Neurite Growth and Polarization on Vitronectin Substrate after in Vitro Trauma is not Enhanced after IGF Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Primary Hippocampal Cell Culture
2.2. In Vitro Trauma
2.3. Immunohistochemistry
2.4. Microscopy Analysis
2.5. Statistical Analysis
3. Results
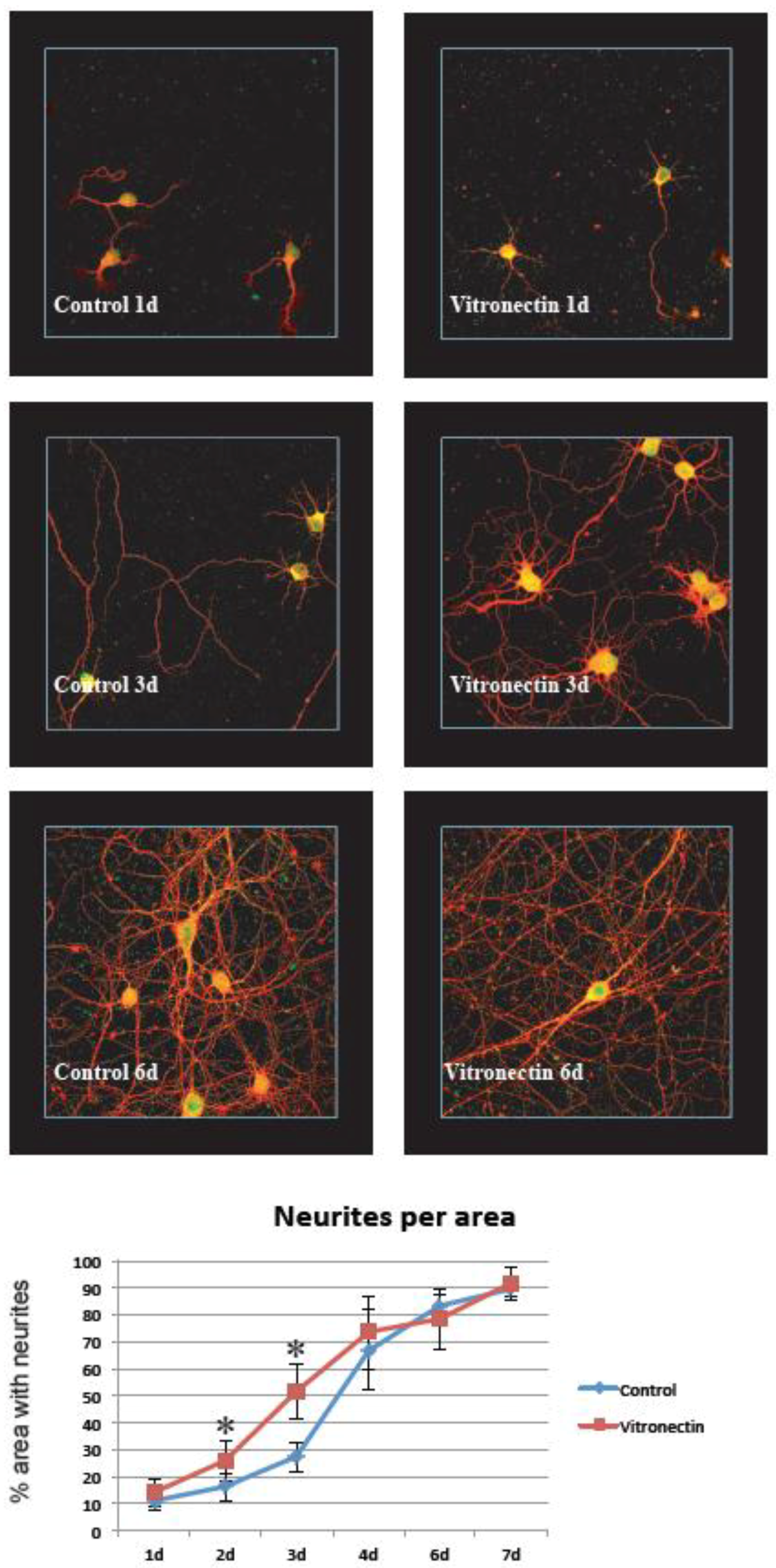
3.1. Neurite Growth on Poly-L-lysin Compared to Vitronectin
3.2. Neurite Growth after In Vitro Trauma
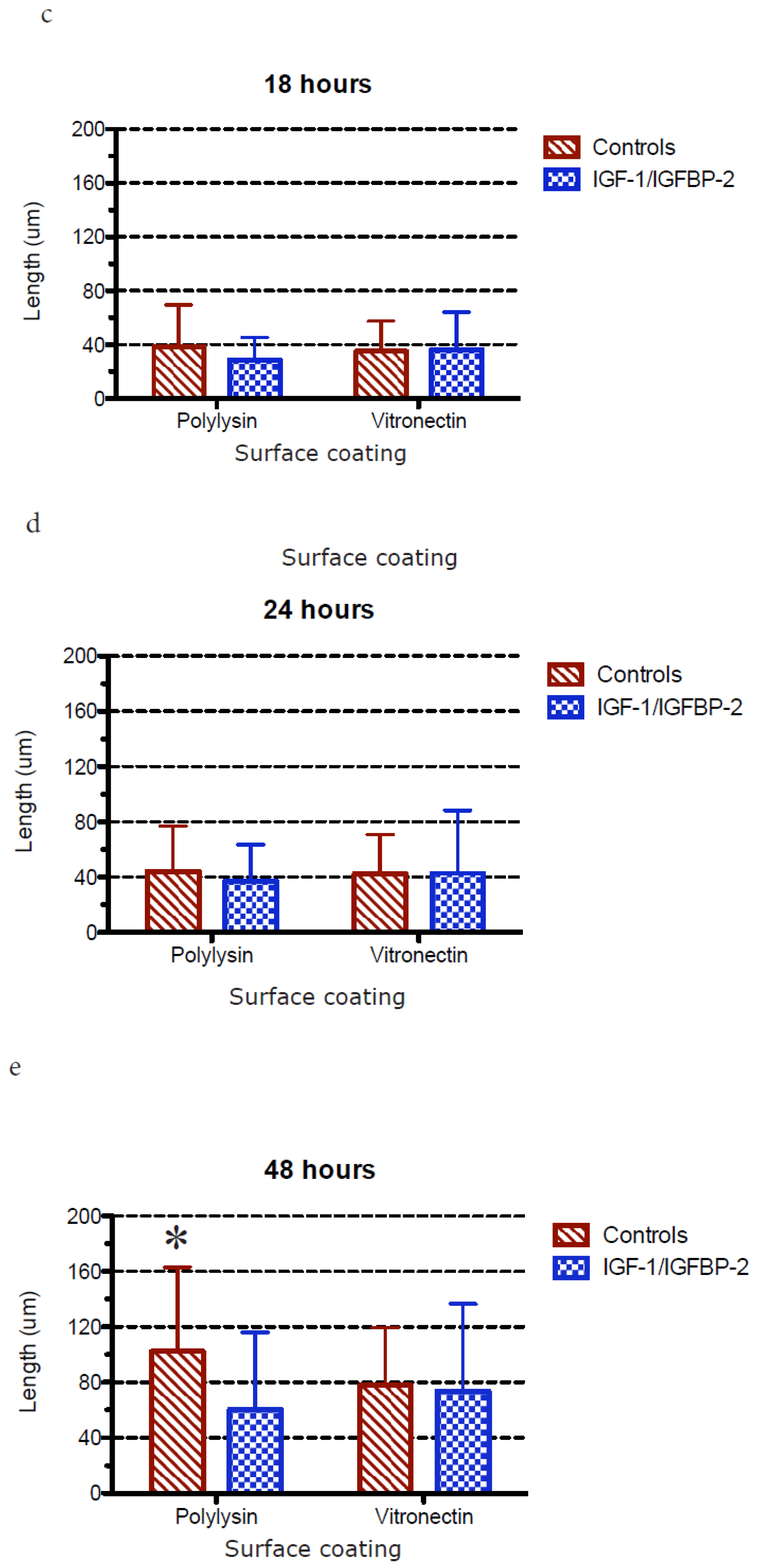
3.3. Measurements of Neurite Polarization
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sandberg Nordqvist, A.C.; von Holst, H.; Holmin, S.; Sara, V.R.; Bellander, B.M.; Schalling, M. Increase of insulin-like growth factor (IGF)-1, IGF binding protein-2 and -4 mRNAs following cerebral contusion. Brain Res. Mol. Brain Res. 1996, 38, 285–293. [Google Scholar] [CrossRef]
- Schober, M.E.; Ke, X.; Block, B.P.; Requena, D.F.; McKnight, R.; Lane, R.H. Traumatic brain injury increased IGF-1B mRNA and altered IGF-1 exon 5 and promoter region epigenetic characteristics in the rat pup hippocampus. J. Neurotrauma 2012, 29, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Bach, M.A.; Shen-Orr, Z.; Lowe, W.L., Jr.; Roberts, C.T., Jr.; LeRoith, D. Insulin-like growth factor I mRNA levels are developmentally regulated in specific regions of the rat brain. Brain Res. Mol. Brain Res. 1991, 10, 43–48. [Google Scholar] [CrossRef]
- Madathil, S.K.; Evans, H.N.; Saatman, K.E. Temporal and regional changes in IGF-1/IGF-1R signaling in the mouse brain after traumatic brain injury. J. Neurotrauma 2010, 27, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Pomytkin, I.; Costa-Nunes, J.P.; Kasatkin, V.; Veniaminova, E.; Demchenko, A.; Lyundup, A.; Lesch, K.P.; Ponomarev, E.D.; Strekalova, T. Insulin receptor in the brain: Mechanisms of activation and the role in the CNS pathology and treatment. CNS Neurosci. Ther. 2018. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/cns.12866. [CrossRef] [PubMed]
- Pons, S.; Torres-Aleman, I. Insulin-like growth factor-I stimulates dephosphorylation of ikappa B through the serine phosphatase calcineurin (protein phosphatase 2B). J. Biol. Chem. 2000, 275, 38620–38625. [Google Scholar] [CrossRef] [PubMed]
- Clawson, T.F.; Vannucci, S.J.; Wang, G.M.; Seaman, L.B.; Yang, X.L.; Lee, W.H. Hypoxia-ischemia-induced apoptotic cell death correlates with IGF-1 mRNA decrease in neonatal rat brain. Biol. Signals Recept. 1999, 8, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Wang, G.M.; Yang, X.L.; Seaman, L.B.; Vannucci, S.I. Perinatal hypoxia-ischemia decreased neuronal but increased cerebral vascular endothelial IGFBP3 expression. Endocrine 1999, 11, 181–188. [Google Scholar] [CrossRef]
- Breese, C.R.; D’Costa, A.; Rollins, Y.D.; Adams, C.; Booze, R.M.; Sonntag, W.E.; Leonard, S. Expression of insulin-like growth factor-1 (IGF-1) and IGF-binding protein 2 (IGF-BP2) in the hippocampus following cytotoxic lesion of the dentate gyrus. J. Comp. Neurol. 1996, 369, 388–404. [Google Scholar] [CrossRef]
- Morel, G.R.; Leon, M.L.; Uriarte, M.; Reggiani, P.C.; Goya, R.G. Therapeutic potential of IGF-1 on hippocampal neurogenesis and function during aging. Neurogenesis (Austin) 2017, 4, e1259709. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.W.; Saatman, K.E. Central Infusion of Insulin-Like Growth Factor-1 Increases Hippocampal Neurogenesis and Improves Neurobehavioral Function after Traumatic Brain Injury. J. Neurotrauma. 2018, 35, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Saatman, K.E.; Contreras, P.C.; Smith, D.H.; Raghupathi, R.; McDermott, K.L.; Fernandez, S.C.; Sanderson, K.L.; Voddi, M.; McIntosh, T.K. Insulin-like growth factor-1 (IGF-1) improves both neurological motor and cognitive outcome following experimental brain injury. Exp. Neurol. 1997, 147, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Loddick, S.A.; Liu, X.J.; Lu, Z.X.; Liu, C.; Behan, D.P.; Chalmers, D.C.; Foster, A.C.; Vale, W.W.; Ling, N.; De Souza, E.B. Displacement of insulin-like growth factors from their binding proteins as a potential treatment for stroke. Proc. Natl. Acad. Sci. USA 1998, 95, 1894–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatton, J.; Rapp, R.P.; Kudsk, K.A.; Brown, R.O.; Luer, M.S.; Bukar, J.G.; Chen, S.A.; McClain, C.J.; Gesundheit, N.; Dempsey, R.J.; et al. Intravenous insulin-like growth factor-I (IGF-1) in moderate-to-severe head injury: a phase II safety and efficacy trial. J. Neurosurg. 1997, 86, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Aberg, M.A.; Aberg, N.D.; Hedbacker, H.; Oscarsson, J.; Eriksson, P.S. Peripheral infusion of IGF-1 selectively induces neurogenesis in the adult rat hippocampus. J. Neurosci. 2000, 20, 2896–2903. [Google Scholar] [CrossRef] [PubMed]
- Borasio, G.D.; Robberecht, W.; Leigh, P.N.; Emile, J.; Guiloff, R.J.; Jerusalem, F.; Silani, V.; Vos, P.E.; Wokke, J.H.; Dobbins, T. A placebo-controlled trial of insulin-like growth factor-I in amyotrophic lateral sclerosis. European ALS/IGF-1 Study Group. Neurology 1998, 51, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Wilczak, N.; de Vos, R.A.; De Keyser, J. Free insulin-like growth factor (IGF)-I and IGF binding proteins 2, 5, and 6 in spinal motor neurons in amyotrophic lateral sclerosis. Lancet 2003, 361, 1007–1011. [Google Scholar] [CrossRef]
- Yoshimura, T.; Arimura, N.; Kaibuchi, K. Signaling networks in neuronal polarization. J. Neurosci. 2006, 26, 10626–10630. [Google Scholar] [CrossRef] [PubMed]
- Ozdinler, P.H.; Macklis, J.D. IGF-1 specifically enhances axon outgrowth of corticospinal motor neurons. Nat. Neurosci. 2006, 9, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Sosa, L.; Dupraz, S.; Laurino, L.; Bollati, F.; Bisbal, M.; Cáceres, A.; Pfenninger, K.H.; Quiroga, S. IGF-1 receptor is essential for the establishment of hippocampal neuronal polarity. Nat. Neurosci. 2006, 9, 993–995. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, S.E.; Doe, C.Q. Microtubule-induced cortical cell polarity. Genes Dev. 2007, 21, 483–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainscough, S.L.; Barnard, Z.; Upton, Z.; Harkin, D.G. Vitronectin supports migratory responses of corneal epithelial cells to substrate bound IGF-1 and HGF, and facilitates serum-free cultivation. Exp. Eye Res. 2006, 83, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Van Lonkhuyzen, D.R.; Hollier, B.G.; Shooter, G.K.; Leavesley, D.I.; Upton, Z. Chimeric vitronectin: insulin-like growth factor proteins enhance cell growth and migration through co-activation of receptors. Growth Factors 2007, 25, 295–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pons, S.; Marti, E. Sonic hedgehog synergizes with the extracellular matrix protein vitronectin to induce spinal motor neuron differentiation. Development 2000, 127, 333–342. [Google Scholar] [PubMed]
- Hashimoto, K.; Sakane, F.; Ikeda, N.; Akiyama, A.; Sugahara, M.; Miyamoto, Y. Vitronectin promotes the progress of the initial differentiation stage in cerebellar granule cells. Mol. Cell Neurosci. 2016, 70, 76–85. [Google Scholar] [CrossRef] [PubMed]
- von Gertten, C.; Flores Morales, A.; Holmin, S.; Mathiesen, T.; Nordqvist, A.C. Genomic responses in rat cerebral cortex after traumatic brain injury. BMC Neurosci. 2005, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Risling, M.; Malm, E.; Sondén, A.; Bolling, M.F.; Sköld, M.K. Cellular High-Energy Cavitation Trauma - Description of a Novel In Vitro Trauma Model in Three Different Cell Types. Front Neurol. 2016, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Sonden, A.; Svensson, B.; Roman, N.; Ostmark, H.; Brismar, B.; Palmblad, J.; Kjellström, B.T. Laser-induced shock wave endothelial cell injury. Lasers Surg. Med. 2000, 26, 364–375. [Google Scholar] [CrossRef]
- Sonden, A.; Svensson, B.; Roman, N.; Brismar, B.; Palmblad, J.; Kjellström, B.T. Mechanisms of shock wave induced endothelial cell injury. Lasers Surg. Med. 2002, 31, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Kricker, J.A.; Towne, C.L.; Firth, S.M.; Herington, A.C.; Upton, Z. Structural and functional evidence for the interaction of insulin-like growth factors (IGFs) and IGF binding proteins with vitronectin. Endocrinology 2003, 144, 2807–2815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kricker, J.A.; Hyde, C.E.; Van Lonkhuyzen, D.R.; Hollier, B.G.; Shooter, G.K.; Leavesley, D.I.; Herington, A.C.; Upton, Z. Mechanistic investigations into interactions between IGF-1 and IGFBPs and their impact on facilitating cell migration on vitronectin. Growth Factors 2010, 28, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, A.S.; Hollier, B.G.; Manton, K.J.; Satyamoorthy, K.; Leavesley, D.I.; Upton, Z. Insulin-like growth factor-I: vitronectin complex-induced changes in gene expression effect breast cell survival and migration. Endocrinology 2011, 152, 1388–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, V.C.; Schütt, B.S.; Andaloro, E.; Ymer, SI.; Hoeflich, A.; Ranke, M.B.; Bach, L.A.; Werther, G.A. Insulin-like growth factor binding protein-2 binding to extracellular matrix plays a critical role in neuroblastoma cell proliferation, migration, and invasion. Endocrinology 2005, 146, 4445–4455. [Google Scholar] [CrossRef] [PubMed]
- Parvaneh Tafreshi, A.; Talebi, F.; Ghorbani, S.; Bernard, C.; Noorbakhsh, F. Altered expression of IGF-1 system in neurons of the inflamed spinal cord during acute experimental autoimmune encephalomyelitis. J. Comp. Neurol. 2017, 525, 3072–3082. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, D.R. Insulin-like growth factor binding proteins and their role in controlling IGF actions. Cytokine Growth Factor Rev. 1997, 8, 45–62. [Google Scholar] [CrossRef]
- Jones, J.I.; Clemmons, D.R. Insulin-like growth factors and their binding proteins: biological actions. Endocr. Rev. 1995, 16, 3–34. [Google Scholar] [PubMed]
- Bianchi, V.E.; Locatelli, V.; Rizzi, L. Neurotrophic and Neuroregenerative Effects of GH/IGF1. Int. J. Mol. Sci. 2017, 18, 2441. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.I.; Gockerman, A.; Busby, W.H., Jr.; Camacho-Hubner, C.; Clemmons, D.R. Extracellular matrix contains insulin-like growth factor binding protein-5: potentiation of the effects of IGF-1. J. Cell Biol. 1993, 121, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Allard, J.B.; Duan, C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front Endocrinol (Lausanne) 2018, 9, 117. [Google Scholar] [CrossRef] [PubMed]









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergen, K.; Frödin, M.; Von Gertten, C.; Sandberg-Nordqvist, A.-C.; Sköld, M.K. Neurite Growth and Polarization on Vitronectin Substrate after in Vitro Trauma is not Enhanced after IGF Treatment. Brain Sci. 2018, 8, 151. https://doi.org/10.3390/brainsci8080151
Bergen K, Frödin M, Von Gertten C, Sandberg-Nordqvist A-C, Sköld MK. Neurite Growth and Polarization on Vitronectin Substrate after in Vitro Trauma is not Enhanced after IGF Treatment. Brain Sciences. 2018; 8(8):151. https://doi.org/10.3390/brainsci8080151
Chicago/Turabian StyleBergen, K., M. Frödin, C. Von Gertten, A. -C. Sandberg-Nordqvist, and M. K. Sköld. 2018. "Neurite Growth and Polarization on Vitronectin Substrate after in Vitro Trauma is not Enhanced after IGF Treatment" Brain Sciences 8, no. 8: 151. https://doi.org/10.3390/brainsci8080151



