuse and a Pattern Analysis of the Literature. Int. J. Adv. Nurs. Stud. 2012, 1, 22–42. [Google Scholar] [CrossRef]
- Kim, E.-J.; Kim, S.-H.; Hyun, Y.-J.; Noh, Y.-K.; Jung, H.-S.; Han, S.-Y.; Park, C.-H.; Choi, B.-M.; Noh, G. Clinical and psychological characteristics of propofol abusers in Korea: A survey of propofol abuse in 38, non-healthcare professionals. Korean J. Anesthesiol. 2015, 68, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Zacny, J.P.; Lichtor, J.L.; Thompson, W.; Apfelbaum, J.L. Propofol at a subanesthetic dose may have abuse potential in healthy volunteers. Anesth. Analg. 1993, 77, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Earley, P.H.; Finver, T. Addiction to propofol: A study of 22 treatment cases. J. Addict. Med. 2013, 7, 169–176. [Google Scholar] [CrossRef] [PubMed]
- McAuliffe, P.F.; Gold, M.S.; Bajpai, L.; Merves, M.L.; Frost-Pineda, K.; Pomm, R.M.; Goldberger, B.A.; Melker, R.J.; Cendán, J.C. Second-hand exposure to aerosolized intravenous anesthetics propofol and fentanyl may cause sensitization and subsequent opiate addiction among anesthesiologists and surgeons. Med. Hypotheses 2006, 66, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Merlo, L.J.; Goldberger, B.A.; Kolodner, D.; Fitzgerald, K.; Gold, M.S. Fentanyl and Propofol Exposure in the Operating Room: Sensitization Hypotheses and Further Data. J. Addict. Dis. 2008, 27, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Wischmeyer, P.E.; Johnson, B.; Wilson, J.E.; Henthorn, T.K. A survey of propofol abuse in academic anesthesia programs. Anesth. Analg. 2007, 105, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Fry, R.A.; Fry, L.E.; Castanelli, D.J. A retrospective survey of substance abuse in anaesthetists in Australia and New Zealand from 2004 to 2013. Anaesth. Intensive Care 2015, 43, 111–117. [Google Scholar] [PubMed]
- 2009—Final Rule: Placement of Fospropofol into Schedule IV. Available online: https://www.deadiversion.usdoj.gov/fed_regs/rules/2009/fr1006.htm (accessed on 14 December 2017).
- Wozniak, K.M.; Vornov, J.J.; Mistry, B.M.; Wu, Y.; Rais, R.; Slusher, B.S. Gastrointestinal delivery of propofol from fospropofol: Its bioavailability and activity in rodents and human volunteers. J. Transl. Med. 2015, 13, 170. [Google Scholar] [CrossRef] [PubMed]
- 2010—Proposed Rule: Placement of Propofol into Schedule IV. Available online: https://www.deadiversion.usdoj.gov/fed_regs/rules/2010/fr1027.htm (accessed on 14 December 2017).
- ASA Supports DEA Proposal to Schedule Propofol—American Society of Anesthesiologists (ASA). Available online: http://www.asahq.org/about-asa/newsroom/news-releases/2010/11/asa-supports-dea-proposal-to-schedule-propofol (accessed on 11 February 2018).
- Securing Propofol. Available online: https://www.aana.com/docs/default-source/practice-aana-com-web-documents-(all)/securing-propofol.pdf?sfvrsn=2b0049b1_2 (accessed on 11 February 2018).
- LeSage, M.G.; Stafford, D.; Glowa, J.R. Abuse liability of the anesthetic propofol: Self-administration of propofol in rats under fixed-ratio schedules of drug delivery. Psychopharmacology 2000, 153, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Gatch, M.B.; Forster, M.J. Behavioral and toxicological effects of propofol. Behav. Pharmacol. 2011, 22, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, X.; Sun, A.; Xu, L.; Wang, S.; Lin, W.; Lai, M.; Zhu, H.; Zhou, W.; Lian, Q. Extracellular Signal-Regulated Kinase in Nucleus Accumbens Mediates Propofol Self-Administration in Rats. Neurosci. Bull. 2016, 32, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, A.; Uskur, T.; Akkan, A.G.; Çevreli, B.; Uzbay, T. Effects of propofol on conditioned place preference in male rats: Involvement of nitrergic system. Am. J. Drug Alcohol Abuse 2018, 44, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.-J.; Cha, J.-H.; Cho, H.-Y.; Chung, E.-Y.; Kwon, K.-J.; Lee, J.Y.; Jeong, H.-S.; Kim, H.-S.; Chung, H.-J.; Kim, E.J. Dependence Potential of Propofol: Behavioral Pharmacology in Rodents. Biomol. Ther. 2012, 20, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.E.; Berridge, K.C. The psychology and neurobiology of addiction: An incentive-sensitization view. Addiction 2000, 95 (Suppl. 2), S91–S117. [Google Scholar] [PubMed]
- Xiao, C.; Shao, X.M.; Olive, M.F.; Griffin, W.C.; Li, K.Y.; Krnjević, K.; Zhou, C.; Ye, J.H. Ethanol Facilitates Glutamatergic Transmission to Dopamine Neurons in the Ventral Tegmental Area. Neuropsychopharmacology 2009, 34, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, C.A.; Ferris, M.J.; Jones, S.R. Cocaine self-administration disrupts mesolimbic dopamine circuit function and attenuates dopaminergic responsiveness to cocaine. Eur. J. Neurosci. 2015, 42, 2091–2096. [Google Scholar] [CrossRef] [PubMed]
- Hyman, S.E.; Malenka, R.C.; Nestler, E.J. Neural Mechanisms of Addiction: The Role of Reward-Related Learning and Memory. Annu. Rev. Neurosci. 2006, 29, 565–598. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Morales, M. The Brain on Drugs: From Reward to Addiction. Cell 2015, 162, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Pain, L.; Gobaille, S.; Schleef, C.; Aunis, D.; Oberling, P. In vivo dopamine measurements in the nucleus accumbens after nonanesthetic and anesthetic doses of propofol in rats. Anesth. Analg. 2002, 95, 915–919. [Google Scholar] [PubMed]
- Valjent, E.; Pagès, C.; Hervé, D.; Girault, J.A.; Caboche, J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur. J. Neurosci. 2004, 19, 1826–1836. [Google Scholar] [CrossRef] [PubMed]
- Ruffle, J.K. Molecular neurobiology of addiction: What’s all the (Δ)FosB about? Am. J. Drug Alcohol Abuse 2014, 40, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Nye, H.E.; Kelz, M.B.; Nestler, E.J. Regulation of delta FosB and FosB-like proteins by electroconvulsive seizure and cocaine treatments. Mol. Pharmacol. 1995, 48, 880–889. [Google Scholar] [PubMed]
- Ulery, P.G.; Nestler, E.J. Regulation of ΔfosB transcriptional activity by Ser27 phosphorylation. Eur. J. Neurosci. 2007, 25, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J. Review. Transcriptional mechanisms of addiction: Role of DeltaFosB. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008, 363, 3245–3255. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Li, J.; Ye, J.H.; Zhang, C. Upregulation of DeltaFosB by propofol in rat nucleus accumbens. Anesth. Analg. 2011, 113, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-Y.; Xiao, C.; Xiong, M.; Delphin, E.; Ye, J.-H. Nanomolar Propofol Stimulates Glutamate Transmission to Dopamine Neurons: A Possible Mechanism of Abuse Potential? J. Pharmacol. Exp. Ther. 2008, 325, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Ponnudurai, R.; Schoenberg, C.; Zhang, C.; Delphin, E. A prospective controlled study to determine the blood propofol concentration in anesthesiologists exposed to propofol vapor in the expired gases of patients receiving propofol-based intravenous sedation. J. Clin. Anesth. 2011, 23, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Svenningsson, P.; Nairn, A.C.; Greengard, P. DARPP-32 mediates the actions of multiple drugs of abuse. AAPS J. 2005, 7, E353–E360. [Google Scholar] [CrossRef] [PubMed]
- Pavković, Ž.; Smiljanić, K.; Kanazir, S.; Milanović, D.; Pešić, V.; Ruždijić, S. Brain molecular changes and behavioral alterations induced by propofol anesthesia exposure in peripubertal rats. Pediatr. Anesth. 2017, 27, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Steketee, J.D.; Kalivas, P.W. Drug wanting: Behavioral sensitization and relapse to drug-seeking behavior. Pharmacol. Rev. 2011, 63, 348–365. [Google Scholar] [CrossRef] [PubMed]
- Vezina, P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci. Biobehav. Rev. 2004, 27, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Ambroggi, F.; Turiault, M.; Milet, A.; Deroche-Gamonet, V.; Parnaudeau, S.; Balado, E.; Barik, J.; van der Veen, R.; Maroteaux, G.; Lemberger, T.; et al. Stress and addiction: Glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nat. Neurosci. 2009, 12, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Shariff, M.; Bartlett, S.E. The Role of the Glucocorticoids in Developing Resilience to Stress and Addiction. Front. Psychiatry 2013, 4, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, B.; Liang, Y.; Dong, Z.; Chen, Z.; Zhang, G.; Lin, W.; Wang, S.; Wang, B.; Ge, R.-S.; Lian, Q. Glucocorticoid receptor mediated the propofol self-administration by dopamine D1 receptor in nucleus accumbens. Neuroscience 2016, 328, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Lin, W.; Wang, H.; Abdullah, T.; Wang, B.; Su, Y.; Ge, R.-S.; Lian, Q. Glucocorticoid receptor in rat nucleus accumbens: Its roles in propofol addictions. Neurosci. Lett. 2018, 662, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Tonner, P.H.; Scholz, J.; Lamberz, L.; Schlamp, N.; Schulte am Esch, J. Inhibition of nitric oxide synthase decreases anesthetic requirements of intravenous anesthetics in Xenopus laevis. Anesthesiology 1997, 87, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Tezcan, A.H.; Özçetin, A.; Özlü, O.; Çevreli, B.; Uzbay, T. Locomotor stimulation by acute propofol administration in rats: Role of the nitrergic system. Pharmacol. Rep. 2015, 67, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, R. Alcohol and NMDA receptor: Current research and future direction. Front. Mol. Neurosci. 2013, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Morisot, N.; Ron, D. Alcohol-dependent molecular adaptations of the NMDA receptor system. Genes Brain Behav. 2017, 16, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Grasshoff, C.; Gillessen, T. Effects of propofol on N-methyl-D-aspartate receptor-mediated calcium increase in cultured rat cerebrocortical neurons. Eur. J. Anaesthesiol. 2005, 22, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Kingston, S.; Mao, L.; Yang, L.; Arora, A.; Fibuch, E.E.; Wang, J.Q. Propofol inhibits phosphorylation of N-methyl-d-aspartate receptor NR1 subunits in neurons. Anesthesiology 2006, 104, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Li, K.; daGraca, R.L.; Delphin, E.; Xiong, M.; Ye, J.H. Behavior and Cellular Evidence for Propofol-induced Hypnosis Involving Brain Glycine Receptors. Anesthesiology 2009, 110, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Molander, A.; Söderpalm, B. Glycine receptors regulate dopamine release in the rat nucleus accumbens. Alcohol. Clin. Exp. Res. 2005, 29, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Blednov, Y.A.; Benavidez, J.M.; Black, M.; Leiter, C.R.; Osterndorff-Kahanek, E.; Harris, R.A. Glycine Receptors Containing 2 or 3 Subunits Regulate Specific Ethanol-Mediated Behaviors. J. Pharmacol. Exp. Ther. 2015, 353, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, S.; Adermark, L.; Ericson, M.; Söderpalm, B. The involvement of accumbal glycine receptors in the dopamine-elevating effects of addictive drugs. Neuropharmacology 2014, 82, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.R.; Rudolph, U.; Lüscher, C. Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends Neurosci. 2011, 34, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.M.; Engin, E.; Tantillo, G.; Lau, H.M.; Muschamp, J.W.; Carlezon, W.A., Jr.; Rudolph, U. Differential Roles of GABAA Receptor Subtypes in Benzodiazepine-Induced Enhancement of Brain-Stimulation Reward. Neuropsychopharmacology 2012, 37, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- Li, M.D.; van der Vaart, A.D. MicroRNAs in addiction: Adaptation’s middlemen? Mol. Psychiatry 2011, 16, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Jonkman, S.; Kenny, P.J. Molecular, Cellular, and Structural Mechanisms of Cocaine Addiction: A Key Role for MicroRNAs. Neuropsychopharmacology 2013, 38, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Liu, X.; Qin, S.; Guan, Y.; Liu, Y.; Cheng, Y.; Chen, X.; Li, W.; Wang, S.; et al. MicroRNA expression profile and functional analysis reveal that miR-382 is a critical novel gene of alcohol addiction. EMBO Mol. Med. 2013, 5, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Stott, S.R.W.; Ang, S.-L. The Generation of Midbrain Dopaminergic Neurons. In Patterning and Cell Type Specification in the Developing CNS and PNS; Elsevier: Amsterdam, The Netherlands, 2013; pp. 435–453. [Google Scholar]
- Schultz, W. Dopamine neurons and their role in reward mechanisms. Curr. Opin. Neurobiol. 1997, 7, 191–197. [Google Scholar] [CrossRef]
- Bromberg-Martin, E.S.; Matsumoto, M.; Hikosaka, O. Dopamine in Motivational Control: Rewarding, Aversive, and Alerting. Neuron 2010, 68, 815–834. [Google Scholar] [CrossRef] [PubMed]
- Rubino, T.; Forlani, G.; Viganò, D.; Zippel, R.; Parolaro, D. Modulation of extracellular signal-regulated kinases cascade by chronic Δ9-tetrahydrocannabinol treatment. Mol. Cell. Neurosci. 2004, 25, 355–362. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, M.; Shiwalkar, N.; Reddy, K.; Shin, P.; Bekker, A. Neurobiology of Propofol Addiction and Supportive Evidence: What Is the New Development? Brain Sci. 2018, 8, 36. https://doi.org/10.3390/brainsci8020036
Xiong M, Shiwalkar N, Reddy K, Shin P, Bekker A. Neurobiology of Propofol Addiction and Supportive Evidence: What Is the New Development? Brain Sciences. 2018; 8(2):36. https://doi.org/10.3390/brainsci8020036
Chicago/Turabian StyleXiong, Ming, Nimisha Shiwalkar, Kavya Reddy, Peter Shin, and Alex Bekker. 2018. "Neurobiology of Propofol Addiction and Supportive Evidence: What Is the New Development?" Brain Sciences 8, no. 2: 36. https://doi.org/10.3390/brainsci8020036




