The Effect of Axon Resealing on Retrograde Neuronal Death after Spinal Cord Injury in Lamprey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of Nissl-Stained Brain Wholemounts and Calculation of Neuronal Sizes
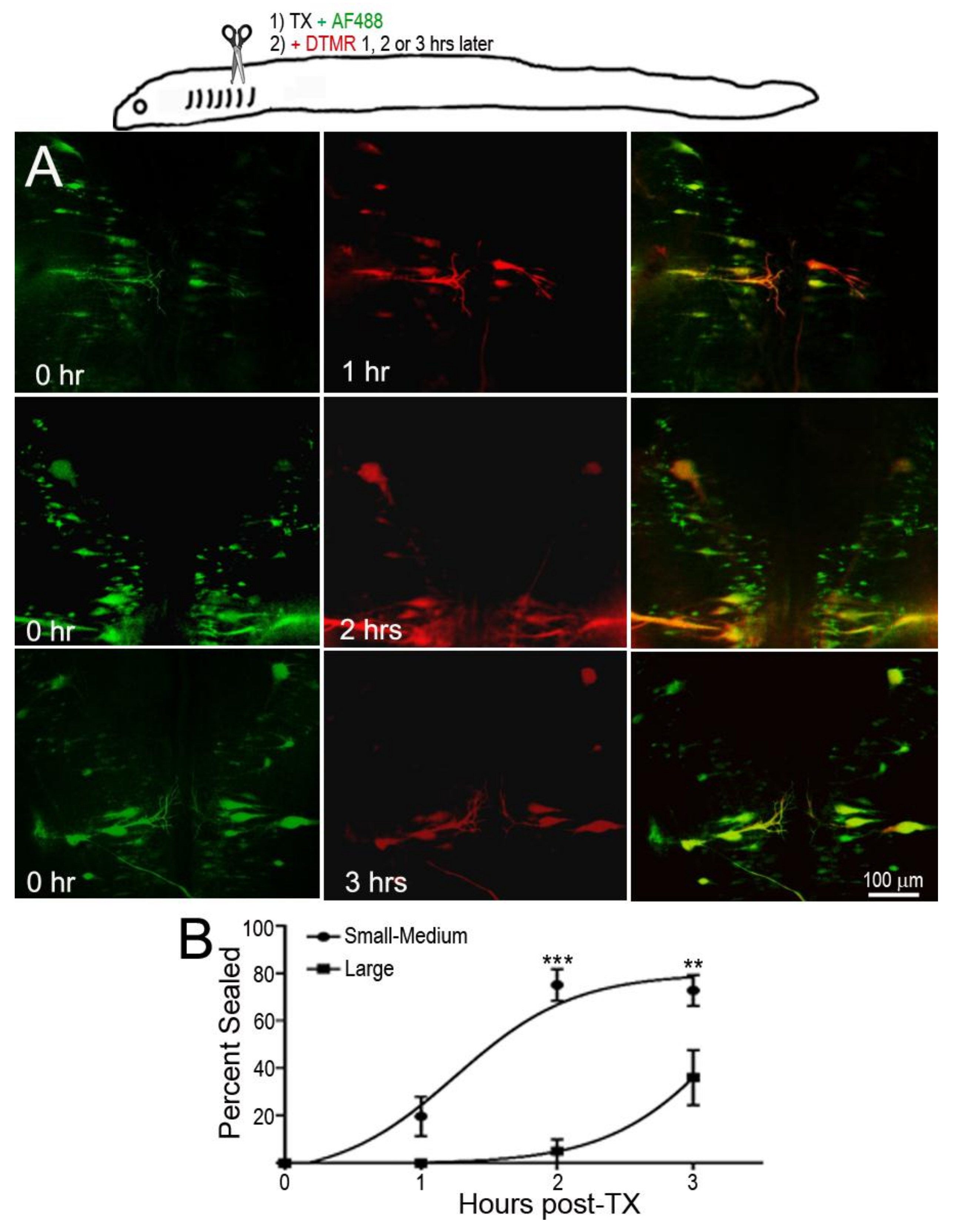
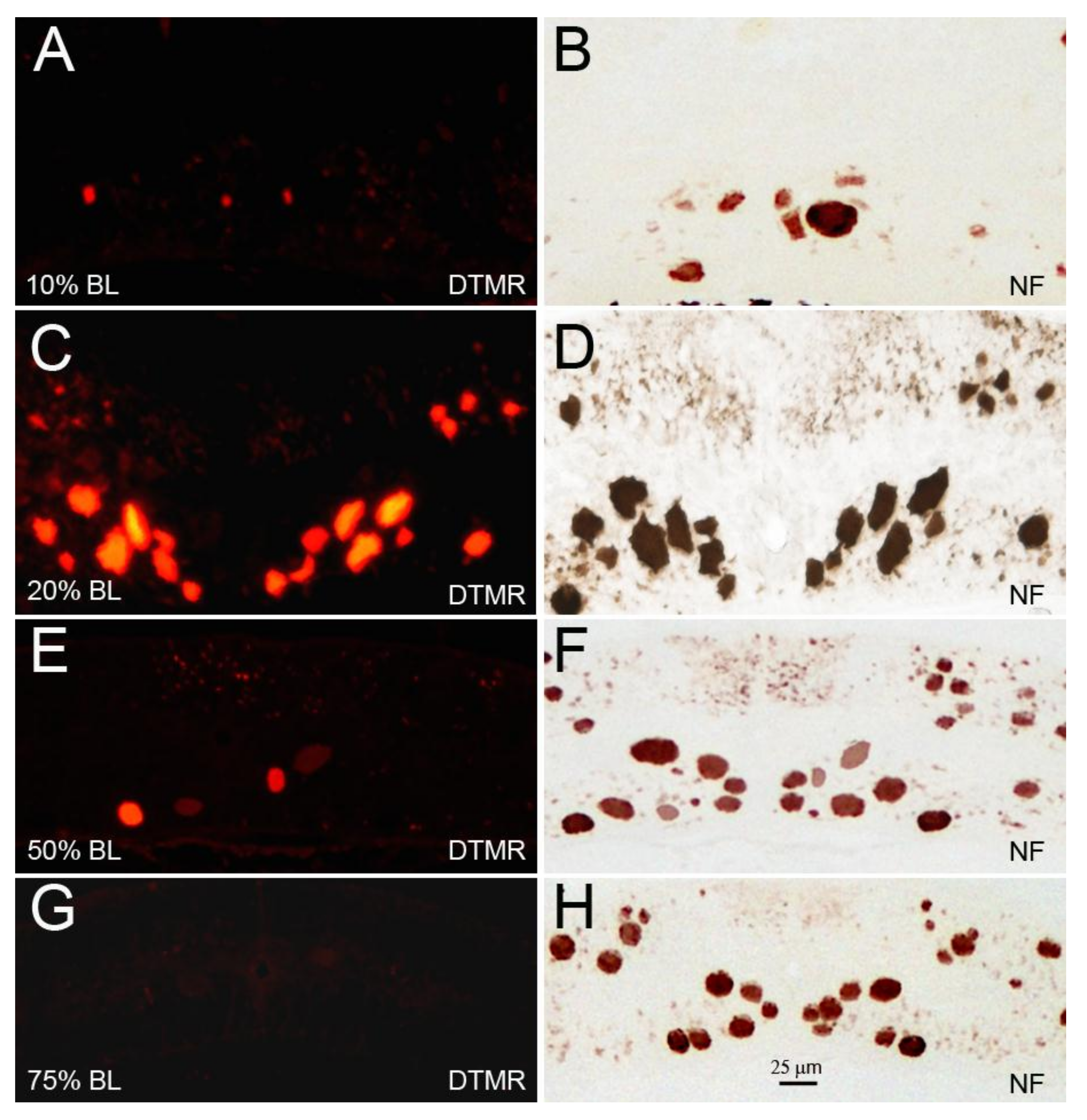
2.3. Spinal Cord Transection and Dye Exclusion Assay to Determine Axon Sealing Kinetics
2.4. Detecting Apoptosis Signaling with Fluorescently-Labeled Inhibitor of Caspases (FLICA)
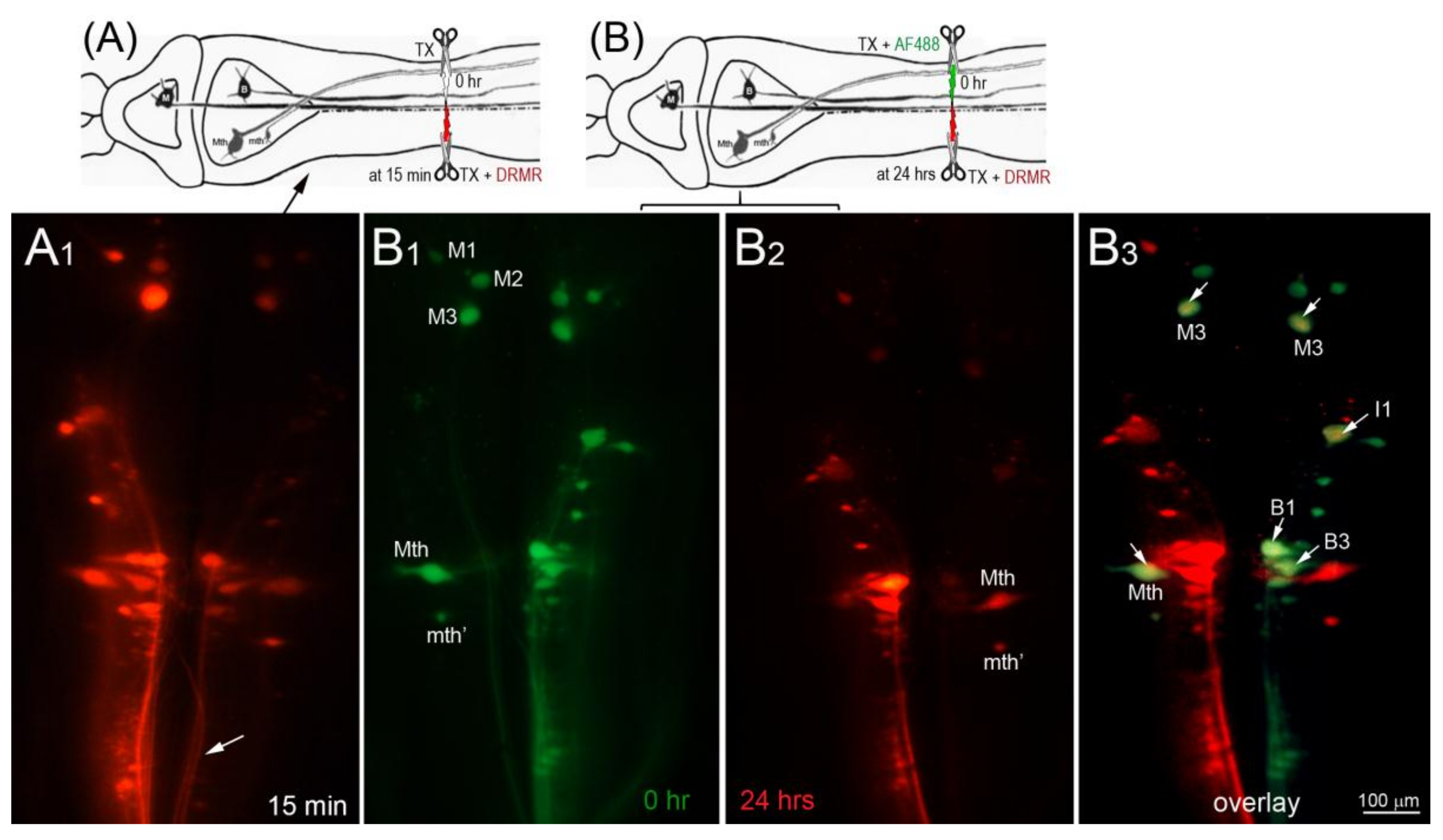
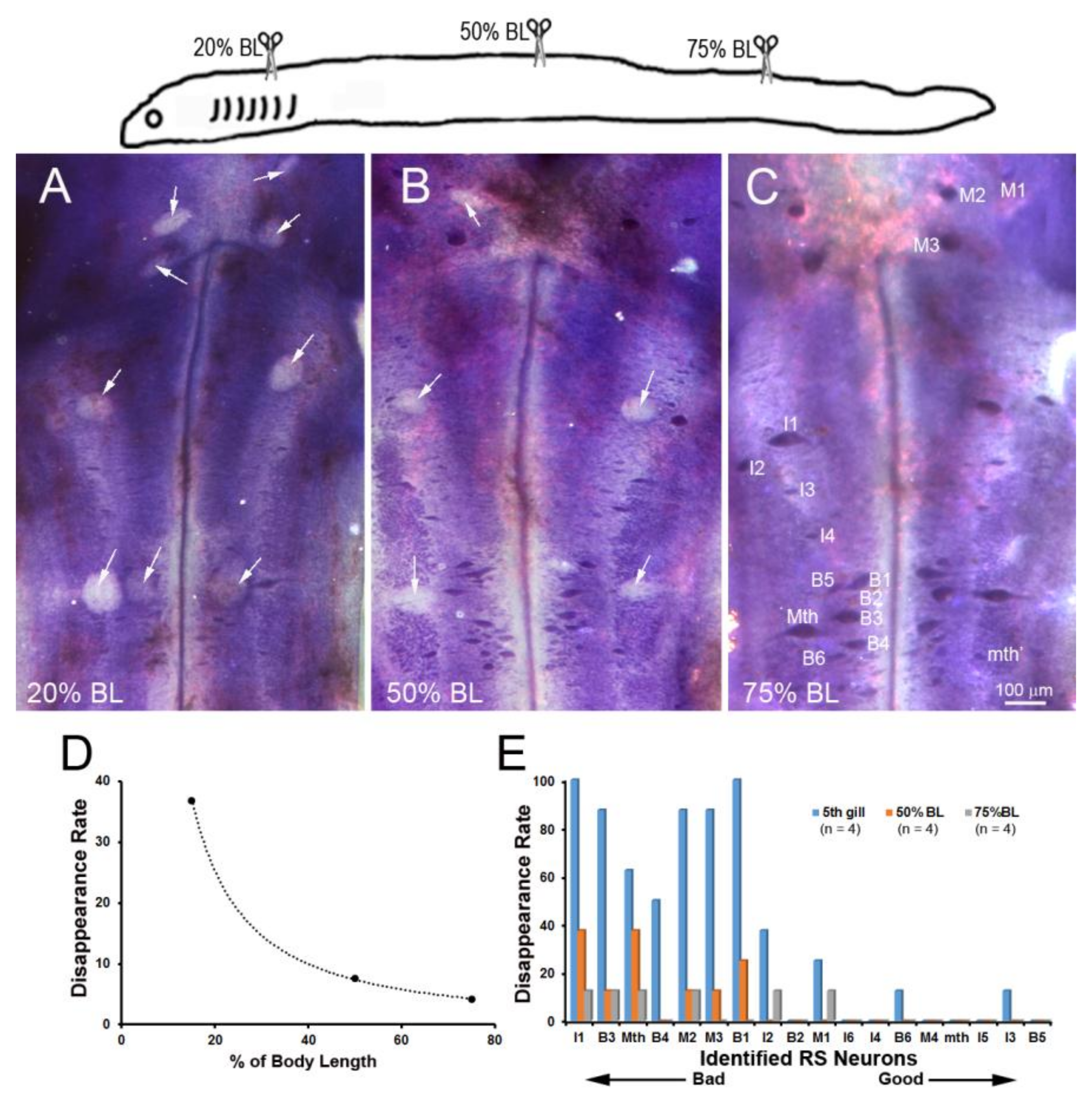
2.5. Measuring the Effect of the Distance of TX from the Cell Body on Retrograde Neuronal Death
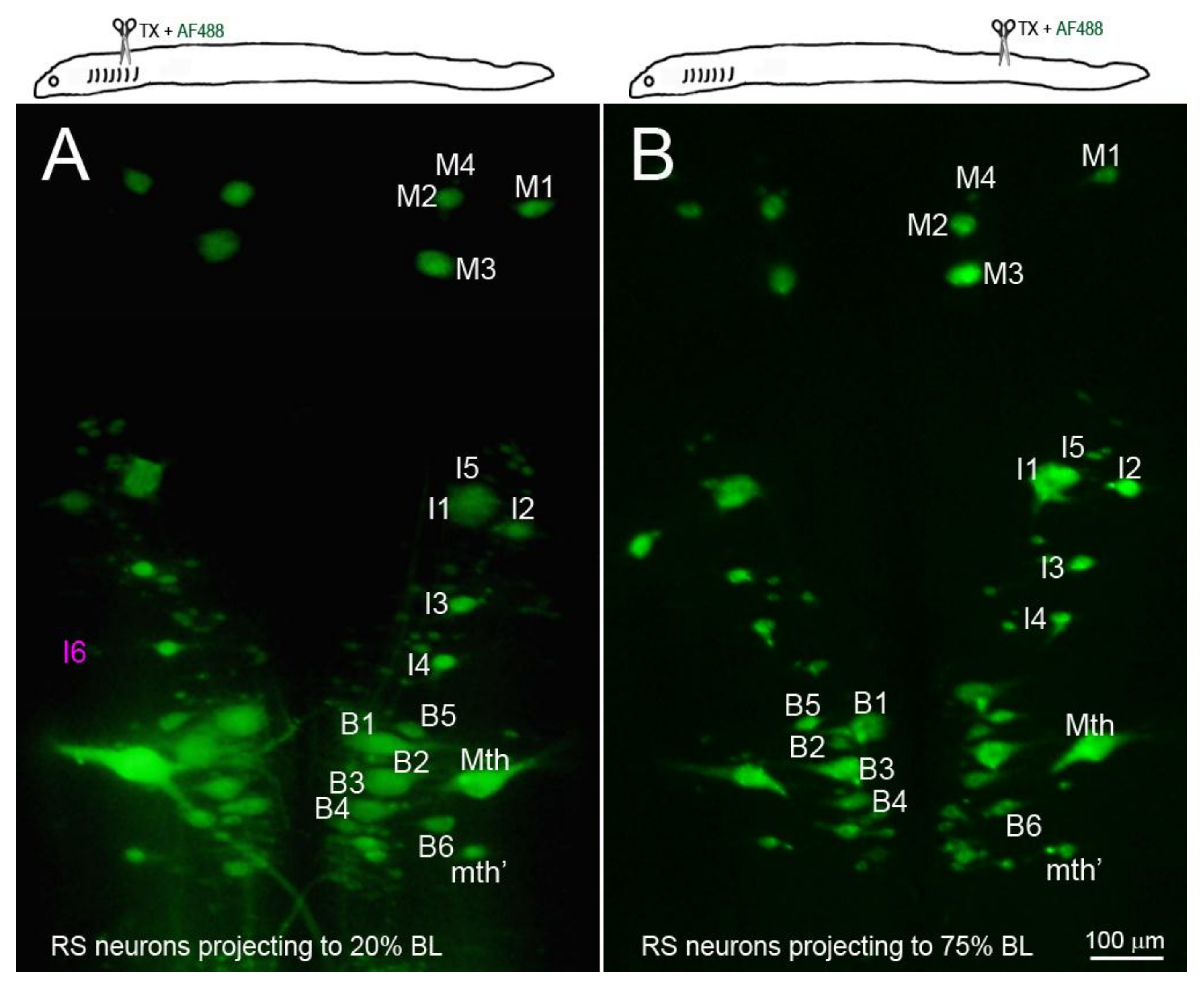
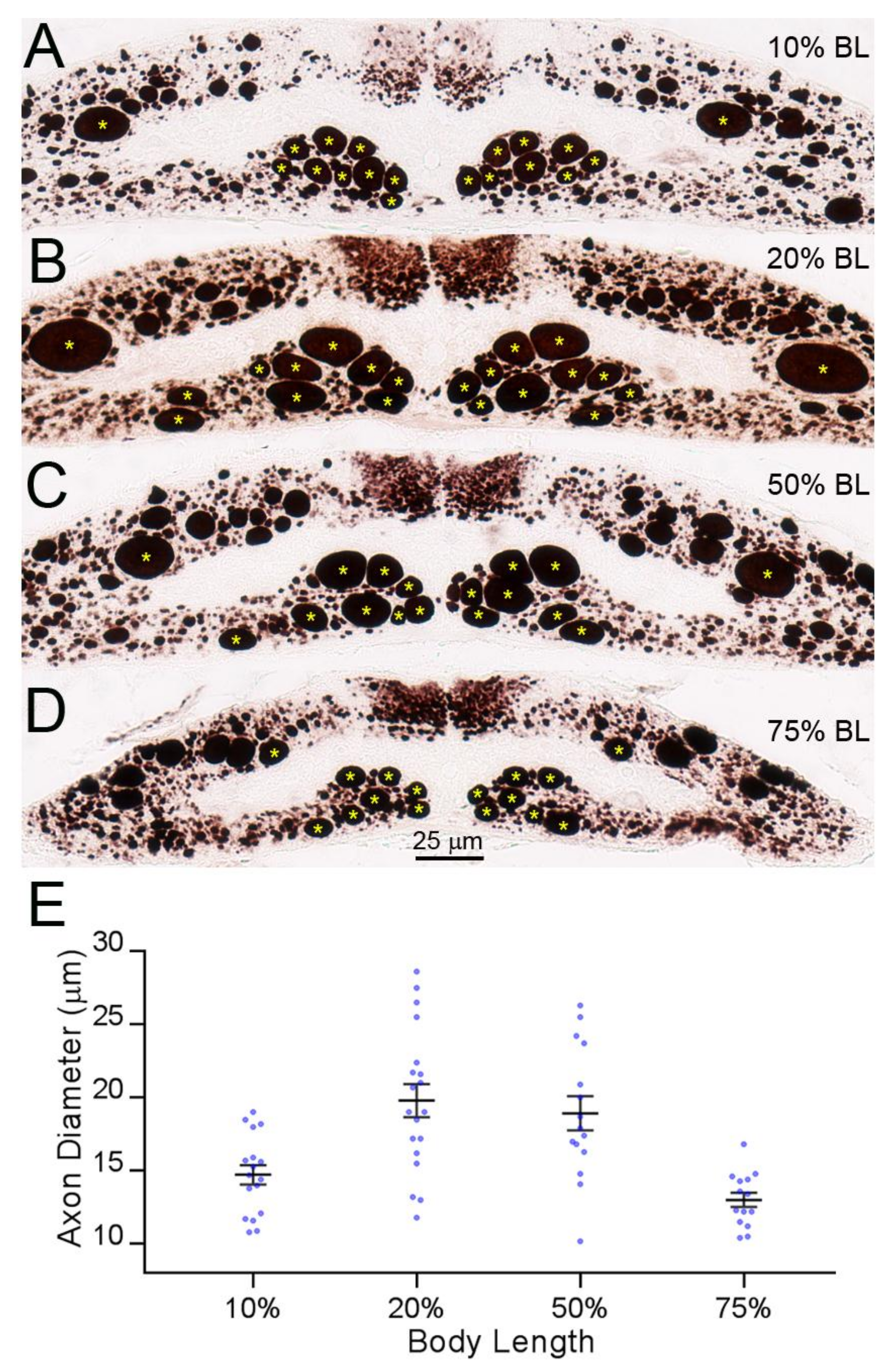
2.6. Distinguishing Distance-Dependence of Retrograde Apoptotic Signaling from the Effects of Variation of Axon Caliber along the Length of the Lamprey’s Body
2.7. Neurofilament (NF) Immunostaining
2.8. Accelerating Axon Sealing with PEG
2.9. Statistical Analysis
3. Results
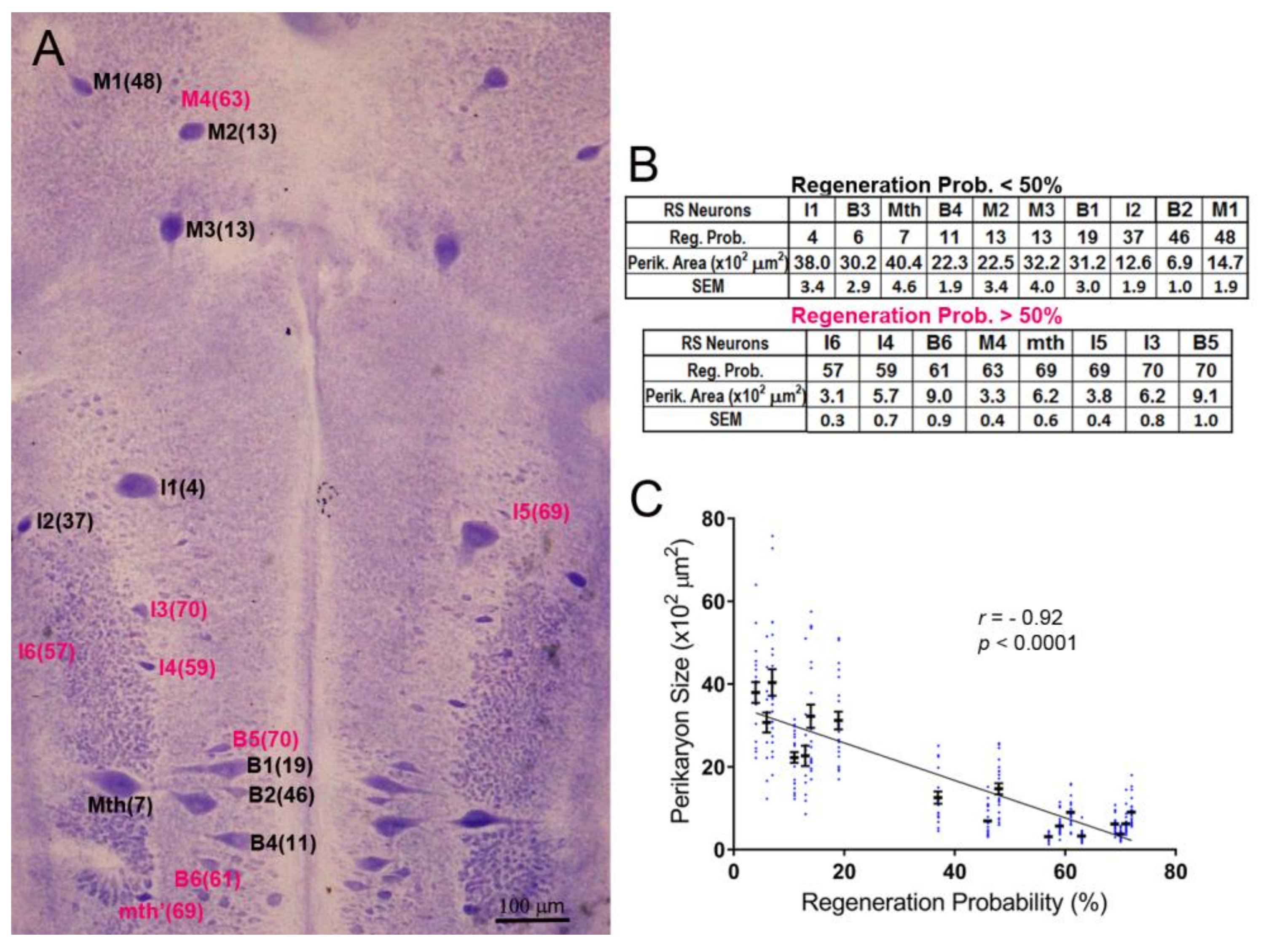
3.1. The Probability of Axonal Regeneration Correlated Inversely with Neuron Size
3.2. Axon Sealing of Identified RS Neurons after Injury
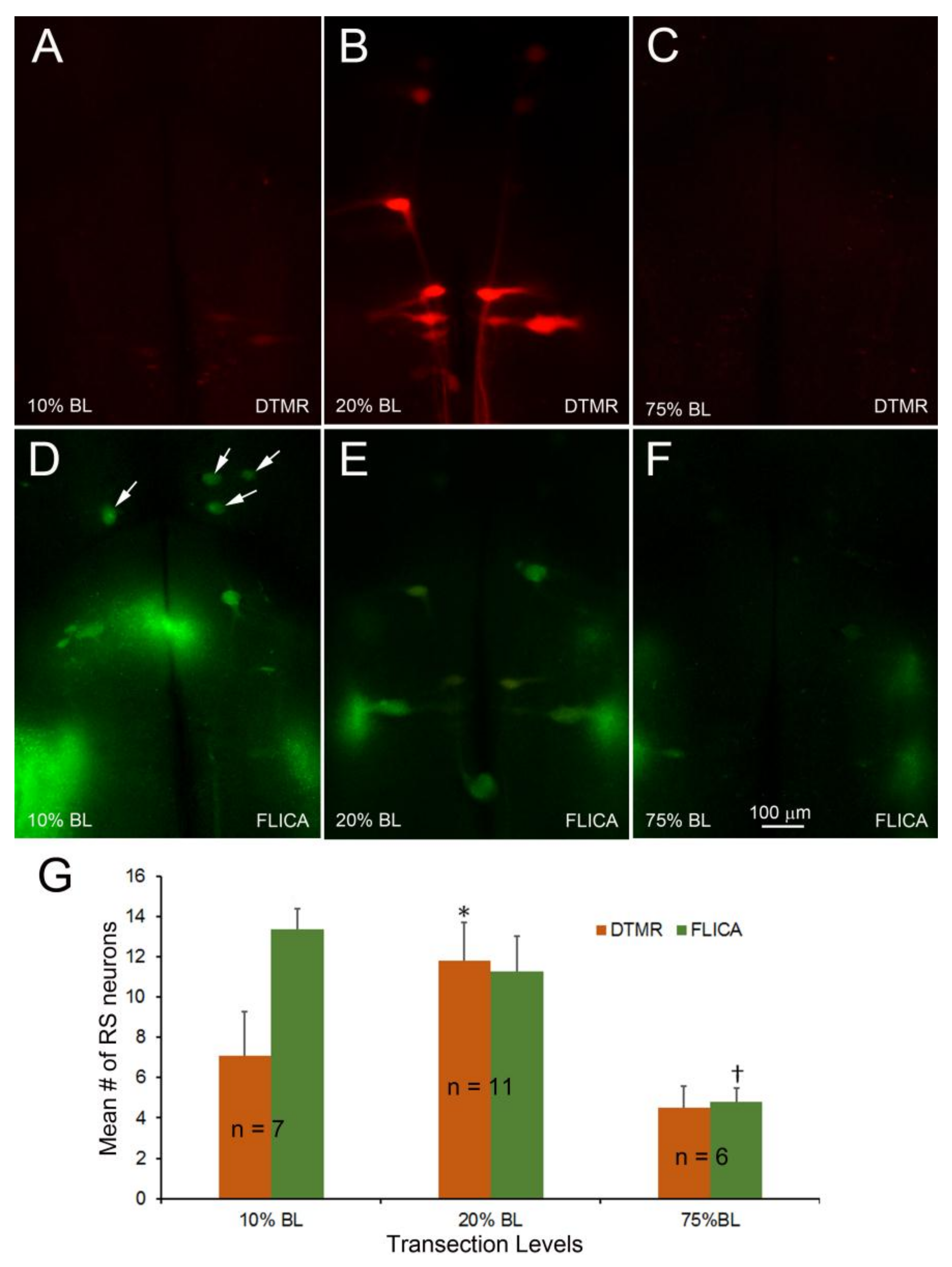
3.3. Most RS Neurons with Delayed Axon Sealing Were FLICA Positive
3.4. Distance-Dependence of Retrograde RS Neuron Death
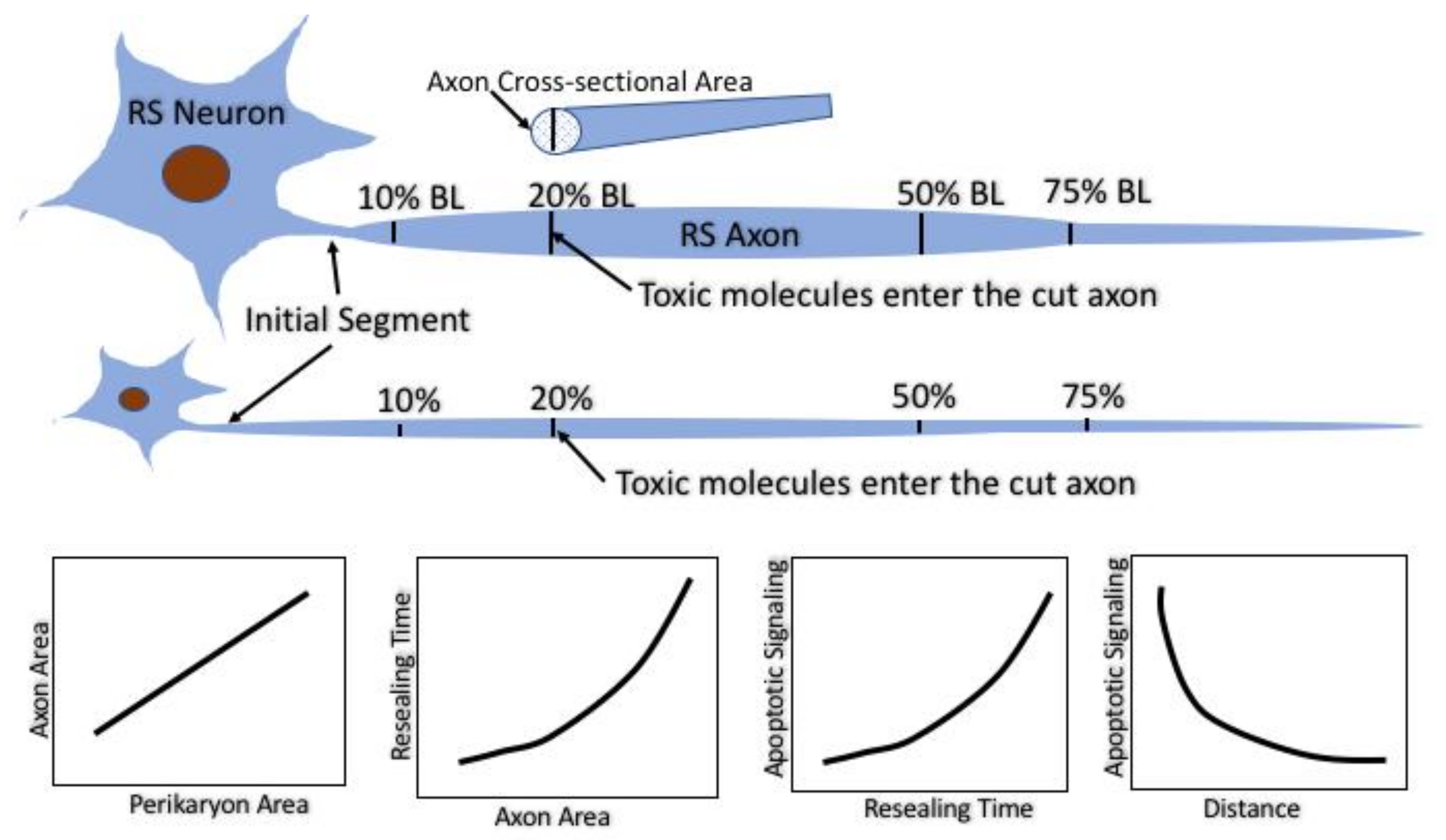
3.5. The Sealing of Axons Depends on Their Caliber, Not on the Distance of Their Axotomy from the Perikaryon
3.6. Axotomy Distance and Axon Sealing Time Have Independent Effects on Retrograde Neuronal Death
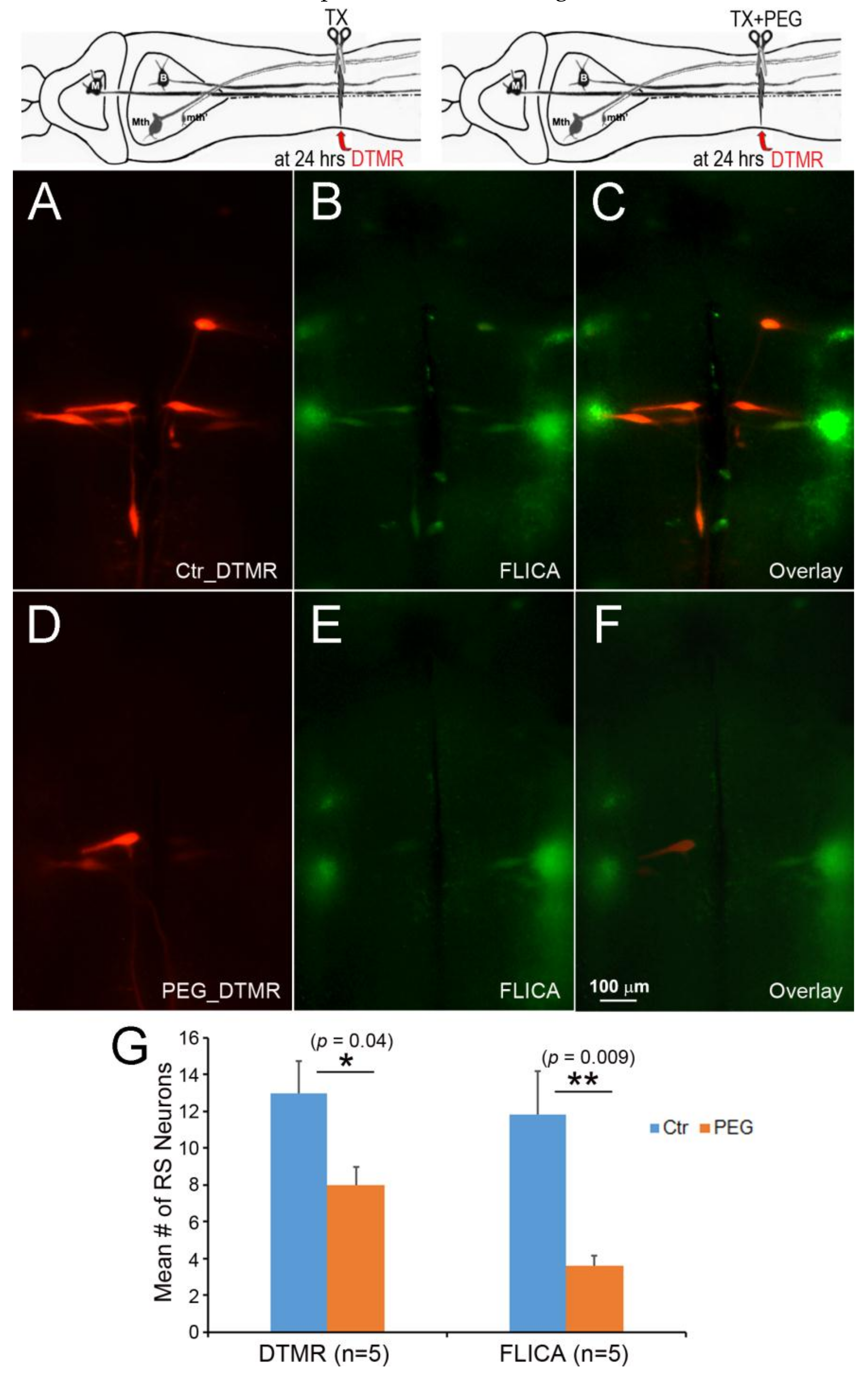
3.7. Facilitation of Axon Sealing with PEG Reduced Retrograde Neuronal Death
4. Discussion
4.1. Axon Caliber is Proportional to the Size of the Perikaryon
4.2. Axon Regeneration and Neuronal Survival after Axotomy Are Inversely Proportional to Neuron Size
4.3. Retrograde Neuronal Death Can Be Caused by Delayed Axon Sealing
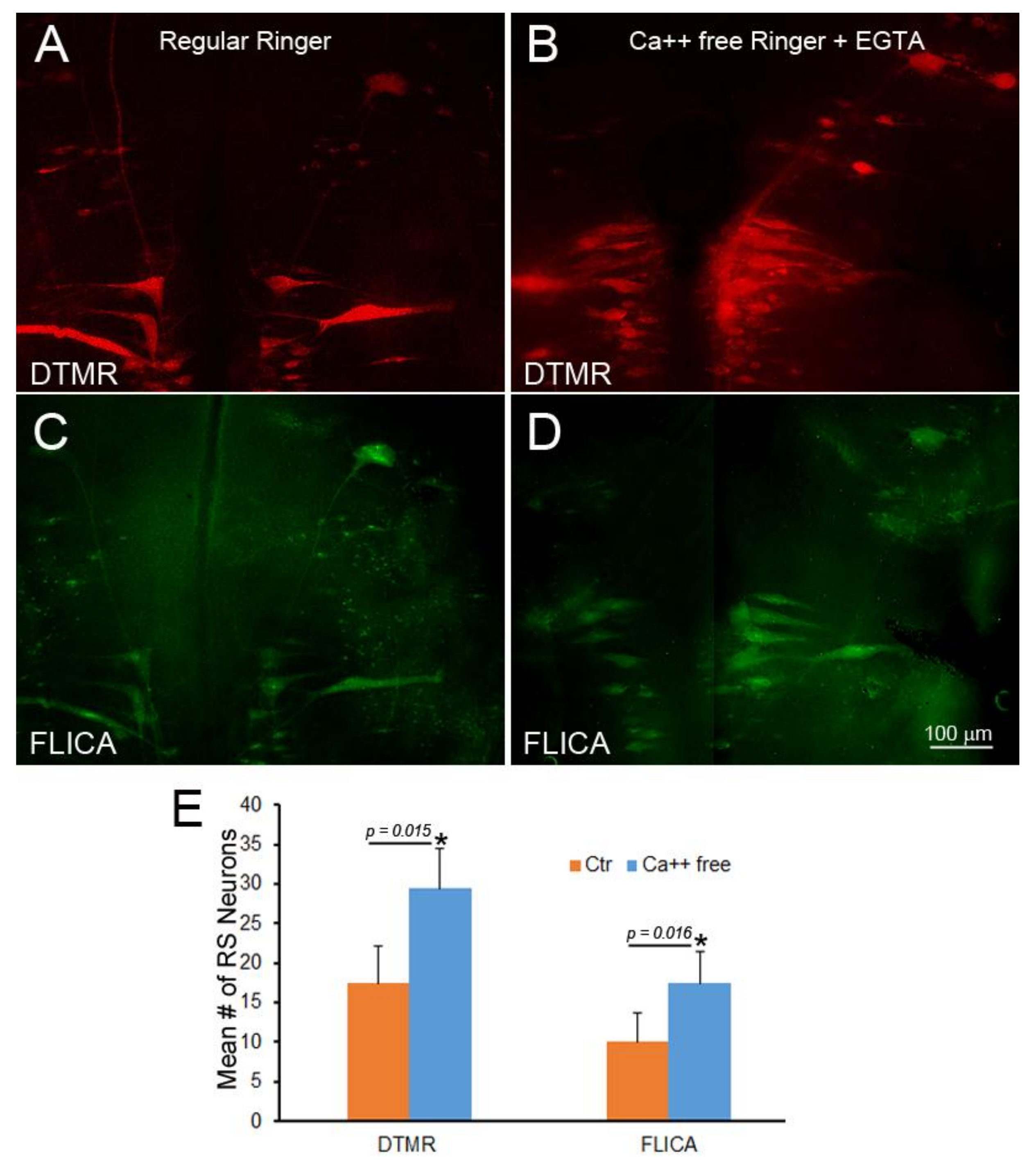
4.4. Mechanisms of Axon Sealing
4.5. Distance-Dependence of Neuronal Death after Axotomy
4.6. PEG Facilitates Axon Resealing and Prevents Retrograde Neuronal Death
4.7. PEG Did Not Prevent Wallerian Degeneration
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cohen, A.H.; Mackler, S.A.; Selzer, M.E. Functional regeneration following spinal transection demonstrated in the isolated spinal cord of the larval sea lamprey. Proc. Natl. Acad. Sci. USA 1986, 83, 2763–2766. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.R., Jr.; McClellan, A.D. Extent and time course of restoration of descending brainstem projections in spinal cord-transected lamprey. J. Comp. Neurol. 1994, 344, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Mackler, S.A.; Selzer, M.E. Regeneration of functional synapses between individual recognizable neurons in the lamprey spinal cord. Science 1985, 229, 774–776. [Google Scholar] [CrossRef] [PubMed]
- Mackler, S.A.; Selzer, M.E. Specificity of synaptic regeneration in the spinal cord of the larval sea lamprey. J. Physiol. 1987, 388, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Rovainen, C.M. Regeneration of Muller and Mauthner axons after spinal transection in larval lampreys. J. Comp. Neurol. 1976, 168, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Selzer, M.E. Mechanisms of functional recovery and regeneration after spinal cord transection in larval sea lamprey. J. Physiol. 1978, 277, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.R.; Cohen, M.J. Synaptic regeneration in identified neurons of the lamprey spinal cords. Science 1979, 206, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.S.; Selzer, M.E. Axonal regeneration in lamprey spinal cord. J. Neurosci. 1983, 3, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Oliphint, P.A.; Alieva, N.; Foldes, A.E.; Tytell, E.D.; Lau, B.Y.; Pariseau, J.S.; Cohen, A.H.; Morgan, J.R. Regenerated synapses in lamprey spinal cord are sparse and small even after functional recovery from injury. J. Comp. Neurol. 2010, 518, 2854–2872. [Google Scholar] [CrossRef] [PubMed]
- Shifman, M.I.; Zhang, G.; Selzer, M.E. Delayed death of identified reticulospinal neurons after spinal cord injury in lampreys. J. Comp. Neurol. 2008, 510, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hu, J.; Li, S.; Huang, L.; Selzer, M.E. Selective expression of CSPG receptors PTPsigma and LAR in poorly regenerating reticulospinal neurons of lamprey. J. Comp. Neurol. 2014, 522, 2209–2229. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Bottenstein, J.E.; Bittner, G.D.; Fishman, H.M. Survival of mammalian B104 cells following neurite transection at different locations depends on somal Ca2+ concentration. J. Neurobiol. 2004, 60, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.J.; Swain, G.P.; Snedeker, J.A.; Pijak, D.S.; Gladstone, L.J.; Selzer, M.E. Recovery of neurofilament expression selectively in regenerating reticulospinal neurons. J. Neurosci. 1997, 17, 5206–5220. [Google Scholar] [CrossRef] [PubMed]
- Bussieres, N.; Pflieger, J.F.; Dubuc, R. Anatomical study of vestibulospinal neurons in lampreys. J. Comp. Neurol. 1999, 407, 512–526. [Google Scholar] [CrossRef]
- Rovainen, C.M. Physiological and anatomical studies on large neurons of central nervous system of the sea lamprey (Petromyzon marinus). I. Muller and Mauthner cells. J. Neurophysiol. 1967, 30, 1000–1023. [Google Scholar] [CrossRef] [PubMed]
- Swain, G.P.; Snedeker, J.A.; Ayers, J.; Selzer, M.E. Cytoarchitecture of spinal-projecting neurons in the brain of the larval sea lamprey. J. Comp. Neurol. 1993, 336, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.R., Jr.; McClellan, A.D. Long distance axonal regeneration of identified lamprey reticulospinal neurons. Exp. Neurol. 1994, 127, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Shifman, M.I.; Selzer, M.E. Expression of the netrin receptor UNC-5 in lamprey brain: Modulation by spinal cord transection. Neurorehabil. Neural Repair 2000, 14, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Shifman, M.I.; Yumul, R.E.; Laramore, C.; Selzer, M.E. Expression of the repulsive guidance molecule RGM and its receptor neogenin after spinal cord injury in sea lamprey. Exp. Neurol. 2009, 217, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Busch, D.J.; Morgan, J.R. Synuclein accumulation is associated with cell-specific neuronal death after spinal cord injury. J. Comp. Neurol. 2012, 520, 1751–1771. [Google Scholar] [CrossRef] [PubMed]
- Herman, P.E.; Papatheodorou, A.; Bryant, S.A.; Waterbury, C.K.M.; Herdy, J.R.; Arcese, A.A.; Buxbaum, J.D.; Smith, J.J.; Morgan, J.R.; Bloom, O. Highly conserved molecular pathways, including Wnt signaling, promote functional recovery from spinal cord injury in lampreys. Sci. Rep. 2018, 8, 742. [Google Scholar] [CrossRef] [PubMed]
- Tuszynski, M.H.; Steward, O. Concepts and methods for the study of axonal regeneration in the CNS. Neuron 2012, 74, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.A.; Ingoglia, N.A.; Sharma, S.C. Axon resealing following transection takes longer in central axons than in peripheral axons: Implications for axonal regeneration. Exp. Neurol. 2001, 167, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.J.; David, G.; Barrett, J.N. Resealing of transected myelinated mammalian axons in vivo: Evidence for involvement of calpain. Neuroscience 1999, 93, 807–815. [Google Scholar] [CrossRef]
- Duan, X.; Qiao, M.; Bei, F.; Kim, I.J.; He, Z.; Sanes, J.R. Subtype-specific regeneration of retinal ganglion cells following axotomy: Effects of osteopontin and mTOR signaling. Neuron 2015, 85, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.J.; Fan, D.P.; Tsui, B.J.; Cassar, S.L.; Tetzlaff, W. Influence of the axotomy to cell body distance in rat rubrospinal and spinal motoneurons: Differential regulation of GAP-43, tubulins, and neurofilament-M. J. Comp. Neurol. 1999, 414, 495–510. [Google Scholar] [CrossRef]
- You, S.W.; So, K.F.; Yip, H.K. Axonal regeneration of retinal ganglion cells depending on the distance of axotomy in adult hamsters. Investig. Ophthalmol. Visual Sci. 2000, 41, 3165–3170. [Google Scholar]
- Nehrt, A.; Hamann, K.; Ouyang, H.; Shi, R. Polyethylene glycol enhances axolemmal resealing following transection in cultured cells and in ex vivo spinal cord. J. Neurotrauma 2010, 27, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Barreiro-Iglesias, A.; Zhang, G.; Selzer, M.E.; Shifman, M.I. Complete spinal cord injury and brain dissection protocol for subsequent wholemount in situ hybridization in larval sea lamprey. J. Vis. Exp. 2014, 92. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.S.; Bell, E.M. A rapid method for the demonstration of nerve cell bodies in invertebrate central nervous systems. Brain Res. 1973, 63, 487–489. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, G.; Rodemer, W.; Jin, L.Q.; Shifman, M.; Selzer, M.E. The role of RhoA in retrograde neuronal death and axon regeneration after spinal cord injury. Neurobiol. Dis. 2017, 98, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Rovainen, C.M.; Johnson, P.A.; Roach, E.A.; Mankovsky, J.A. Projections of Individual Axons in Lamprey Spinal-Cord Determined by Tracings through Serial Sections. J. Comp. Neurol. 1973, 149, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.L.; Danzer, S.C.; Govind, C.K.; Kirk, M.D. Morphological correlates of neural regeneration in the feeding system of Aplysia californica after central nervous system lesions. J. Comp. Neurol. 1997, 387, 279–290. [Google Scholar] [CrossRef]
- Olby, N.J.; Blakemore, W.F. Primary demyelination and regeneration of ascending axons in the dorsal funiculus of the rat spinal cord following photochemically induced injury. J. Neurocytol. 1996, 25, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Blight, A.R.; Decrescito, V. Morphometric analysis of experimental spinal cord injury in the cat: The relation of injury intensity to survival of myelinated axons. Neuroscience 1986, 19, 321–341. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Wong, T.H.; Tator, C.H.; Tymianski, M. Effect of a direct current field on axons after experimental spinal cord injury, Canadian journal of surgery. J. Chir. 1989, 32, 188–191. [Google Scholar]
- Masland, R.H. The neuronal organization of the retina. Neuron 2012, 76, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Bittner, G.D.; Spaeth, C.S.; Poon, A.D.; Burgess, Z.S.; McGill, C.H. Repair of traumatic plasmalemmal damage to neurons and other eukaryotic cells. Neural Regen. Res. 2016, 11, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Yawo, H.; Kuno, M. How a nerve fiber repairs its cut end: Involvement of phospholipase A2. Science 1983, 222, 1351–1353. [Google Scholar] [CrossRef] [PubMed]
- Spira, M.E.; Benbassat, D.; Dormann, A. Resealing of the proximal and distal cut ends of transected axons: Electrophysiological and ultrastructural analysis. J. Neurobiol. 1993, 24, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Gallant, P.E. Effects of the external ions and metabolic poisoning on the constriction of the squid giant axon after axotomy. J. Neurosci. 1988, 8, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Krause, T.L.; Fishman, H.M.; Ballinger, M.L.; Bittner, G.D. Extent and mechanism of sealing in transected giant axons of squid and earthworms. J. Neurosci. 1994, 14, 6638–6651. [Google Scholar] [CrossRef] [PubMed]
- Eddleman, C.S.; Ballinger, M.L.; Smyers, M.E.; Fishman, H.M.; Bittner, G.D. Endocytotic formation of vesicles and other membranous structures induced by Ca2+ and axolemmal injury. J. Neurosci. 1998, 18, 4029–4041. [Google Scholar] [CrossRef] [PubMed]
- Eddleman, C.S.; Ballinger, M.L.; Smyers, M.E.; Godell, C.M.; Fishman, H.M.; Bittner, G.D. Repair of plasmalemmal lesions by vesicles. Proc. Natl. Acad. Sci. USA 1997, 94, 4745–4750. [Google Scholar] [CrossRef] [PubMed]
- Fishman, H.M.; Bittner, G.D. Vesicle-mediated restoration of a plasmalemmal barrier in severed axons. Physiology 2003, 18, 115–118. [Google Scholar] [CrossRef]
- Hendricks, B.K.; Shi, R. Mechanisms of neuronal membrane sealing following mechanical trauma. Neurosci. Bull. 2014, 30, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Spaeth, C.S.; Spaeth, E.B.; Wilcott, R.W.; Fan, J.D.; Robison, T.; Bittner, G.D. Pathways for plasmalemmal repair mediated by PKA, Epac, and cytosolic oxidation in rat B104 cells in vitro and rat sciatic axons ex vivo. Dev. Neurobiol. 2012, 72, 1399–1414. [Google Scholar] [CrossRef] [PubMed]
- Zuzek, A.; Fan, J.D.; Spaeth, C.S.; Bittner, G.D. Sealing of transected neurites of rat B104 cells requires a diacylglycerol PKC-dependent pathway and a PKA-dependent pathway. Cell. Mol. Neurobiol. 2013, 33, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Detrait, E.R.; Yoo, S.; Eddleman, C.S.; Fukuda, M.; Bittner, G.D.; Fishman, H.M. Plasmalemmal repair of severed neurites of PC12 cells requires Ca2+ and synaptotagmin. J. Neurosci. Res. 2000, 62, 566–573. [Google Scholar] [CrossRef]
- Strautman, A.F.; Cork, R.J.; Robinson, K.R. The distribution of free calcium in transected spinal axons and its modulation by applied electrical fields. J. Neurosci. 1990, 10, 3564–3575. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.R.; Marincu, B.N.; Sorbara, C.D.; Mahler, C.F.; Schumacher, A.M.; Griesbeck, O.; Kerschensteiner, M.; Misgeld, T. A recoverable state of axon injury persists for hours after spinal cord contusion in vivo. Nat. Commun. 2014, 5, 5683. [Google Scholar] [CrossRef] [PubMed]
- Dusart, I.; Schwab, M.E. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur. J. Neurosci. 1994, 6, 712–724. [Google Scholar] [CrossRef] [PubMed]
- Blight, A.R. Macrophages and inflammatory damage in spinal cord injury. J. Neurotrauma 1992, 9 (Suppl. 1), S83–S91. [Google Scholar] [PubMed]
- Blight, A.R.; Cohen, T.I.; Saito, K.; Heyes, M.P. Quinolinic acid accumulation and functional deficits following experimental spinal cord injury. Brain 1995, 118 Pt 3, 735–752. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Selzer, M.E.; Li, S. Scar-mediated inhibition and CSPG receptors in the CNS. Exp. Neurol. 2012, 237, 370–378. [Google Scholar] [CrossRef] [PubMed]
- McKeon, R.J.; Schreiber, R.C.; Rudge, J.S.; Silver, J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J. Neurosci. 1991, 11, 3398–3411. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.H.; Gross, G.W.; Emery, D.G.; Gardner, C.R. Neuronal survival or death after dendrite transection close to the perikaryon: Correlation with electrophysiologic, morphologic, and ultrastructural changes. Cent. Nerv. Syst. Trauma 1985, 2, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.N.; Quinn, J. The relationship between some measures of synaptic ultrastructure as a function of distance from the soma on lamprey reticulospinal neurons. J. Neurocytol. 1979, 8, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, P.; Christensen, B.N. A quantitative analysis of ultrastructural changes induced by electrical stimulation of identified spinal cord axons in the larval lamprey. J. Neurocytol. 1980, 9, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.S.; Jarlfors, U.; Beranek, R. The organization of synaptic axcplasm in the lamprey (Petromyzon marinus) central nervous system. J. Cell Biol. 1970, 46, 199–219. [Google Scholar] [CrossRef] [PubMed]
- Spaeth, C.S.; Robison, T.; Fan, J.D.; Bittner, G.D. Cellular mechanisms of plasmalemmal sealing and axonal repair by polyethylene glycol and methylene blue. J. Neurosci. Res. 2012, 90, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Roederer, E.; Goldberg, N.H.; Cohen, M.J. Modification of retrograde degeneration in transected spinal axons of the lamprey by applied DC current. J. Neurosci. 1983, 3, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jin, L.Q.; Sul, J.Y.; Haydon, P.G.; Selzer, M.E. Live imaging of regenerating lamprey spinal axons. Neurorehabil. Neural Repair 2005, 19, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Bittner, G.D.; Ballinger, M.L.; Raymond, M.A. Reconnection of severed nerve axons with polyethylene glycol. Brain Res. 1986, 367, 351–355. [Google Scholar] [CrossRef]
- Krause, T.L.; Bittner, G.D. Rapid morphological fusion of severed myelinated axons by polyethylene glycol. Proc. Natl. Acad. Sci. USA 1990, 87, 1471–1475. [Google Scholar] [CrossRef] [PubMed]
- Lore, A.B.; Hubbell, J.A.; Bobb, D.S.; Ballinger, M.L.; Loftin, K.L.; Smith, J.W.; Smyers, M.E.; Garcia, H.D.; Bittner, G.D. Rapid induction of functional and morphological continuity between severed ends of mammalian or earthworm myelinated axons. J. Neurosci. 1999, 19, 2442–2454. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Rodemer, W.; Lee, T.; Hu, J.; Selzer, M.E. The Effect of Axon Resealing on Retrograde Neuronal Death after Spinal Cord Injury in Lamprey. Brain Sci. 2018, 8, 65. https://doi.org/10.3390/brainsci8040065
Zhang G, Rodemer W, Lee T, Hu J, Selzer ME. The Effect of Axon Resealing on Retrograde Neuronal Death after Spinal Cord Injury in Lamprey. Brain Sciences. 2018; 8(4):65. https://doi.org/10.3390/brainsci8040065
Chicago/Turabian StyleZhang, Guixin, William Rodemer, Taemin Lee, Jianli Hu, and Michael E. Selzer. 2018. "The Effect of Axon Resealing on Retrograde Neuronal Death after Spinal Cord Injury in Lamprey" Brain Sciences 8, no. 4: 65. https://doi.org/10.3390/brainsci8040065




