Silymarin Activates c-AMP Phosphodiesterase and Stimulates Insulin Secretion in a Glucose-Dependent Manner in HIT-T15 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sources of Reagents
2.2. HIT-T15 Cells and Peroxide Levels
2.3. HIT-T15 Cells and Insulin Secretion and Content
2.4. HIT-T15 Cells and cAMP Phosphodiesterase Activity
2.5. Statistical Analysis
3. Results
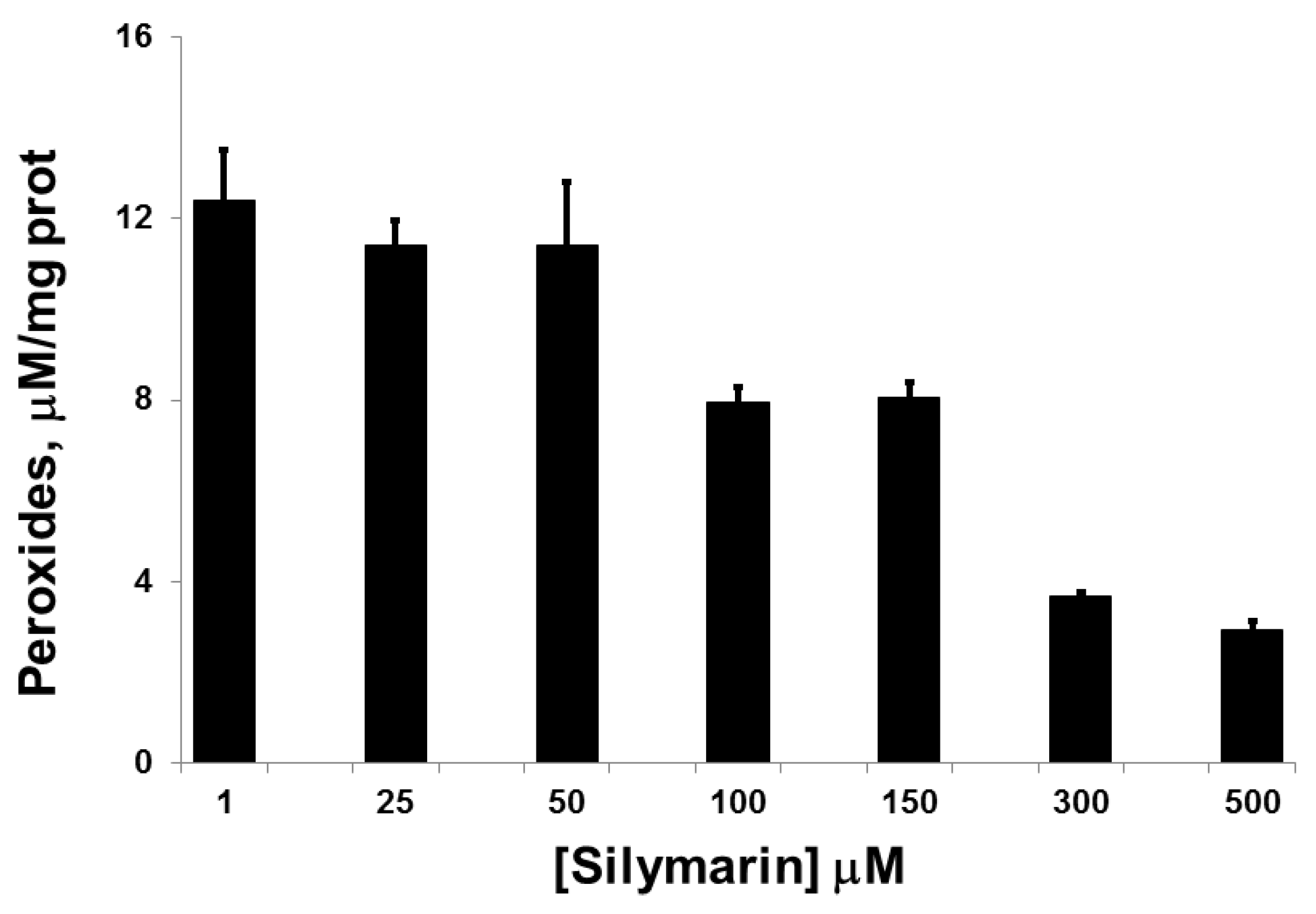
3.1. Silymarin Effects on Intracellular Peroxide Levels
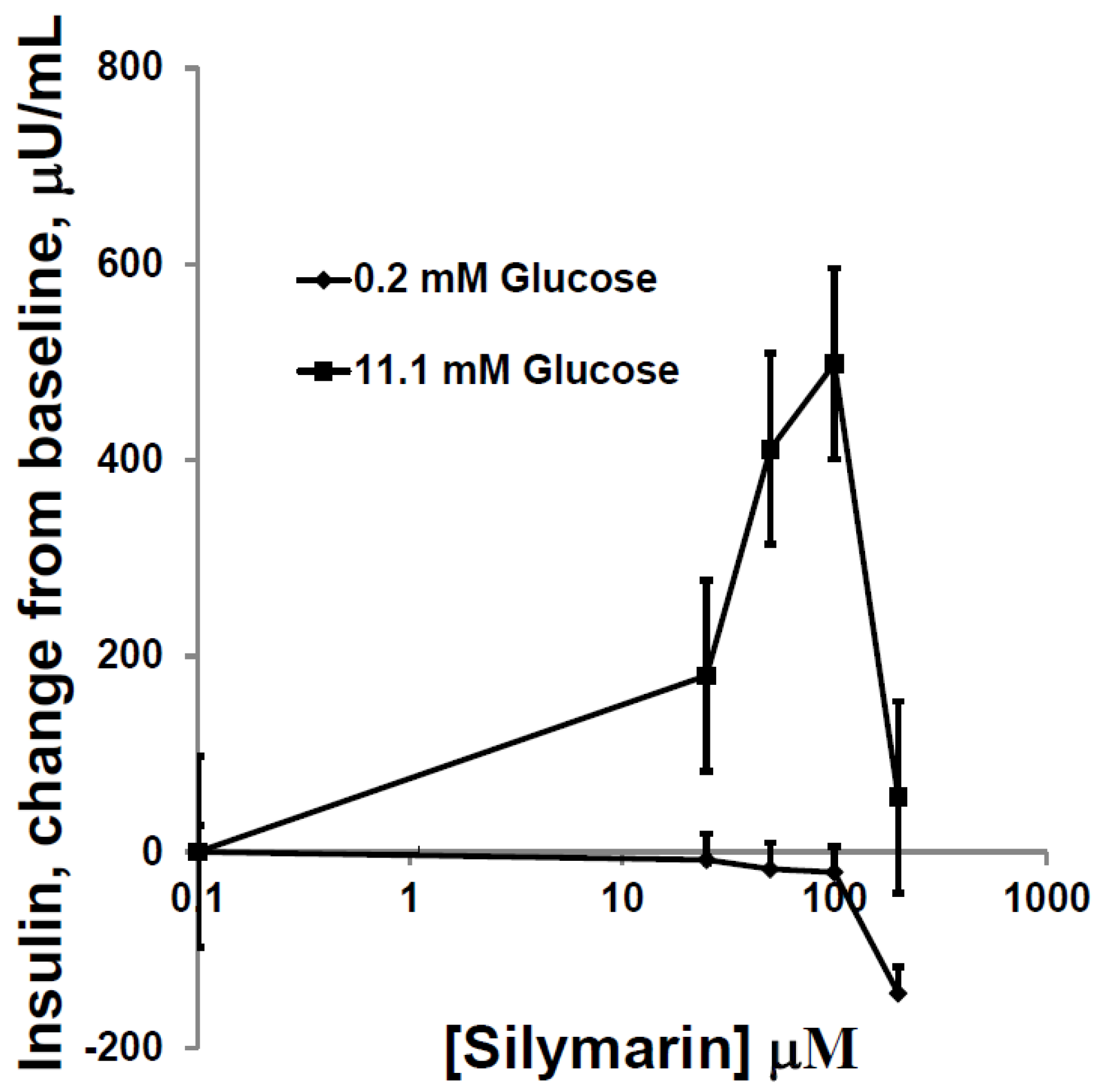
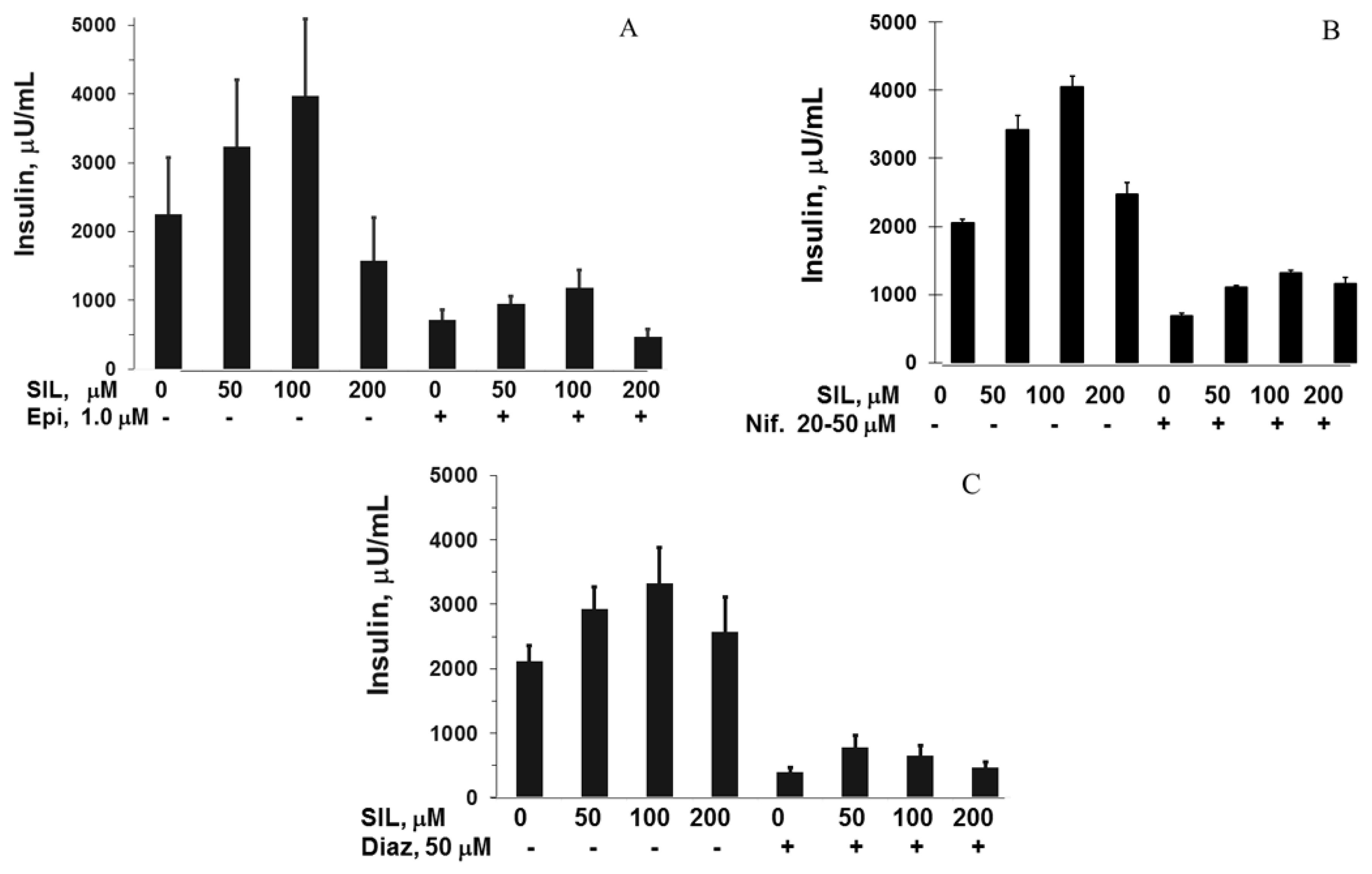
3.2. Silymarin Effects on Insulin Secretion, Insulin Content, and Apoptosis
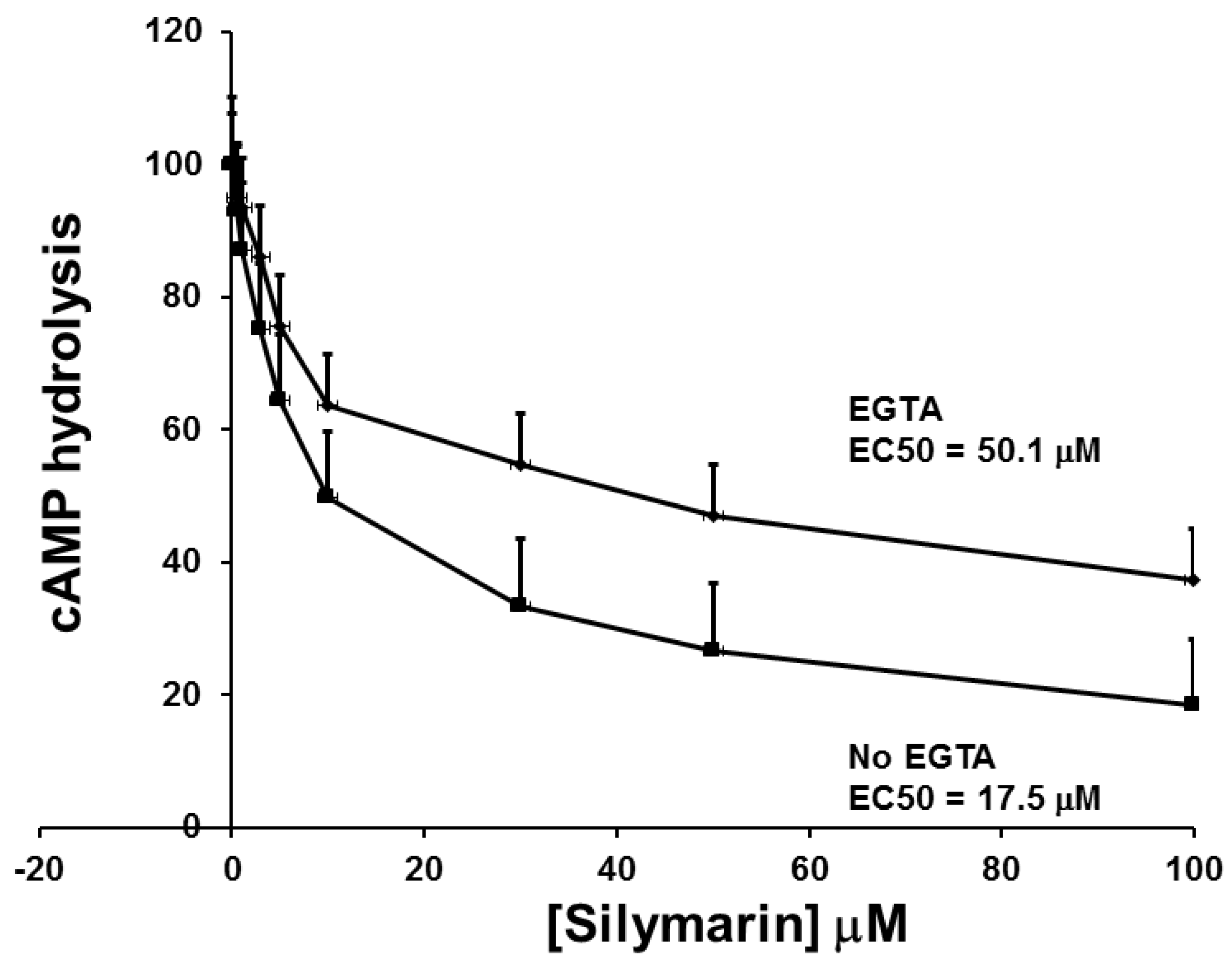
3.3. The Effect of SIL on cAMP Phosphodiesterase Activity and Comparison to other Inhibitors of cAMP Phosphodiestrerase Activity on GSIS and cAMP Phosphodiesterase Activity
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Robertson, R.P. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet β cells in diabetes. J. Biol Chem. 2004, 279, 42351–42354. [Google Scholar] [CrossRef] [PubMed]
- Velussi, M.; Cernigoi, A.M.; de Monte, A.; Dapas, F.; Caffau, C.; Zilli, M. Long-term (12 months) treatment with an anti-oxidant drug (silymarin) is effective on hyperinsulinemia, exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. J. Hepatol. 1997, 26, 871–879. [Google Scholar] [CrossRef]
- Hussain, S.A. Silymarin as an adjunct to glibenclamide therapy improves long-term and postprandial glycemic control and body mass index in type 2 diabetes. J. Med. Food 2007, 10, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Huseini, H.F.; Larijani, B.; Heshmat, R.; Fakhrzadeh, H.; Radjabipour, B.; Toliat, T.; Raza, M. The efficacy of Silybum marianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: A randomized, double-blind, placebo-controlled, clinical trial. Phytother. Res. 2006, 20, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.P.; Bachner, J.; Loffler, E. Silymarin: Potent inhibitor of cyclic AMP phosphodiesterase. Methods Find. Exp. Clin. Pharmacol. 1985, 7, 409–413. [Google Scholar] [PubMed]
- Zhang, H.J.; Walseth, T.F.; Robertson, R.P. Insulin secretion and cAMP metabolism in HIT cells. Reciprocal and serial passage-dependent relationships. Diabetes 1989, 38, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.P.; Zhang, H.J.; Pyzdrowski, K.L.; Walseth, T.F. Preservation of insulin mRNA levels and insulin secretion in HIT cells by avoidance of chronic exposure to high glucose concentrations. J. Clin. Investig. 1992, 90, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.D.; Gupta, Y.K. Determination of hydrogen peroxide by thallium(III) in the presence of iron(II). Talanta 1973, 20, 903–905. [Google Scholar] [CrossRef]
- Mahadevan, J.; Parazzoli, S.; Oseid, E.; Hertzel, A.V.; Bernlohr, D.A.; Vallerie, S.N.; Liu, C.; Lopez, M.; Harmon, J.S.; Robertson, R.P. Ebselen treatment prevents islet apoptosis, maintains intranuclear Pdx-1 and MafA levels, and preserves β-cell mass and function in ZDF rats. Diabetes 2013, 62, 3582–3588. [Google Scholar] [CrossRef] [PubMed]
- Slucca, M.; Harmon, J.S.; Oseid, E.A.; Bryan, J.; Robertson, R.P. ATP-K+ channel mediates the zinc switch-off signal for glucagon response during glucose deprivation. Diabetes 2010, 59, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Rybalkin, S.D.; Hinds, T.R.; Beavo, J.A. Enzyme assays for cGMP hydrolyzing phosphodiesterases. Methods Mol. Biol. 2013, 1020, 51–62. [Google Scholar] [PubMed]
- Robertson, R.P.; Harmon, J.S. Diabetes, glucose toxicity, and oxidative stress: A case of double jeopardy for the pancreatic islet beta cell. Free Radic. Biol. Med. 2006, 41, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Gleason, C.E.; Tran, P.O.; Harmon, J.S.; Robertson, R.P. Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc. Natl. Acad. Sci. USA 1999, 96, 10857–10862. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, R.; Mahadevan, J.; Oseid, E.; Vallerie, S.; Robertson, R.P. Silymarin Activates c-AMP Phosphodiesterase and Stimulates Insulin Secretion in a Glucose-Dependent Manner in HIT-T15 Cells. Antioxidants 2016, 5, 47. https://doi.org/10.3390/antiox5040047
Meng R, Mahadevan J, Oseid E, Vallerie S, Robertson RP. Silymarin Activates c-AMP Phosphodiesterase and Stimulates Insulin Secretion in a Glucose-Dependent Manner in HIT-T15 Cells. Antioxidants. 2016; 5(4):47. https://doi.org/10.3390/antiox5040047
Chicago/Turabian StyleMeng, Ran, Jana Mahadevan, Elizabeth Oseid, Sara Vallerie, and R. Paul Robertson. 2016. "Silymarin Activates c-AMP Phosphodiesterase and Stimulates Insulin Secretion in a Glucose-Dependent Manner in HIT-T15 Cells" Antioxidants 5, no. 4: 47. https://doi.org/10.3390/antiox5040047




