Antioxidant Potential of Bark Extracts from Boreal Forest Conifers
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Plant Material and Preparation of Crude Bark Extract
2.3. Dosing of Total Phenol Content
2.4. ORACFL Assay
2.5. Cell Culture
2.6. Antioxidant Cell-Based Assay
2.7. Cytotoxicity Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yield, Total Phenol Content and Cytotoxicity of Various Conifer Bark Extracts
| Conifer species | Extraction conditions (water:ethanol) | Extraction yield a (g/100 g) | Extract Phenolic content (g GAE/100 g) b | Cytotoxicity (μg/mL) c |
|---|---|---|---|---|
| Pinus banksiana | 100:0 | 9 | 27 ± 2 | >100 |
| 75:25 | 11 | 30 ± 2 | >100 | |
| 50:50 | 12 | 37 ± 4 | >100 | |
| 25:75 | 13 | 45 ± 1 | >200 | |
| 0:100 | 15 | 23 ± 1 | >100 | |
| Pinus resinosa | 100:0 | 5 | 37 ± 4 | >200 |
| 75:25 | 7 | 61 ± 6 | >100 | |
| 50:50 | 7 | 61 ± 4 | >100 | |
| 25:75 | 11 | 46 ± 3 | >100 | |
| 0:100 | 10 | 42 ± 5 | >50 | |
| Pinus strobus | 100:0 | 7 | 21 ± 3 | >50 |
| 75:25 | 9 | 26 ± 1 | >200 | |
| 50:50 | 15 | 22 ± 1 | >50 | |
| 25:75 | 9 | 15 ± 2 | >50 | |
| 0:100 | 9 | 10 ± 1 | >50 | |
| Picea glauca | 100:0 | 17 | 59 ± 5 | >100 |
| 75:25 | 21 | 51 ± 4 | >100 | |
| 50:50 | 24 | 55 ± 9 | >50 | |
| 25:75 | 29 | 36 ± 4 | >200 | |
| 0:100 | 26 | 48 ± 5 | >100 | |
| Picea mariana | 100:0 | 14 | 50 ± 5 | >200 |
| 75:25 | 17 | 55 ± 6 | >100 | |
| 50:50 | 23 | 48 ± 4 | >200 | |
| 25:75 | 24 | 46 ± 5 | >200 | |
| 0:100 | 23 | 43 ± 3 | >100 | |
| Larix laricina | 100:0 | 11 | 34 ± 2 | >50 |
| 75:25 | 15 | 27 ± 2 | >12.5 | |
| 50:50 | 24 | 29 ± 3 | >50 | |
| 25:75 | 30 | 26 ± 3 | >50 | |
| 0:100 | 30 | 29 ± 3 | >50 | |
| Abies balsamea | 100:0 | 7 | 6 ± 1 | >100 |
| 75:25 | 9 | 21 ± 1 | >100 | |
| 50:50 | 7 | 32 ± 2 | >100 | |
| 25:75 | 17 | 21 ± 2 | >50 | |
| 0:100 | 20 | 20 ± 1 | >50 |
3.2. Evaluation of Antioxidant Activity of Extracts Using ORACFL and Cell-Based Assay
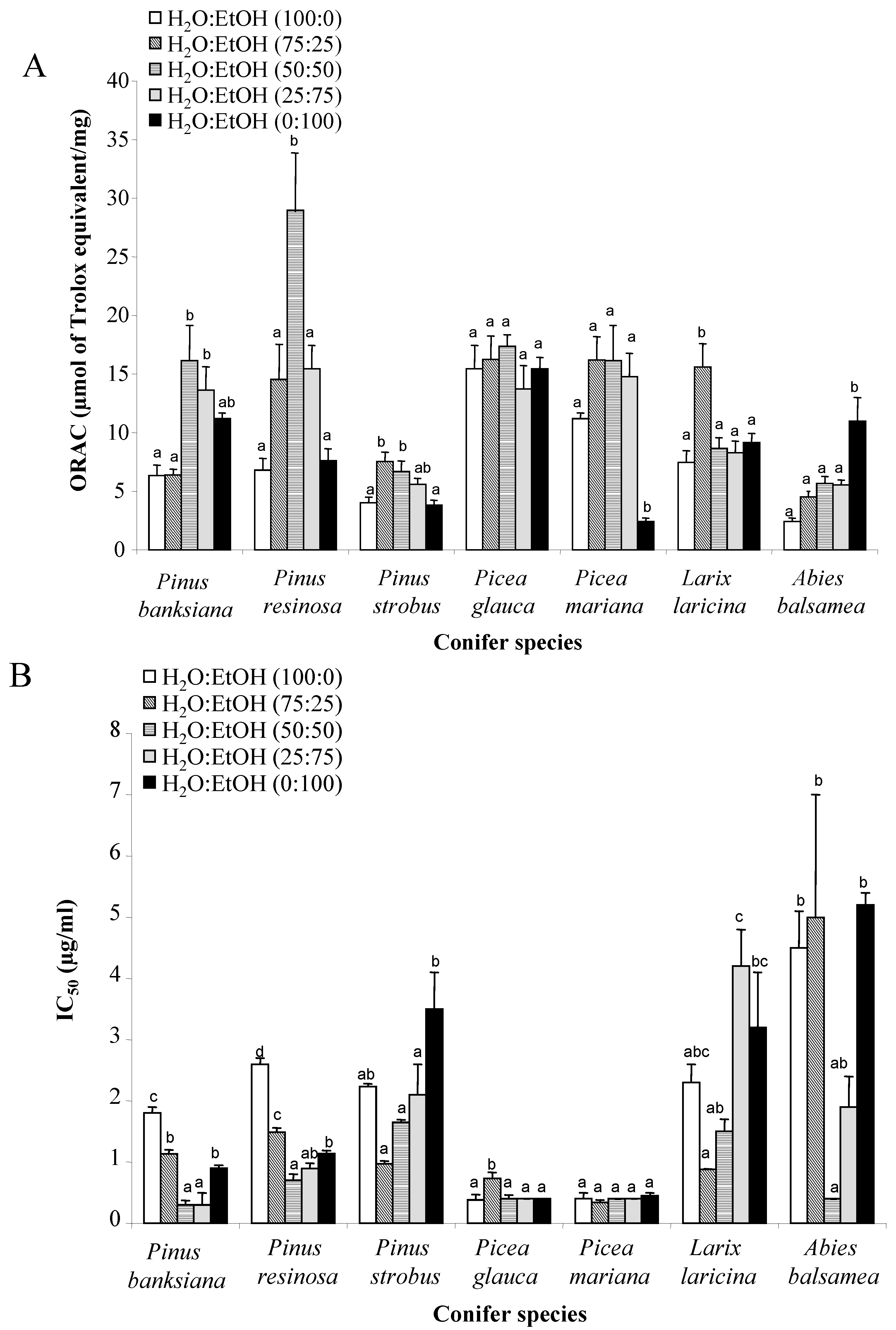
3.3. Relationship between Total Phenol Content and Antioxidant Activities
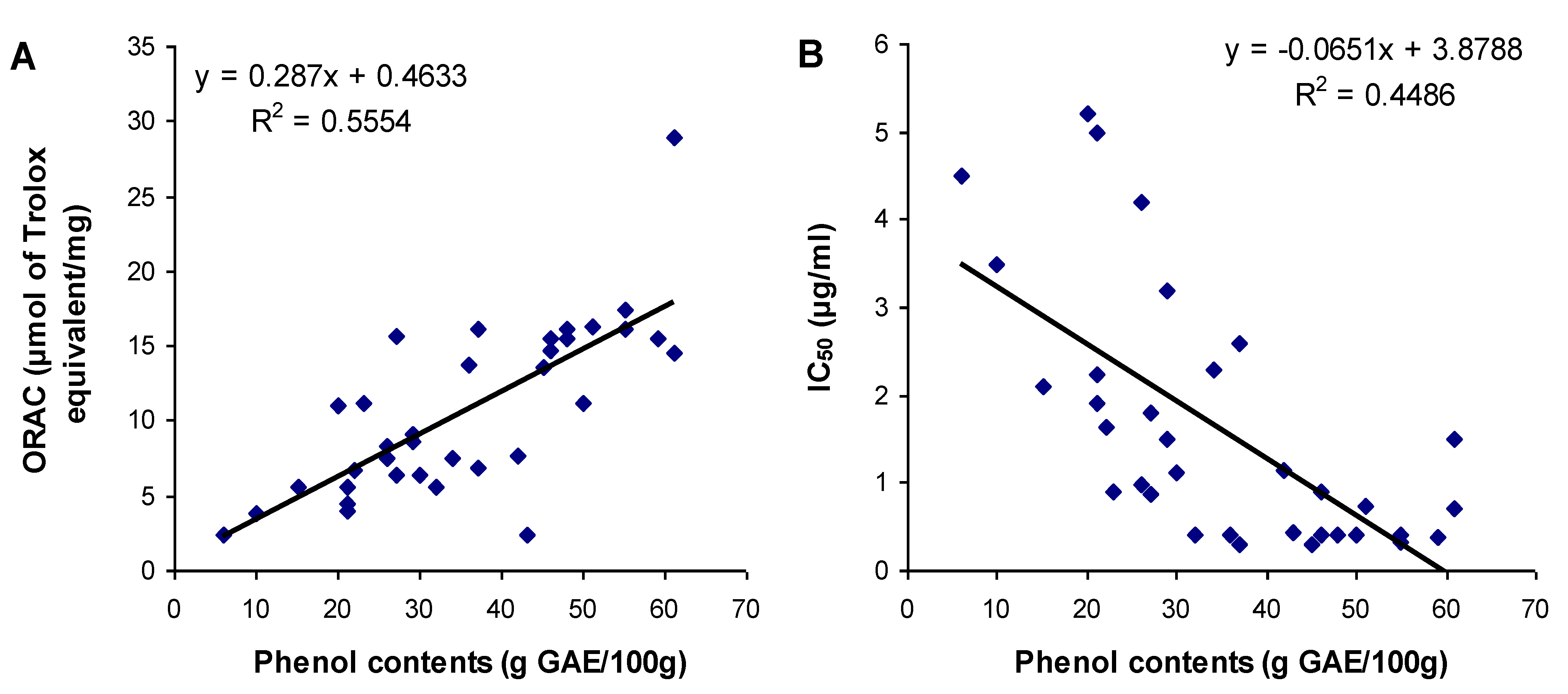
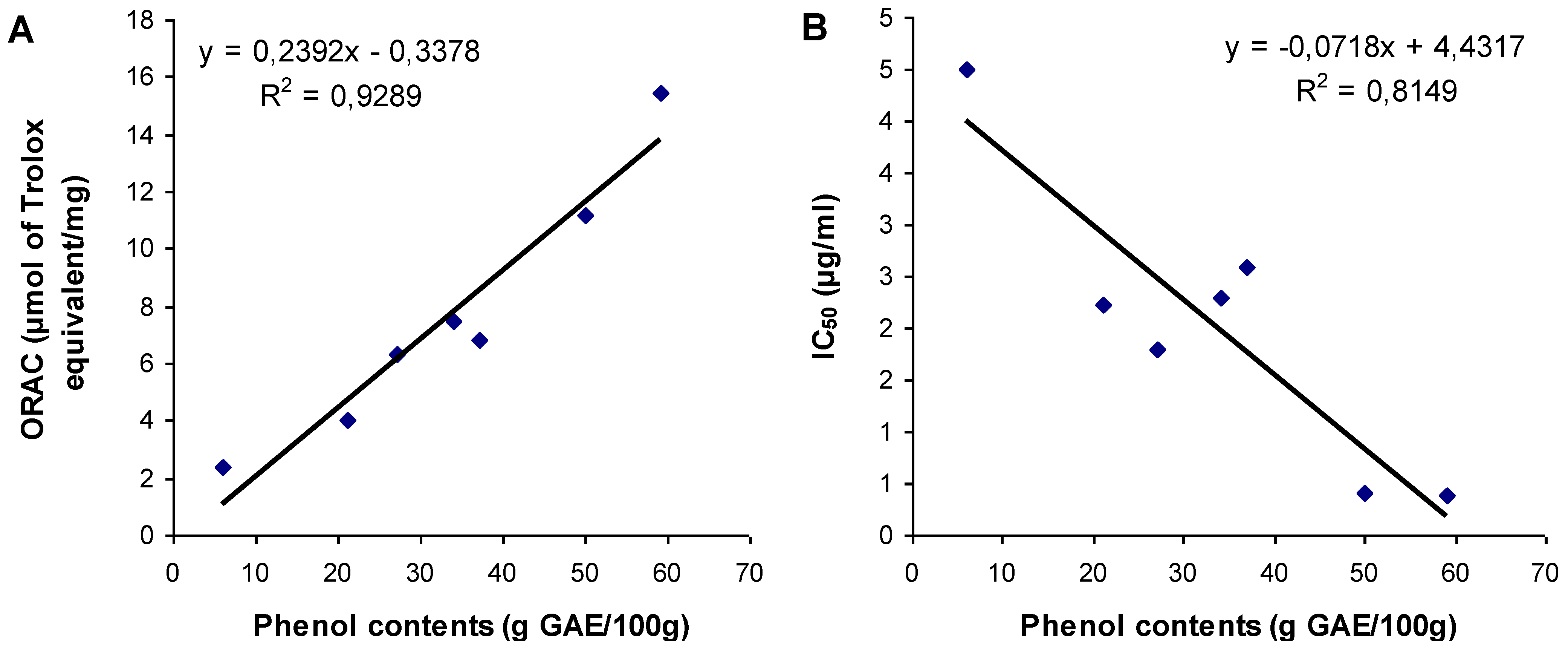
4. Conclusions
Acknowlegments
Conflict of Interest
References
- Moerman, D.E. Native American Ethnobotany, 3rd ed; Timber Press Inc.: Portland, OR, USA, 2000. [Google Scholar]
- Chandler, R.F.; Freeman, L.; Hooper, S.N. Herbal remedies of the maritime indians. J. Ethnopharmacol. 1979, 1, 49–68. [Google Scholar] [CrossRef]
- Gottesfeld, L.M.J. The importance of bark products in the aboriginal economies of Northwestern British Columbia. Can. Econ. Bot. 1992, 46, 148–157. [Google Scholar] [CrossRef]
- Mechling, W.H. The malecite indians with notes on the micmacs. Anthropologica 1959, 8, 239–263. [Google Scholar]
- Palmer, G. Shuswap Indian ethnobotany. Syesis 1975, 8, 29–51. [Google Scholar]
- Reagan, A.B. Plants used by the bois fort chippewa (Ojibwa) Indians of Minnesota. Wis. Archeol. 1928, 7, 230–248. [Google Scholar]
- Carr, L.G.; Westey, C. Surviving folktales & herbal lore among the shinnecock Indians. J. Am. Folk. 1945, 58, 113–123. [Google Scholar] [CrossRef]
- Chapple, I.L. Reactive oxygen species and antioxidants in inflammatory diseases. J. Clin. Periodontol. 1997, 24, 287–296. [Google Scholar] [CrossRef]
- Nagata, M. Inflammatory cells and oxygen radicals. Curr. Drug Targets Inflamm. Allergy 2005, 4, 503–504. [Google Scholar] [CrossRef]
- Hitchon, C.A.; El-Gabalawy, H.S. Oxidation in rheumatoid arthritis. Arthritis Res. Ther. 2004, 6, 265–278. [Google Scholar] [CrossRef] [Green Version]
- MacNee, W. Pulmonary and systemic oxidant/antioxidant imbalance in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2005, 2, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Repine, J.E.; Bast, A.; Lankhorst, I. Oxidative stress in chronic obstructive pulmonary disease: The Oxidative Stress Study Group. Am. J. Respir. Crit. Care Med. 1997, 156, 341–357. [Google Scholar] [CrossRef]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [PubMed]
- Rahman, I.; Kilty, I. Antioxidant therapeutic targets in COPD. Curr. Drug Targets 2006, 7, 707–720. [Google Scholar] [CrossRef]
- Santus, P.; Sola, A.; Carlucci, P.; Fumagalli, F.; di Gennaro, A.; Mondoni, M.; Carnini, C.; Centanni, S.; Sala, A. Lipid peroxidation and 5-lipoxygenase activity in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005, 171, 838–843. [Google Scholar] [CrossRef]
- Tabak, C.; Arts, I.C.W.; Smit, H.A.; Heederik, D.; Kromhout, D. Chronic obstructive pulmonary disease and intake of catechins, flavonols, and flavones. Am. J. Respir. Crit. Care Med. 2001, 164, 61–64. [Google Scholar] [CrossRef]
- Packer, L.; Rimbach, G.; Virgili, F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus maritima) bark, pycnogenol. Free Radic. Biol. Med. 1999, 27, 704–724. [Google Scholar] [CrossRef]
- Devaraj, S.; Vega-López, S.; Kaul, N.; Schönlau, F.; Rohdewald, P.; Jialal, I. Supplementation with a pine bark extract rich in polyphenols increases plasma antioxidant capacity and alters the plasma lipoprotein profile. Lipids 2002, 37, 931–934. [Google Scholar] [CrossRef]
- Lau, B.H.; Riesen, S.K.; Truong, K.P.; Lau, E.W.; Rohdewald, P.; Barreta, R.A. Pycnogenol as an adjunct in the management of childhood asthma. J. Asthma 2004, 41, 825–832. [Google Scholar] [CrossRef]
- Diouf, P.N.; Stevanovic, T.; Cloutier, A. Study on chemical composition, antioxidant and anti-inflammatory activities of hot water extract from Picea mariana bark and its proanthocyanidin-rich fractions. Food Chem. 2009, 113, 897–902. [Google Scholar] [CrossRef]
- García-Pérez, M.-E.; Royer, M.; Duque-Fernandez, A.; Diouf, P.N.; Stevanovic, T.; Pouliot, R. Antioxidant, toxicological and antiproliferative properties of Canadian polyphenolic extracts on normal and psoriatic keratinocytes. J. Ethnopharmacol. 2010, 132, 251–258. [Google Scholar] [CrossRef]
- Royer, M.; Prado, M.; Garcia-Pérez, M.-E.; Diouf, P.N.; Stevanovic, T. Study of nutraceutical, nutricosmetics and cosmeceutical potentials of polyphenolic bark extracts from Canadian forest species. PharmaNutrition 2013, in press. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Ou, B.; Hampsch-Woodhill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Girard-Lalancette, K.; Pichette, A.; Legault, J. Sensitive cell-based assay using DCFH oxidation for the determination or pro- and antioxidant properties of compounds and mixtures: Analysis of fruit and vegetable juices. Food Chem. 2009, 115, 720–726. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Ustun, O.; Senol, F.S.; Kurkcuaglu, M.; Orhan, I.E.; Kartal, M.; Can Baser, K.H. Investigation on chemical composition, anticholinesterase and antioxidant activities of extracts and essential oils of Turkish Pinus species and pycnogenol. Ind. Crop. Prod. 2012, 38, 115–123. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woilles, M.; Mérillon, J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- García-Pérez, M.-E.; Royer, M.; Herbette, G.; Desjardins, Y.; Pouliot, R.; Stevanovic, T. Picea mariana bark: A new source of trans-resveratrol and other bioactive polyphenols. Food Chem. 2012, 135, 1173–1182. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Legault, J.; Girard-Lalancette, K.; Dufour, D.; Pichette, A. Antioxidant Potential of Bark Extracts from Boreal Forest Conifers. Antioxidants 2013, 2, 77-89. https://doi.org/10.3390/antiox2030077
Legault J, Girard-Lalancette K, Dufour D, Pichette A. Antioxidant Potential of Bark Extracts from Boreal Forest Conifers. Antioxidants. 2013; 2(3):77-89. https://doi.org/10.3390/antiox2030077
Chicago/Turabian StyleLegault, Jean, Karl Girard-Lalancette, Dominic Dufour, and André Pichette. 2013. "Antioxidant Potential of Bark Extracts from Boreal Forest Conifers" Antioxidants 2, no. 3: 77-89. https://doi.org/10.3390/antiox2030077




