The Effect of Lactobacillus plantarum ATCC 8014 and Lactobacillus acidophilus NCFM Fermentation on Antioxidant Properties of Selected in Vitro Sprout Culture of Orthosiphon aristatus (Java Tea) as a Model Study
Abstract
:Abbreviations
| RA | Rosmarinic Acid |
| IOSC | in vitro Sprout Culture of Orthosiphon aristatus |
| LAB | Lactic Acid Bacteria |
| SSF | Solid State Fermentations |
| LSF | Liquid State Fermentations |
| LP | Lactobacillus plantarum |
| LA | Lactobacillus acidophilus |
| OD | Optical Density |
| LP-SSF | Solid State Fermentations inoculated with Lactobacillus plantarum |
| LA-SSF | Solid State Fermentations inoculated with Lactobacillus acidophilus |
| LP-LSF | Liquid State Fermentations inoculated with Lactobacillus plantarum |
| LA-LSF | Liquid State Fermentations inoculated with Lactobacillus acidophilus |
| DPPH | 1,1-diphenyl-2- picrylhydrazyl |
| TEAC | Trolox Equivalent Antioxidant Capacity |
| SOD | Superoxide Dismutase |
| JA | MS medium with Jasmonic Acid |
| YE | MS medium with Yeast Eextract |
| TE | Trolox Equivalent |
| GAE | Gallic Acid Equivalent |
| QE | Quercetin Equivalent |
| DW | Dry Weight |
1. Introduction
2. Experimental Section
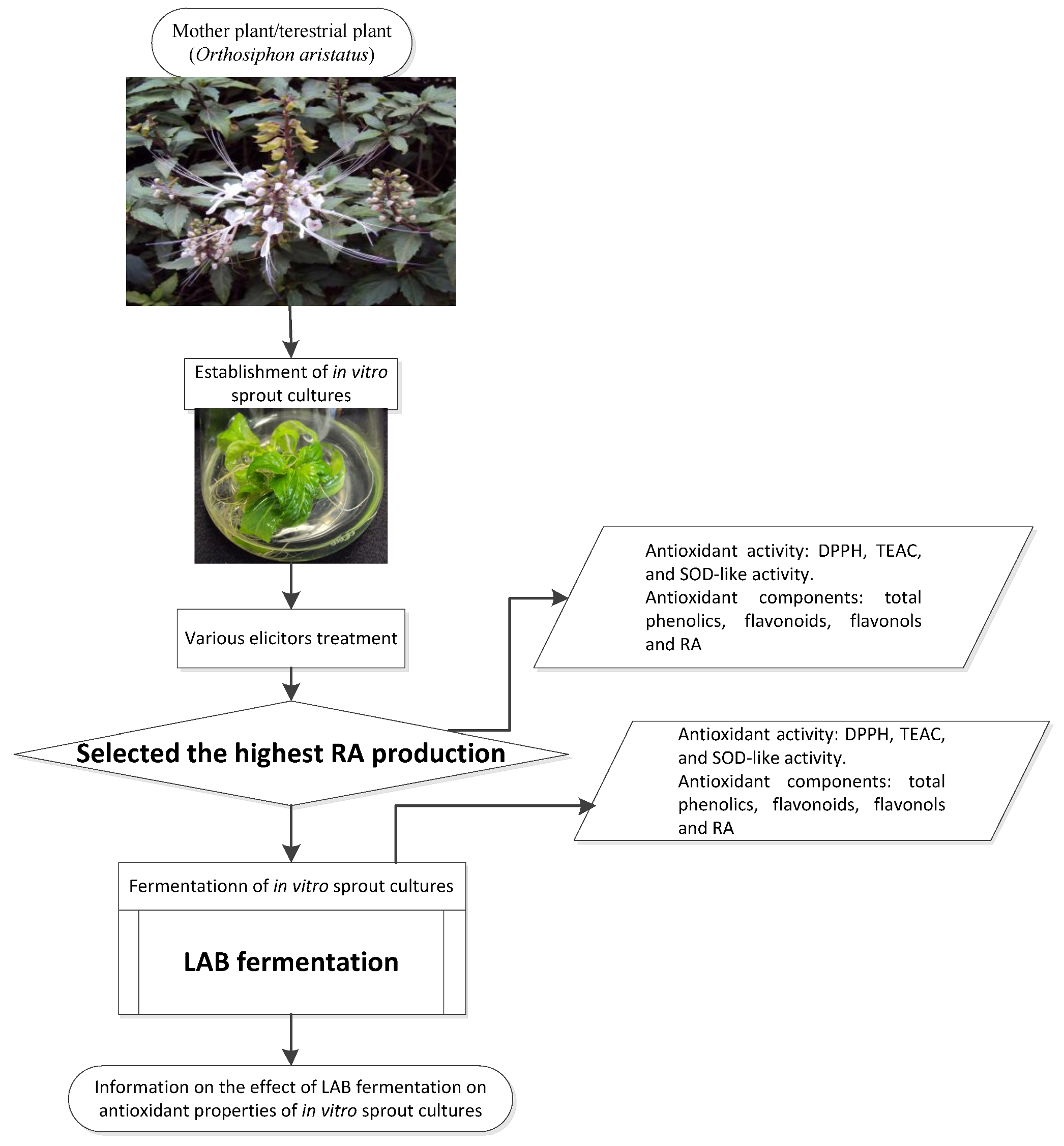
2.1. Materials
2.1.1. Plant Materials
2.1.2. Culture Media
2.1.3. Chemicals for Analysis
2.2. Methods
2.2.1. Establishment of IOSC
2.2.2. Fermentation Study of Selected IOSCs
2.2.3. LAB Fermentation of IOSC
2.2.4. Inoculums Preparation
2.2.5. The Effect of IOSC Extract on Viable Cell LAB Strains Growth
2.2.6. Solid State Fermentations (SSF)
2.2.7. Total LAB
2.2.8. Liquid State Fermentations (LSF)
2.2.9. Viable Cells LAB Growth
2.2.10. Sample Preparation and Extraction of the IOSC
2.2.11. Antioxidant Properties of the IOSC

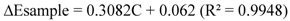




2.2.12. HPLC Phenolic acids in IOSC Extract
2.3. Statistical Analysis
3. Results and Discussion
3.1. Selection of High RA Line and Antioxidant Properties of IOSCs by Elicitation for the LAB Fermentation Study
| Sample group | IOSC Length | Number of leaves | Number of roots | Fresh weight (FW) (g) |
|---|---|---|---|---|
| Control | 5.02 ± 0.58 | 9.40 ± 2.88 | 4.40 ± 1.14 | 6.29 ± 1.44 |
| JA | 3.88 ± 0.94 | 5.80 ± 1.30 | 3.80 ± 2.17 | 4.23 ± 0.86 |
| YE | 3.10 ± 0.80 | 5.20 ± 1.30 | 3.60 ± 1.95 | 5.40 ± 1.18 |
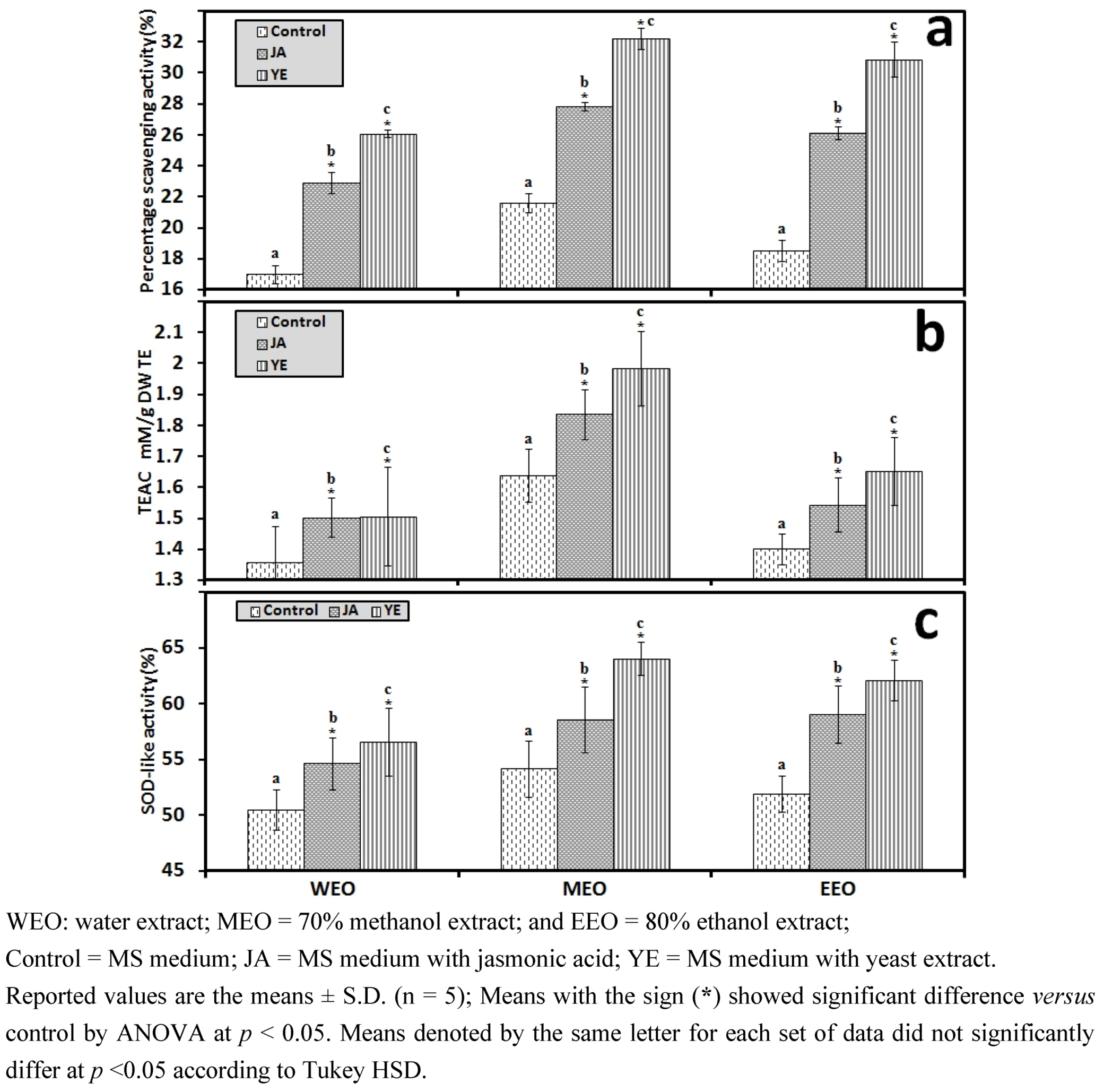
3.1.1. Antioxidant Activity of IOSC after Elicitation
3.1.2. Antioxidant Components of IOSC after Elicitation
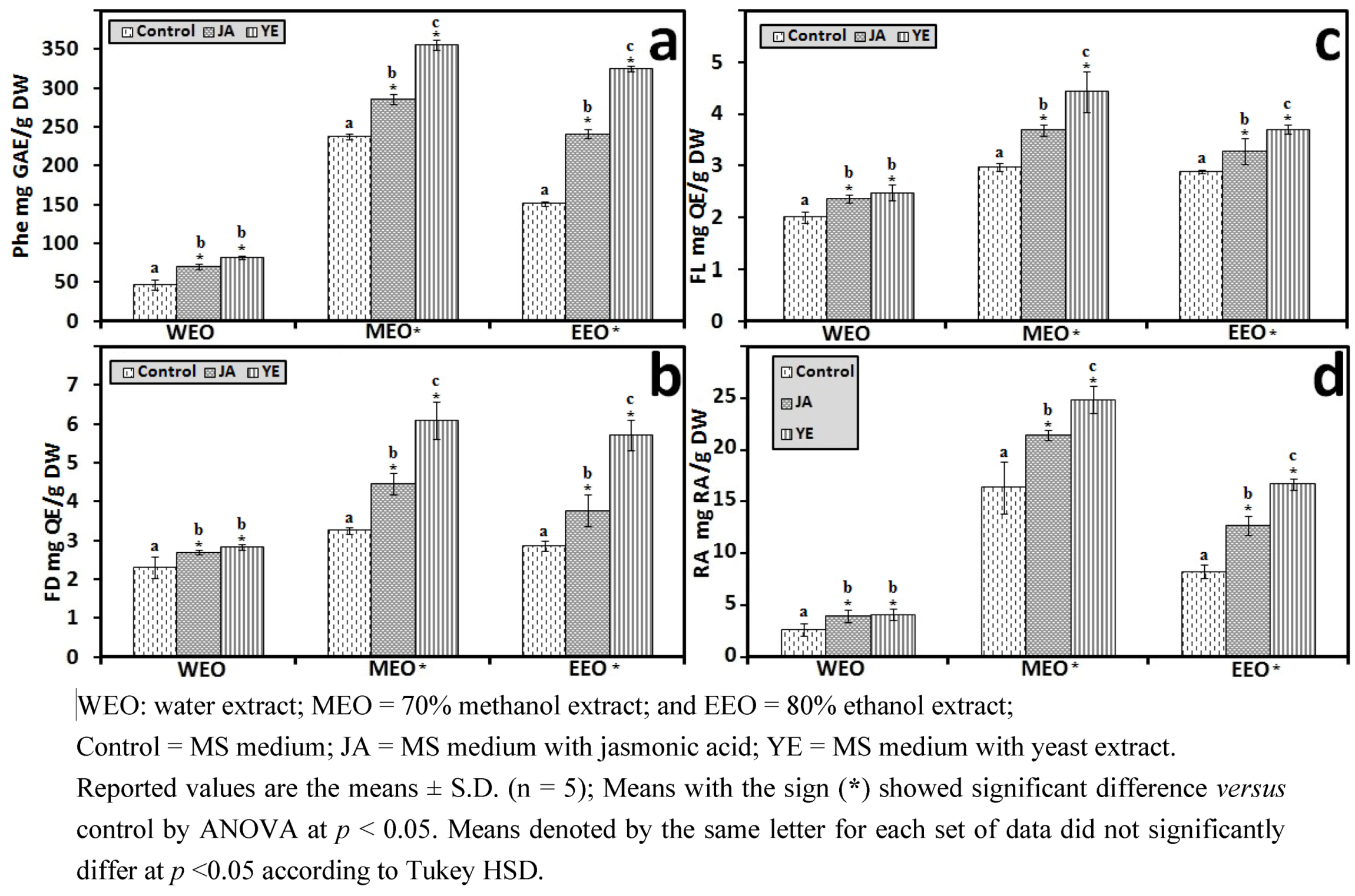
3.1.3. Solvent for Extraction
3.1.4. HPLC Phenolic Acids of IOSC after Elicitation
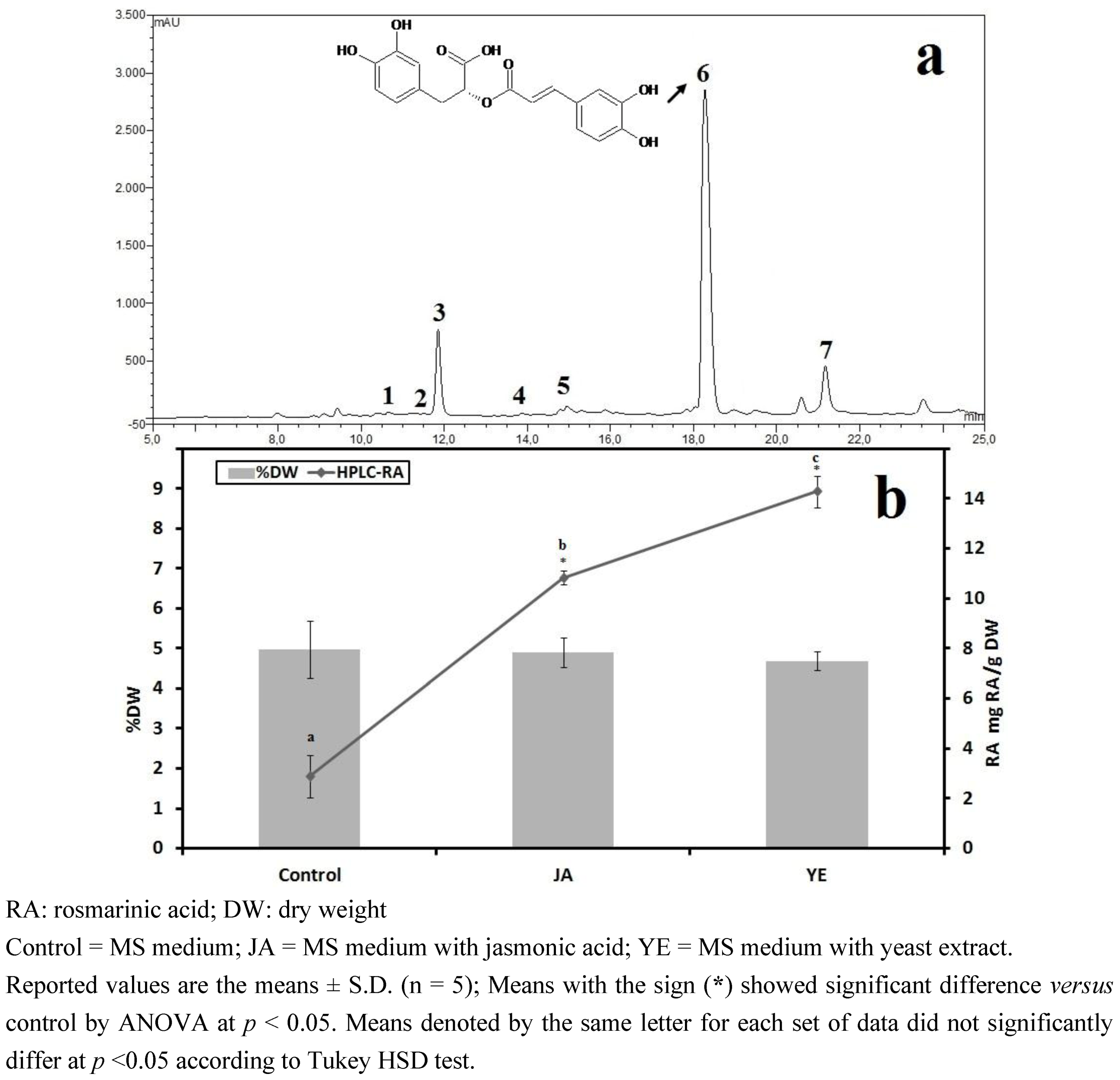
| Other phenolic acids | Control(mg/g·DW) | Fold of control | |
|---|---|---|---|
| JA | YE | ||
| Vanillic acid | 2.28 ± 0.27 | 2.80 | 3.72 |
| Chlorogenic acid | 0.49 ± 0.07 | 1.10 | 1.90 |
| Caffeic acid | 4.62 ± 0.15 | 1.30 | 3.46 |
| p-Coumaric acid | 0.27 ± 0.03 | 2.40 | 3.50 |
| Sinapic acid | 1.12 ± 0.04 | 2.5 | 1.82 |
3.2. LAB Fermentation Study of Selected IOSC
3.2.1. The Effects of IOSC Extract on Viable Cell LAB Growth
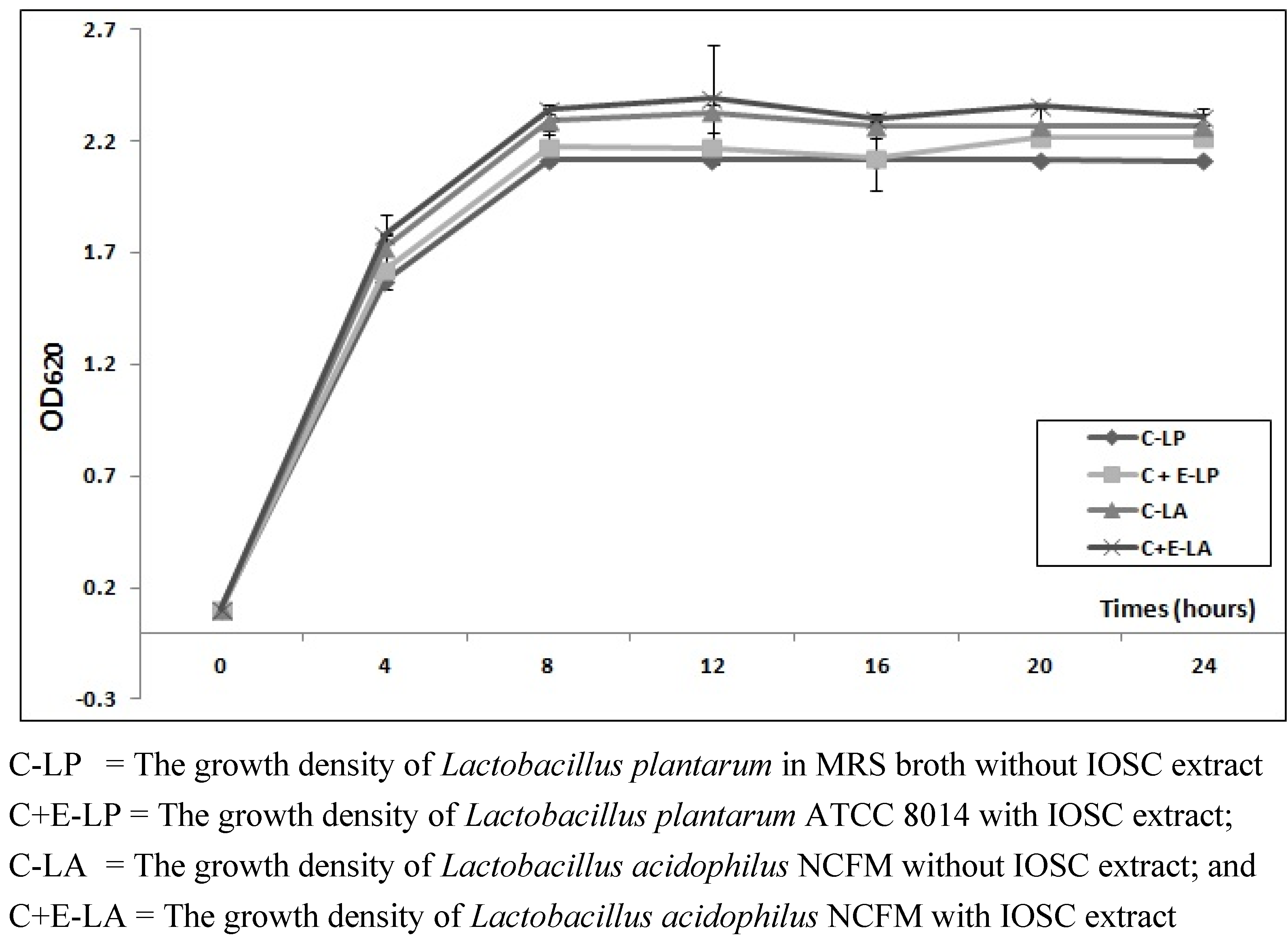
3.2.2. Viable Cell Growth of SSF and LSF
| Strains | Time (h) | Total LAB * |
|---|---|---|
| L. plantarum | Inoculated OD620 0.1 = 3.2 × 105 cfu/g | |
| 24 | 8.1 × 105 cfu/g | |
| 48 | 5.2 × 104 cfu/g | |
| 72 | 2.5 × 104 cfu/g | |
| L. acidophilus | Inoculated OD620 0.1 = 5.8 × 105 cfu/g | |
| 24 | 3.2 × 105 cfu/g | |
| 48 | 1.8 × 104 cfu/g | |
| 72 | 3.0 × 104 cfu/g | |
| MRS broths | Time (h) | OD620 |
|---|---|---|
| L. plantarum (without IOSC; control) | 24 | 2.221 ± 0.010 |
| 48 | 1.661 ± 0.009 | |
| 72 | 1.777 ± 0.013 | |
| L. plantarum with IOSC | 24 | 2.225 ± 0.021 |
| 48 | 1.664 ± 0.011 | |
| 72 | 1.871 ± 0.011 | |
| L. acidophilus (without IOSC; control) | 24 | 2.151 ± 0.008 |
| 48 | 1.558 ± 0.015 | |
| 72 | 1.831 ± 0.007 | |
| L. acidophilus with IOSC | 24 | 2.226 ± 0.009 |
| 48 | 1.560 ± 0.012 | |
| 72 | 1.839 ± 0.014 |
3.2.3. Solid State Fermentation (SSF) of IOSC
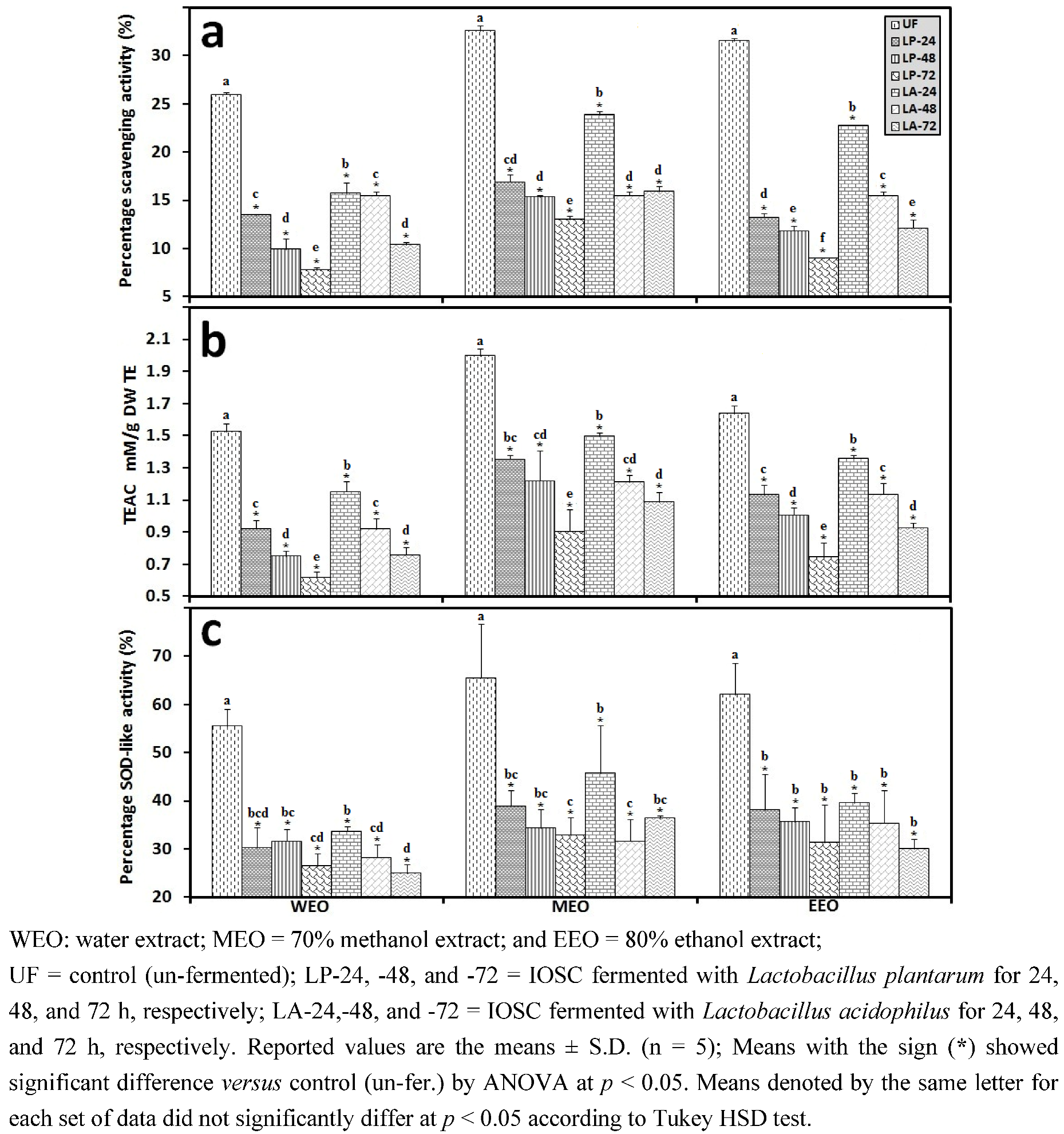
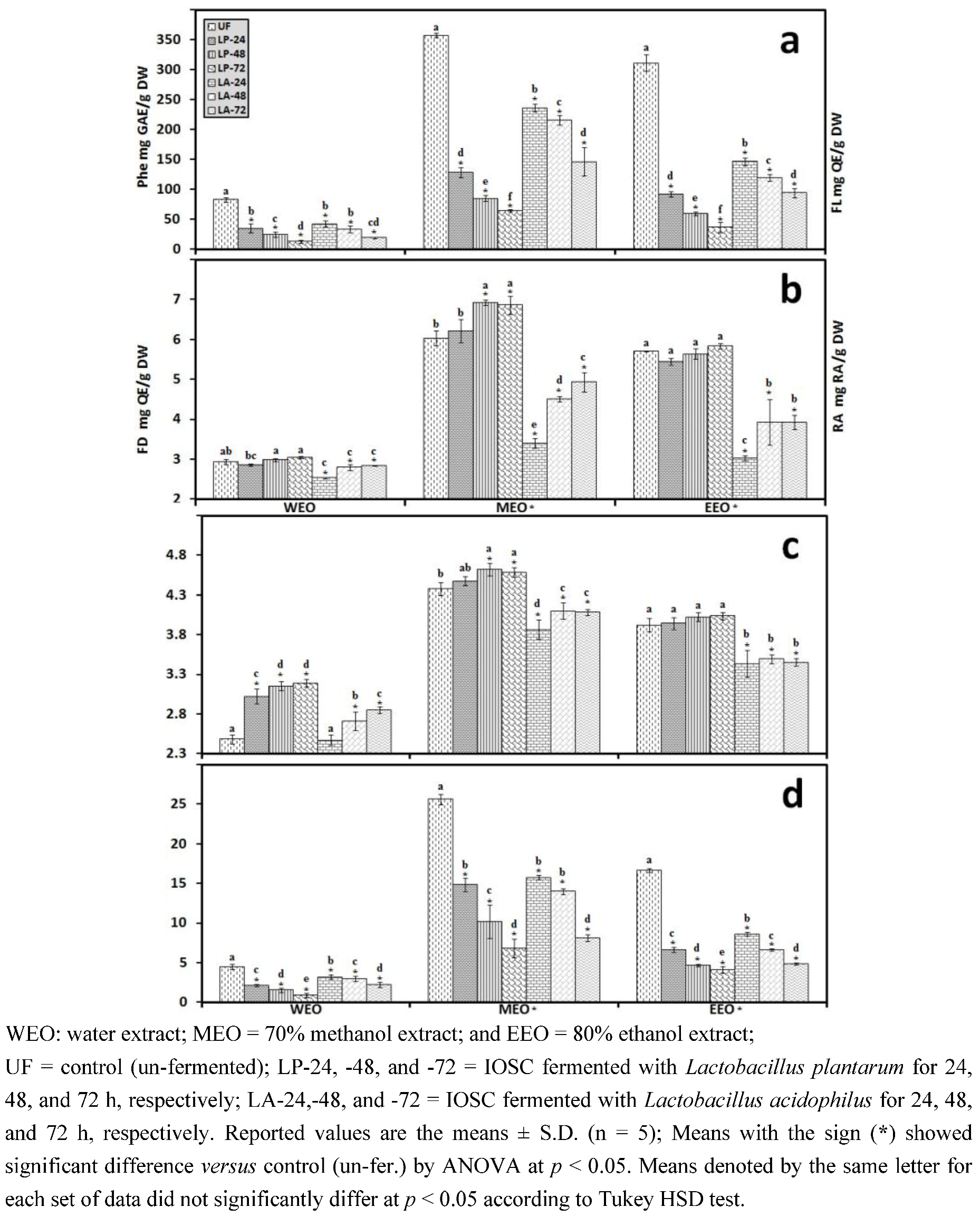
3.2.4. Liquid State Fermentation (LSF) of IOSC
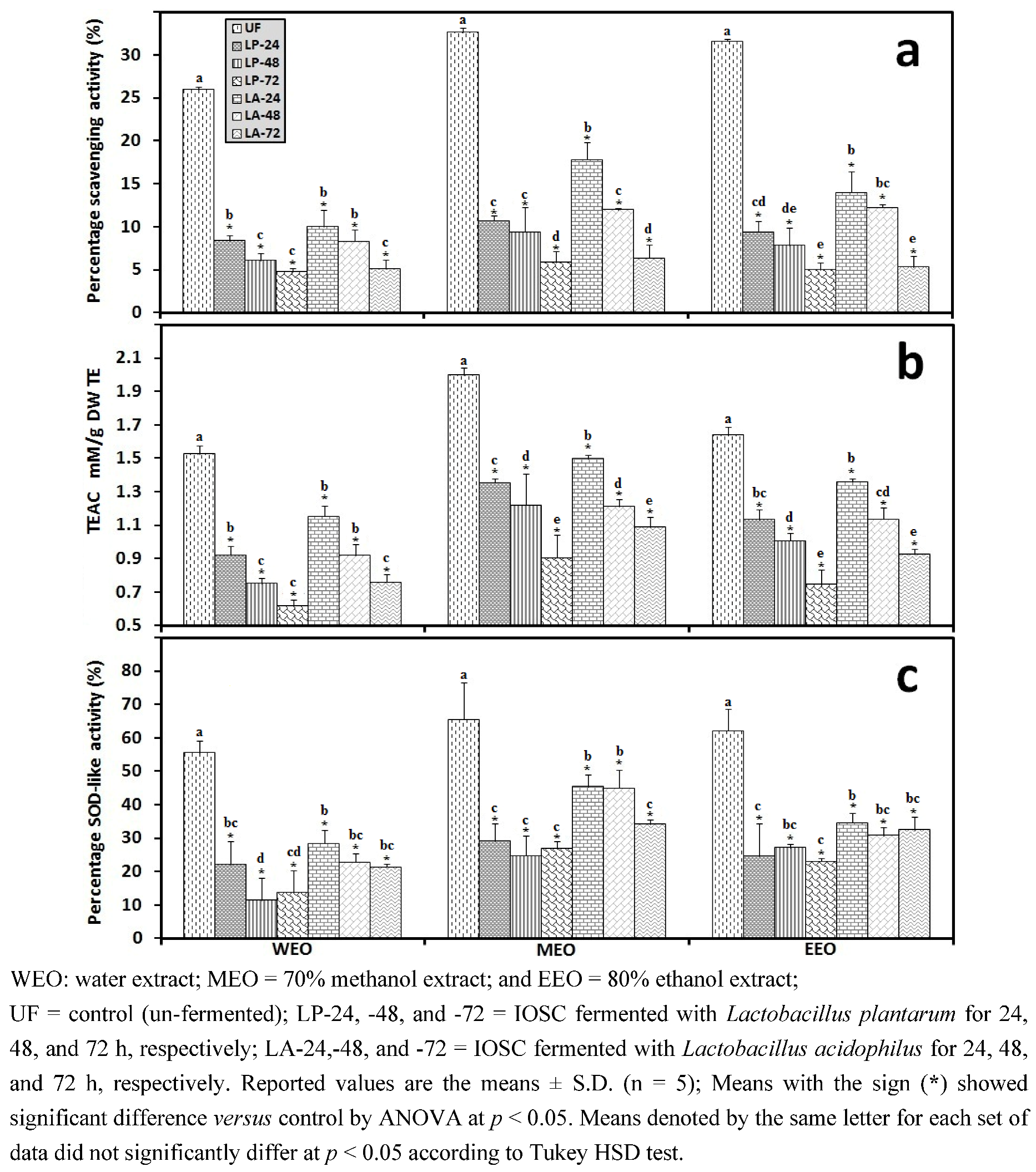
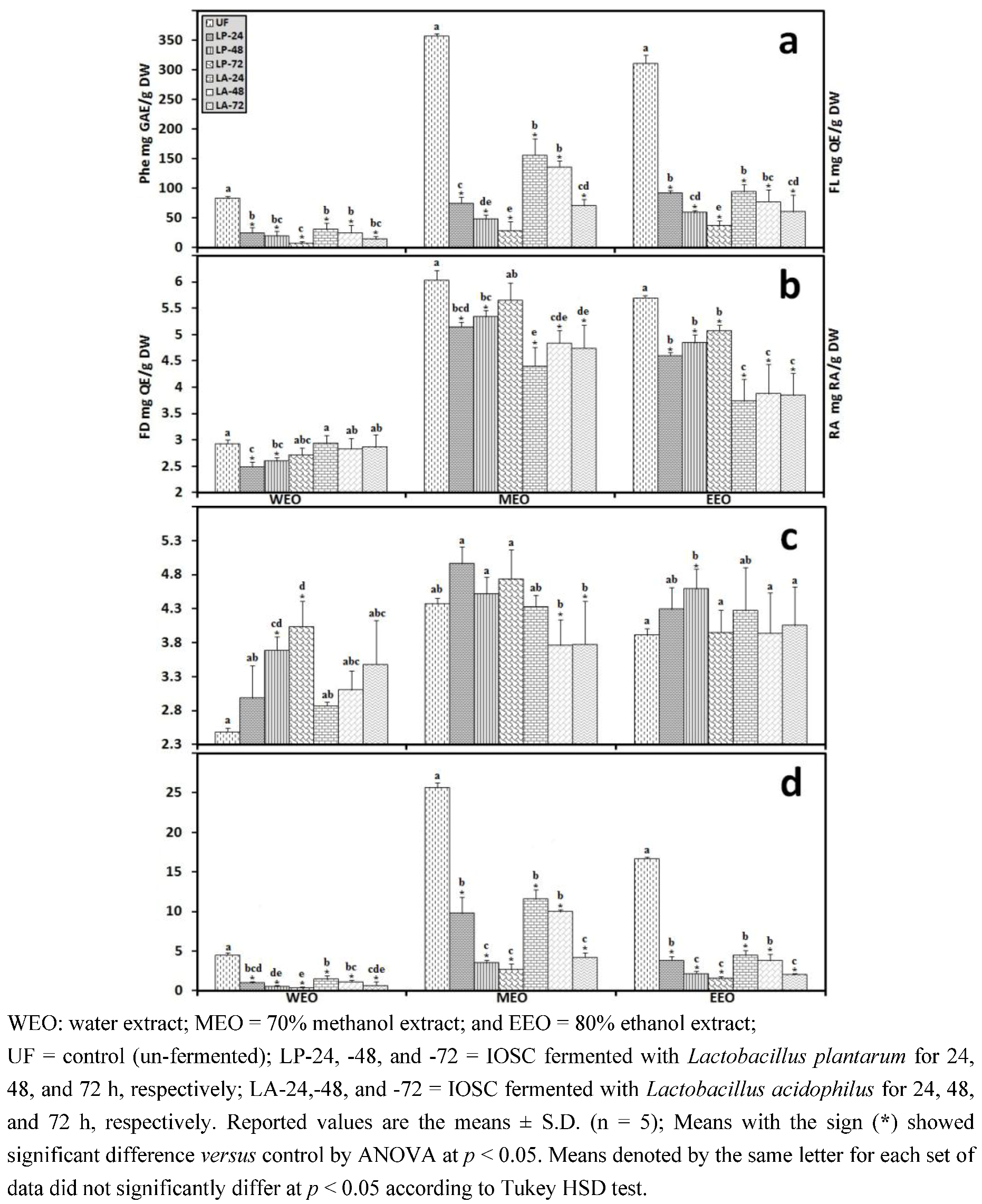
3.2.5. HPLC Analysis of IOSC after LAB Fermentation
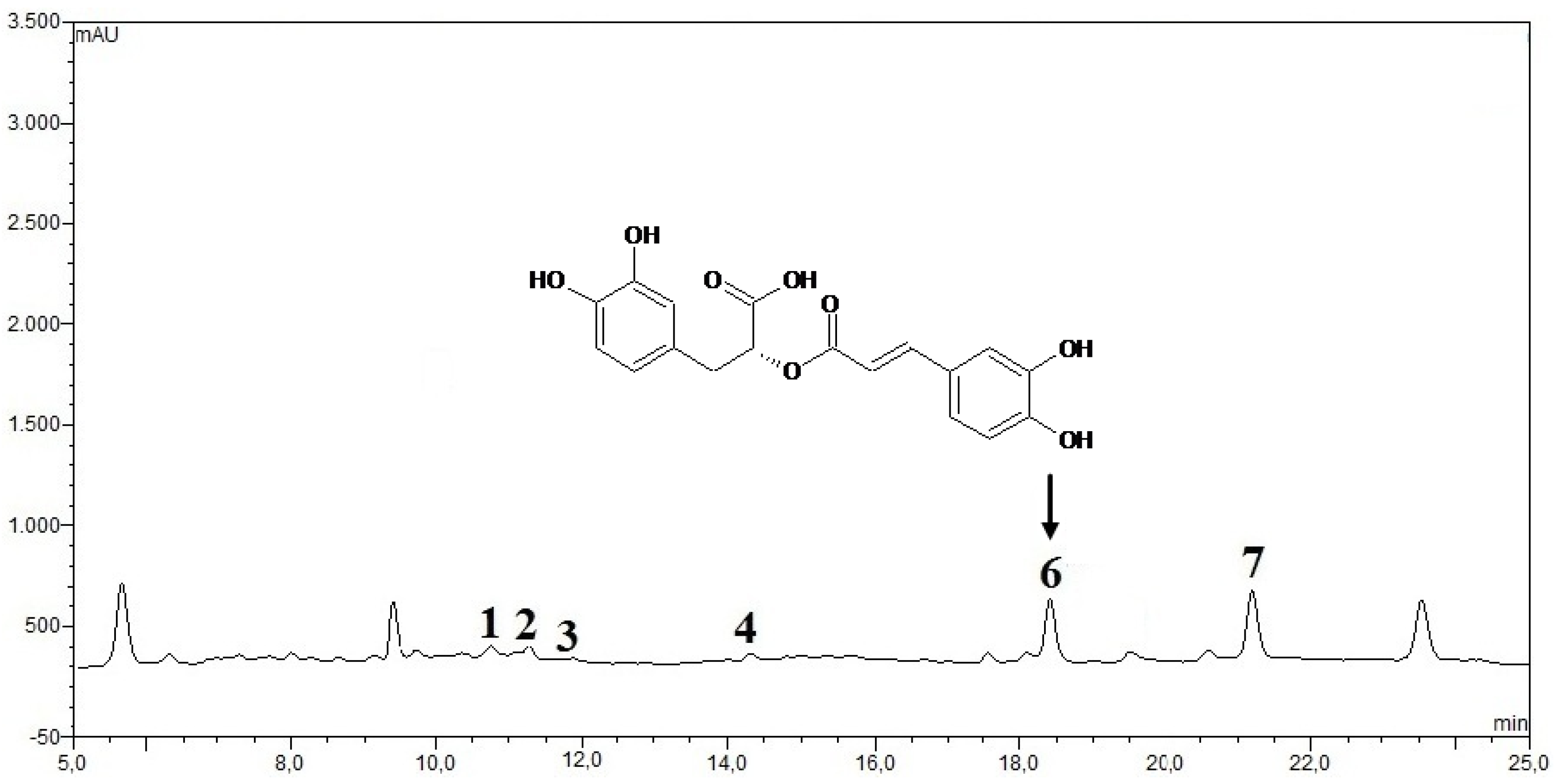
| Phenolic acids | Control (un-fermented) | % decrease L. plantarum ATCC 8014 | % decrease L. acidophilus NCFM | ||
|---|---|---|---|---|---|
| (mg/g·DW) | SSF | LSF | SSF | LSF | |
| Rosmarinic acid | 14.28 ± 0.64 | 55.7 | 72.3 | 46.8 | 56.2 |
| vanillic acid | 8.48 ± 0.06 | 23.5 | 43.2 | 20.4 | 24.2 |
| chlorogenic acid | 0.93 ± 0.11 | 27.6 | 40.1 | 23.6 | 35.6 |
| caffeic acid | 9.06 ± 0.33 | 42.4 | 49.5 | 37.3 | 44.9 |
| p-coumaric acid | 0.95 ± 0.07 | 29.6 | 37.6 | 28.6 | 32.8 |
| sinapic acid | 2.04 ± 0.03 | 26.8 | 33.9 | 21.6 | 29.8 |
3.2.6. Correlation Analysis between the Experimental Variables
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
References
- Park, S.U.; Uddin, M.R.; Xu, H.; Kim, Y.K.; Lee, S.Y. Biotechnological applications for rosmarinic acid production in plant. Afr. J. Biotechnol. 2008, 7, 4959–4965. [Google Scholar]
- Petersen, M.; Simmonds, M.S.J. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Akowuah, G.A.; Ismail, Z.; Norhayati, I.; Sadikun, A. The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical-scavenging activity. Food Chem. 2005, 93, 311–317. [Google Scholar] [CrossRef]
- Fadhlina, A. The Effects of Temperature and Fermentation Time on Selected Cchemical Composition, Antioxidant and Quality of Cat Whiskers (Orthosiphon stamineus) Tea; University Sains Malaysia: Pulau Pinang, Malaysia, 2008. [Google Scholar]
- Shevchenko, Y.; Smetanska, I.; Wendt, A. Sprout culture of Stevia rebaudiana Bertoni. In Stevia Science; Euprint: Heverlee, Belgium, 2010; pp. 5–26. [Google Scholar]
- Cai, Z. Effects Elicitors on Secondary Metabolism of Plant Suspension Cultures of Vitis vinifera and Malus domestica and their Exudates; Berlin University of Technology: Berlin, Germany, 2012. [Google Scholar]
- Sumaryono, W.; Proksch, P.; Hartmann, T.; Nimtz, M.; Wray, V. Induction of rosmarinic acid accumulation in cell suspension cultures of Orthosiphon aristatus after treatment with yeast extract. Phytochemistry 1991, 30, 3267–3271. [Google Scholar] [CrossRef]
- Mewis, I.; Smetanska, I.; Müller, C.; Ulrichs, C. Specific poly-phenolic compounds in cell culture of Vitis vinifera L. cv. Gamay Fréaux. Appl. Biochem. Biotechnol. 2011, 164, 148–161. [Google Scholar] [CrossRef]
- Dordevic, T.M.; Siler-Marinkovic, S.S.; Dimitrijevic-Brankovic, S.I. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010, 119, 957–963. [Google Scholar] [CrossRef]
- Maifreni, M.; Marino, M.; Conte, L. Lactic acid fermentation of Brassica rapa: Chemical and microbial evaluation of a typical Italian product (brovada). Eur. Food Res. Technol. 2004, 218, 469–473. [Google Scholar] [CrossRef]
- Katina, K.; Laitila, A.; Juvonen, R.; Liukkonen, K.H.; Kariluoto, S.; Piironen, V.; Landberg, R.; Aman, P.; Poutanen, K. Bran fermentation as a means to enhance technological properties and bioactivity of rye. Food Microbiol. 2007, 24, 175–186. [Google Scholar] [CrossRef]
- Katina, K.; Liukkonen, K.H.; Kaukovirta-Norja, A.; Adlercreutz, H.; Heinonen, S.M.; Lampi, A.M.; Pihlava, J.M.; Poutanen, K. Fermentation-induced changes in the nutritional value of native or germinated rye. J. Cereal Sci. 2007, 46, 348–355. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Chou, C.-C.; Yu, R.-C. Antioxidant activity of lactic-fermented Chinese cabbage. Food Chem. 2009, 115, 912–917. [Google Scholar] [CrossRef]
- Zhao, D.; Ding, X. Studies on the low-salt Chinese potherb mustard (Brassica juncea, Coss.) pickle. I-The effect of a homofermentative L(+)-lactic acid producer Bacillus coagulans on starter culture in the low-salt Chinese potherb mustard pickle fermentation. LWT-Food Sci. Technol. 2008, 41, 474–482. [Google Scholar] [CrossRef]
- Liu, J.-G.; Hou, C.-W.; Lee, S.-Y.; Chuang, Y.; Lin, C.-C. Antioxidant effects and UVB protective activity of Spirulina (Arthrospira platensis) products fermented with lactic acid bacteria. Process Biochem. 2011, 46, 1405–1410. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Peñas, E.; Sidro, B.; Ullate, M.; Frias, J.; Vidal-Valverde, C. White cabbage fermentation improves ascorbigen content, antioxidant and nitric oxide production inhibitory activity in LPS-induced macrophages. LWT-Food Sci. Technol. 2012, 46, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.; Wang, O.; Wu, W.; Zhu, S.; Zhou, F.; Ji, B.; Gao, F.; Zhang, D.; Liu, J.; Cheng, Q. Comparative study of the effects of solid-state fermentation with three filamentous fungi on the Total Phenolics Content (TPC), flavonoids, and antioxidant activities of subfractions from Oats (Avena sativa L.). J. Agr. Food Chem. 2011, 60, 507–513. [Google Scholar]
- Cho, K.M.; Lee, J.H.; Yun, H.D.; Ahn, B.Y.; Kim, H.; Seo, W.T. Changes of phytochemical constituents (isoflavones, flavanols, and phenolic acids) during cheonggukjang soybeans fermentation using potential probiotics Bacillus subtilis CS90. J. Food Compos. Anal. 2011, 24, 402–410. [Google Scholar] [CrossRef]
- Ng, C.-C.; Wang, C.-Y.; Wang, Y.-P.; Tzeng, W.-S.; Shyu, Y.-T. Lactic acid bacterial fermentation on the production of functional antioxidant herbal Anoectochilus formosanus Hayata. J. Biosci. Bioeng. 2011, 111, 289–293. [Google Scholar] [CrossRef]
- Wu, S.-C.; Su, Y.-S.; Cheng, H.-Y. Antioxidant properties of Lactobacillus-fermented and non-fermented Graptopetalum paraguayense E. Walther at different stages of maturity. Food Chem. 2011, 129, 804–809. [Google Scholar] [CrossRef]
- He, X.; Zou, Y.; Yoon, W.-B.; Park, S.-J.; Park, D.-S.; Ahn, J. Effects of probiotic fermentation on the enhancement of biological and pharmacological activities of Codonopsis lanceolata extracted by high pressure treatment. J. Biosci. Bioeng. 2011, 112, 188–193. [Google Scholar] [CrossRef]
- Wang, N.-F.; Yan, Z.; Li, C.-Y.; Jiang, N.; Liu, H.-J. Antioxidant activity of peanut flour fermented with Lactic Acid Bacteria. J. Food Biochem. 2011, 35, 1514–1521. [Google Scholar] [CrossRef]
- Alberto, M.R.; Farías, M.E.; Manca de Nadra, M.C. Effect of gallic acid and catechin on Lactobacillus hilgardii 5w growth and metabolism of organic compounds. J. Agr. Food Chem. 2001, 49, 4359–4363. [Google Scholar] [CrossRef]
- Sunny-Roberts, E.O. Application of Disaccarides Pre-Treatment in Improving Tolerances of Lactobacillus rhamnosus Strains to Environmental Stresses or during Vacuum- and Spray Drying Processes; Berlin University of Technology: Berlin, Germany, 2009. [Google Scholar]
- Mohdaly, A.A.; Sarhan, M.A.; Smetanska, I.; Mahmoud, A. Antioxidant properties of various solvent extracts of potato peel, sugar beet pulp and sesame cake. J. Sci. Food Agr. 2010, 90, 218–226. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Debnath, T.; Park, P.-J.; Deb Nath, N.C.; Samad, N.B.; Park, H.W.; Lim, B.O. Antioxidant activity of Gardenia jasminoides Ellis fruit extracts. Food Chem. 2011, 128, 697–703. [Google Scholar] [CrossRef]
- Kiong, A.L.P.; Lai, A.G.; Hussein, S.; Harun, A.R. Physiological responses of Orthosiphon stamineus plantles to Gamma Irradiation. AEJSA 2008, 2, 135–149. [Google Scholar]
- Lee, I.H.; Hung, Y.-H.; Chou, C.-C. Solid-state fermentation with fungi to enhance the antioxidative activity, total phenolic and anthocyanin contents of black bean. Int. J. Food Microbiol. 2008, 121, 150–156. [Google Scholar] [CrossRef]
- Azlim Almey, A.A.; Ahmed Jalal Khan, C.; Syed Zahir, I.; Mustapha Suleiman, K.; Aisyah, M.R.; Kamarul Rahim, K. Total phenolic content and primary antioxidant activity of methanolic and ethanolic extracts of aromatic plants’ leaves. Int. Food Res. J. 2010, 17, 1077–1084. [Google Scholar]
- Ogata, A.; Tsuruga, A.; Matsuno, M.; Mizukami, H. Elicitor-induced rosmarinic acid biosynthesis in Lithospermum erythrorhizon cell suspension cultures: Activities of rosmarinic acid synthase and the final two cytochrome P450-catalyzed hydroxylations. Plant Biotechnol. 2004, 21, 393–396. [Google Scholar] [CrossRef]
- Kim, H.K.; Oh, S.-R.; Lee, H.-K.; Huh, H. Benzothiadiazole enhances the elicitation of rosmarinic acid production in a suspension culture of Agastache rugosa O. Kuntze. Biotechnol. Lett. 2001, 23, 55–60. [Google Scholar] [CrossRef]
- Mizukami, H.; Ogawa, T.; Ohashi, H.; Ellis, B.E. Induction of rosmarinic acid biosynthesis in Lithospermum erythrorhizon cell suspension cultures by yeast extract. Plant Cell Rep. 1992, 11, 480–483. [Google Scholar] [PubMed]
- Gänzle, M.G.; Ehmann, M.; Hammes, W.P. Modeling of growth of Lactobacillus sanfranciscensis and Candida milleri in response to process parameters of sourdough fermentation. Appl. Environ. Microbiol. 1998, 64, 2616–2623. [Google Scholar] [PubMed]
- Cuppers, H.G.A.M.; Smelt, J.P.P.M. Time to turbidity measurement as a tool for modeling spoilage by Lactobacillus. J. Ind. Microbiol. Biotechnol. 1993, 12, 168–171. [Google Scholar]
- Chen, C.-P.; Lin, C.-C.; Tsuneo, N. Screening of Taiwanese crude drugs for antibacterial activity against Streptococcus mutans. J. Ethnopharmacol. 1989, 27, 285–295. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef]
- Doblado, R.; Zielinski, H.; Piskula, M.; Kozlowska, H.; Munoz, R.; Frias, J.; Vidal-Valverde, C. Effect of processing on the antioxidant vitamins and antioxidant capacity of Vigna sinensis Var. Carilla. J. Agr. Food Chem. 2005, 53, 1215–1222. [Google Scholar] [CrossRef]
- Fang, Z.; Hu, Y.; Liu, D.; Chen, J.; Ye, X. Changes of phenolic acids and antioxidant activities during potherb mustard (Brassica juncea, Coss.) pickling. Food Chem. 2008, 108, 811–817. [Google Scholar] [CrossRef]
- Othman, N.B.; Roblain, D.; Chammen, N.; Thonart, P.; Hamdi, M. Antioxidant phenolic compounds loss during the fermentation of Chétoui olives. Food Chem. 2009, 116, 662–669. [Google Scholar] [CrossRef]
- Juan, M.-Y.; Chou, C.-C. Enhancement of antioxidant activity, total phenolic and flavonoid content of black soybeans by solid state fermentation with Bacillus subtilis BCRC 14715. Food Microbiol. 2010, 27, 586–591. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Bioaccessibility and antioxidant potential of millet grain phenolics as affected by simulated in vitro digestion and microbial fermentation. J. Funct. Foods 2012, 4, 226–237. [Google Scholar] [CrossRef]
- Singh, H.B.; Singh, B.N.; Singh, S.P.; Nautiyal, C.S. Solid-state cultivation of Trichoderma harzianum NBRI-1055 for modulating natural antioxidants in soybean seed matrix. Bioresource Technol. 2010, 101, 6444–6453. [Google Scholar] [CrossRef]
- Cho, K.M.; Hong, S.Y.; Math, R.K.; Lee, J.H.; Kambiranda, D.M.; Kim, J.M.; Islam, S.M.A.; Yun, M.G.; Cho, J.J.; Lim, W.J.; Yun, H.D. Biotransformation of phenolics (isoflavones, flavanols and phenolic acids) during the fermentation of cheonggukjang by Bacillus pumilus HY1. Food Chem. 2009, 114, 413–419. [Google Scholar] [CrossRef]
- Rodriguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gomez-Cordoves, C.; Mancheno, J.M.; Munoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Orozco, R.; Frias, J.; Muñoz, R.; Zielinski, H.; Piskula, M.; Kozlowska, H.; Vidal-Valverde, C. Effect of fermentation conditions on the antioxidant compounds and antioxidant capacity of Lupinus angustifolius cv. zapaton. Eur. Food Res. Technol. 2008, 227, 979–988. [Google Scholar] [CrossRef]
- Frias, J.; Miranda, M.L.; Doblado, R.; Vidal-Valverde, C. Effect of germination and fermentation on the antioxidant vitamin content and antioxidant capacity of Lupinus albus L. var. Multolupa. Food Chem. 2005, 92, 211–220. [Google Scholar] [CrossRef]
- Torres, A.; Frias, J.; Granito, M.; Vidal-Valverde, C. Fermented pigeon pea (Cajanus cajan) ingredients in pasta products. J. Agr. Food Chem. 2006, 54, 6685–6691. [Google Scholar] [CrossRef]
- Fernandez-Orozco, R.; Frias, J.; Muñoz, R.; Zielinski, H.; Piskula, M.K.; Kozlowska, H.; Vidal-Valverde, C. Fermentation as a bio-process to obtain functional soybean flours. J. Agr. Food Chem. 2007, 55, 8972–8979. [Google Scholar] [CrossRef]
- Chang, C.T.; Hsu, C.K.; Chou, S.T.; Chen, Y.C.; Huang, F.S.; Chung, Y.C. Effect of fermentation time on the antioxidant activities of tempeh prepared from fermented soybean using Rhizopus oligosporus. Int. J. Food Sci. Technol. 2009, 44, 799–806. [Google Scholar] [CrossRef]
- Landete, J.M.; Rodríguez, H.; De Las Rivas, B.; Muñoz, R. High-added-value antioxidants obtained from the degradation of wine phenolics by Lactobacillus plantarum. J. Food Protect. 2007, 70, 2670–2675. [Google Scholar]
- Jamal, P.; Idris, Z.M.; Alam, M.Z. Effects of physicochemical parameters on the production of phenolic acids from palm oil mill effluent under liquid-state fermentation by Aspergillus niger IBS-103ZA. Food Chem. 2011, 124, 1595–1602. [Google Scholar] [CrossRef]
- Hegde, S.; Kavitha, S.; Varadaraj, M.C.; Muralikrishna, G. Degradation of cereal bran polysaccharide-phenolic acid complexes by Aspergillus niger CFR 1105. Food Chem. 2006, 96, 14–19. [Google Scholar] [CrossRef]
- Justesen, U.; Arrigoni, E.; Larsen, B.R.; Amado, R. Degradation of flavonoid glycosides and aglycones during in vitro fermentation with human faecal flora. LWT-Food Sci. Technol. 2000, 33, 424–430. [Google Scholar] [CrossRef]
- Podsedek, A.; Sosnowska, D.; Redzynia, M.; Anders, B. Antioxidant capacity and content of Brassica oleracea dietary antioxidants. Int. J. Food Sci. Technol. 2006, 41, 49–58. [Google Scholar] [CrossRef]
- Sumaryono, W.; Proksch, P.; Wray, V.; Witte, L.; Hartmann, T. Qualitative and quantitative analysis of the phenolic constituents from Orthosiphon aristatus. Planta Med. 1991, 57, 176–180. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Gómez-Plaza, E.; Martínez, A.; López-Roca, J.M. Evolution of phenolic compounds during wine fermentation and post-fermentation: Influence of grape temperature. J. Food Compos. Anal. 1999, 12, 259–272. [Google Scholar] [CrossRef]
- Ju, H.K.; Cho, E.J.; Jang, M.H.; Lee, Y.Y.; Hong, S.S.; Park, J.H.; Kwon, S.W. Characterization of increased phenolic compounds from fermented Bokbunja (Rubus coreanus Miq.) and related antioxidant activity. J. Pharmaceut. Biomed. Anal. 2009, 49, 820–827. [Google Scholar] [CrossRef]
- Hernández, T.; Estrella, I.; Carlavilla, D.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V. Phenolic compounds in red wine subjected to industrial malolactic fermentation and ageing on lees. Anal. Chim. Acta 2006, 563, 116–125. [Google Scholar] [CrossRef]
- Rodríguez, H.; Landete, J.M.; Rivas, B.D.L.; Muñoz, R. Metabolism of food phenolic acids by Lactobacillus plantarum CECT 748T. Food Chem. 2008, 107, 1393–1398. [Google Scholar] [CrossRef] [Green Version]
- Curiel, J.A.; Rodríguez, H.; Landete, J.M.; de las Rivas, B.; Muñoz, R. Ability of Lactobacillus brevis strains to degrade food phenolic acids. Food Chem. 2010, 120, 225–229. [Google Scholar] [CrossRef]
- Fletcher, R.S.; Slimmon, T.; McAuley, C.Y.; Kott, L.S. Heat stress reduces the accumulation of rosmarinic acid and the total antioxidant capacity in spearmint (Mentha spicata L). J. Sci. Food Agr. 2005, 85, 2429–2436. [Google Scholar] [CrossRef]
- Ariffin, F.; Heong Chew, S.; Bhupinder, K.; Karim, A.A.; Huda, N. Antioxidant capacity and phenolic composition of fermented Centella asiatica herbal teas. J. Sci. Food Agr. 2011, 91, 2731–2739. [Google Scholar] [CrossRef]
- Coinu, R.; Carta, S.; Urgeghe, P.P.; Mulinacci, N.; Pinelli, P.; Franconi, F.; Romani, A. Dose-effect study on the antioxidant properties of leaves and outer bracts of extracts obtained from Violetto di Toscana artichoke. Food Chem. 2007, 101, 524–531. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. Fund. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Tepe, B. Antioxidant potentials and rosmarinic acid levels of the methanolic extracts of Salvia virgata (Jacq), Salvia staminea (Montbret & Aucher ex Bentham) and Salvia verbenaca (L.) from Turkey. Bioresource Technol. 2008, 99, 1584–1588. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hunaefi, D.; Akumo, D.N.; Riedel, H.; Smetanska, I. The Effect of Lactobacillus plantarum ATCC 8014 and Lactobacillus acidophilus NCFM Fermentation on Antioxidant Properties of Selected in Vitro Sprout Culture of Orthosiphon aristatus (Java Tea) as a Model Study. Antioxidants 2012, 1, 4-32. https://doi.org/10.3390/antiox1010004
Hunaefi D, Akumo DN, Riedel H, Smetanska I. The Effect of Lactobacillus plantarum ATCC 8014 and Lactobacillus acidophilus NCFM Fermentation on Antioxidant Properties of Selected in Vitro Sprout Culture of Orthosiphon aristatus (Java Tea) as a Model Study. Antioxidants. 2012; 1(1):4-32. https://doi.org/10.3390/antiox1010004
Chicago/Turabian StyleHunaefi, Dase, Divine N. Akumo, Heidi Riedel, and Iryna Smetanska. 2012. "The Effect of Lactobacillus plantarum ATCC 8014 and Lactobacillus acidophilus NCFM Fermentation on Antioxidant Properties of Selected in Vitro Sprout Culture of Orthosiphon aristatus (Java Tea) as a Model Study" Antioxidants 1, no. 1: 4-32. https://doi.org/10.3390/antiox1010004




