Effects of Different Drying Methods and Storage Time on Free Radical Scavenging Activity and Total Phenolic Content of Cosmos Caudatus
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Plant Material
2.3. Drying Process
2.4. Extraction of Antioxidant Compounds
2.5. Free Radical Scavenging Assay
2.6. Total Phenolic Content Assay
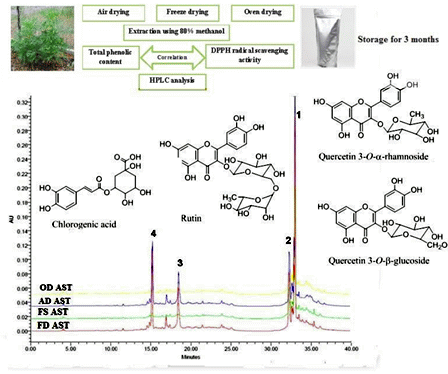
2.7. HPLC Method
2.8. Statistical Analysis
3. Results and Discussion
3.1. Variation of Dry Weight and Moisture Content among the Dried Samples
| Samples | Dry Weight (g) | Moisture Content (%) |
|---|---|---|
| Air dried | 21.2 a ± 0.4 | 8.8 a |
| Freeze dried | 21.09 a ± 0.6 | 8.5 a |
| Oven dried | 20.77 a ± 0.3 | 9.4 a |
3.2. Drying Influence on Phenolic Compounds

3.3. Drying Impact on DPPH Radical Scavenging Activity
3.4. Effect of Storage


3.5. Correlation between DPPH and TPC
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of interest
References
- Abas, F.; Shaari, K.; Lajis, N.H.; Israf, D.A.; Umi Kalsom, Y. Antioxidative and radical scavenging properties of the constituents isolated from Cosmos caudatus kunth. Nat. Prod. Sci. 2003, 9, 245–248. [Google Scholar]
- Oliveira, W.P.; Bott, R.F.; Souza, C.R.F. Manufacture of standardized dried extracts from medicinal brazilian plants. Dry. Technol. 2006, 24, 523–533. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Murtijaya, J. Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT-Food Sci. Technol. 2007, 40, 1664–1669. [Google Scholar] [CrossRef]
- Hossain, M.B.; Barry-Ryan, C.; Martin-Diana, A.B.; Brunton, N.P. Effect of drying method on the antioxidant capacity of six lamiaceae herbs. Food Chem. 2010, 123, 85–91. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Wong, S.K.; Lim, K.K.; Tan, S.P.; Lianto, F.S.; Yong, M.Y. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 2009, 113, 166–172. [Google Scholar] [CrossRef]
- Asami, D.K.; Hong, Y.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef]
- Chang, C.; Lin, H.; Chang, C.; Liu, Y.C. Comparisons on the antioxidant properties of fresh, freeze-dried and hot-air-dried tomatoes. J. Food Eng. 2006, 77, 478–485. [Google Scholar] [CrossRef]
- Roy, M.K.; Takenaka, M.; Isobe, S.; Tsushida, T. Antioxidant potential, anti-proliferative activities, and phenolic content in water-soluble fractions of some commonly consumed vegetables: Effects of thermal treatment. Food Chem. 2007, 103, 106–114. [Google Scholar] [CrossRef]
- Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic. Biol. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef]
- Surveswaran, S.; Cai, Y.; Corke, H.; Sun, M. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem. 2007, 102, 938–953. [Google Scholar] [CrossRef]
- Clarke, G.; Ting, K.; Wiart, C.; Fry, J. High correlation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, ferric reducing activity potential and total phenolics content indicates redundancy in use of all three assays to screen for antioxidant activity of extracts of plants from the Malaysian rainforest. Antioxidants 2013, 2, 1–10. [Google Scholar] [CrossRef]
- Abas, F.; Lajis, N.H.; Israf, D.A.; Khozirah, S.; Umi Kalsom, Y. Antioxidant and nitric oxide inhibition activities of selected malay traditional vegetables. Food Chem. 2006, 95, 566–573. [Google Scholar] [CrossRef]
- Mediani, A.; Abas, F.; Khatib, A.; Tan, C. Cosmos caudatus as a potential source of polyphenolic compounds: Optimisation of oven drying conditions and characterisation of its functional properties. Molecules 2013, 18, 10452–10464. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Method of Analysis, 18th ed.; Association of Officiating Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Mustafa, R.A.; Hamid, A.A.; Mohamed, S.; Abu Bakar, F. Total phenolic compounds, flavonoids, and radical scavenging activity of 21 selected tropical plants. J. Food Sci. 2010, 75, C28–C35. [Google Scholar] [CrossRef]
- Georgé, S.; Tourniaire, F.; Gautier, H.; Goupy, P.; Rock, E.; Caris-Veyrat, C. Changes in the contents of carotenoids, phenolic compounds and vitamin C during technical processing and lyophilisation of red and yellow tomatoes. Food Chem. 2011, 124, 1603–1611. [Google Scholar] [CrossRef]
- Arabhosseini, A.; Huisman, W.; van Boxtel, A.; Muller, J. Long-term effects of drying conditions on the essential oil and color of tarragon leaves during storage. J. Food Eng. 2007, 79, 561–566. [Google Scholar] [CrossRef]
- Arslan, D.; Özcan, M.M. Dehydration of red bell-pepper (Capsicum annuum L.): Change in drying behavior, colour and antioxidant content. Food Bioprod. Process. 2010, 89, 504–513. [Google Scholar] [CrossRef]
- Kong, K.W.; Ismail, A.; Tan, C.P.; Rajab, F. Optimization of oven drying conditions for lycopene content and lipophilic antioxidant capacity in a by-product of the pink guava puree industry using response surface methodology. LWT-Food Sci. Technol. 2010, 43, 729–735. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Anese, M.; Parpinel, M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Harbourne, N.; Marete, E.; Jacquier, J.C.; O’Riordan, D. Effect of drying methods on the phenolic constituents of meadowsweet (Filipendula ulmaria) and willow (Salix alba). LWT-Food Sci. Technol. 2009, 42, 1468–1473. [Google Scholar] [CrossRef]
- Spigno, G.; de Faveri, D.M. Microwave-assisted extraction of tea phenols: A phenomenological study. J. Food Eng. 2009, 93, 210–217. [Google Scholar] [CrossRef]
- Suvarnakuta, P.; Chaweerungrat, C.; Devahastin, S. Effects of drying methods on assay and antioxidant activity of xanthones in mangosteen rind. Food Chem. 2011, 125, 240–247. [Google Scholar] [CrossRef]
- Paixão, N.; Perestrelo, R.; Marques, J.C.; Câmara, J.S. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007, 105, 204–214. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Yu, L.; Haley, S.; Perret, J.; Harris, M. Free radical scavenging properties of wheat extracts. J. Agric. Food Chem. 2002, 50, 1619–1624. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mediani, A.; Abas, F.; Tan, C.P.; Khatib, A. Effects of Different Drying Methods and Storage Time on Free Radical Scavenging Activity and Total Phenolic Content of Cosmos Caudatus. Antioxidants 2014, 3, 358-370. https://doi.org/10.3390/antiox3020358
Mediani A, Abas F, Tan CP, Khatib A. Effects of Different Drying Methods and Storage Time on Free Radical Scavenging Activity and Total Phenolic Content of Cosmos Caudatus. Antioxidants. 2014; 3(2):358-370. https://doi.org/10.3390/antiox3020358
Chicago/Turabian StyleMediani, Ahmed, Faridah Abas, Chin Ping Tan, and Alfi Khatib. 2014. "Effects of Different Drying Methods and Storage Time on Free Radical Scavenging Activity and Total Phenolic Content of Cosmos Caudatus" Antioxidants 3, no. 2: 358-370. https://doi.org/10.3390/antiox3020358