Inorganic Reactive Sulfur-Nitrogen Species: Intricate Release Mechanisms or Cacophony in Yellow, Blue and Red?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. UV/VIS Spectroscopy
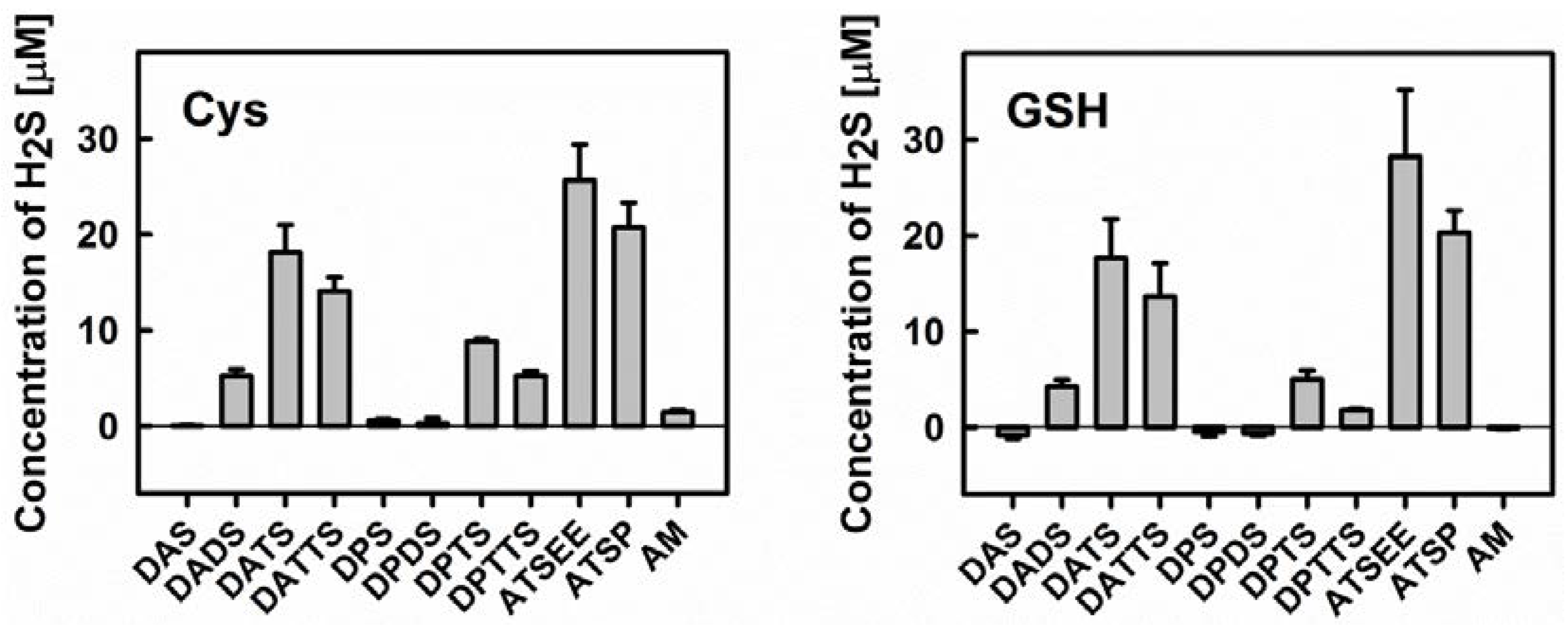
2.3. Determination of Sulfide Release from Organic Polysulfanes by the Methylene Blue Method
3. Results and Discussion
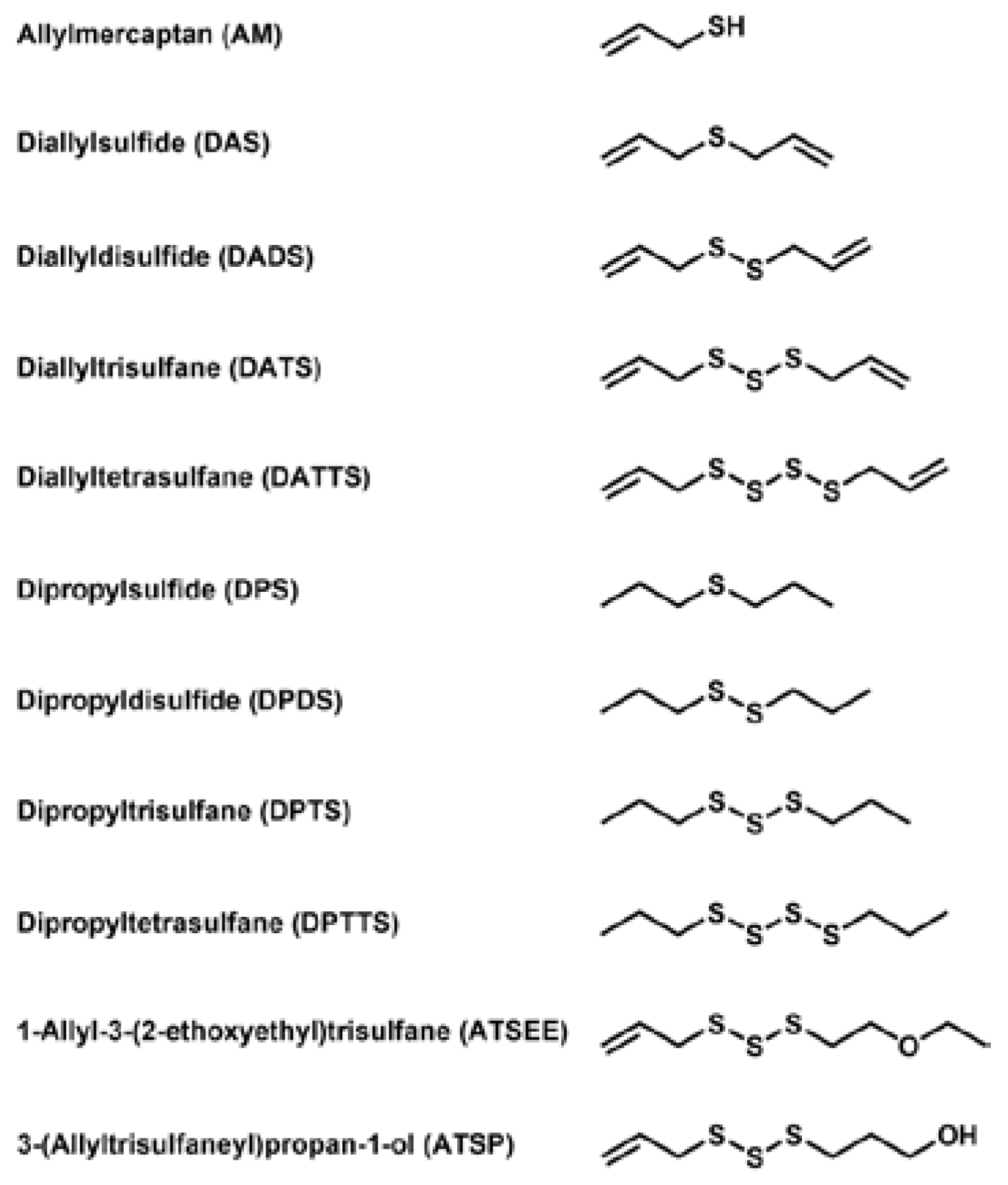
3.1. Selection and Acquisition of Sulfur Compounds
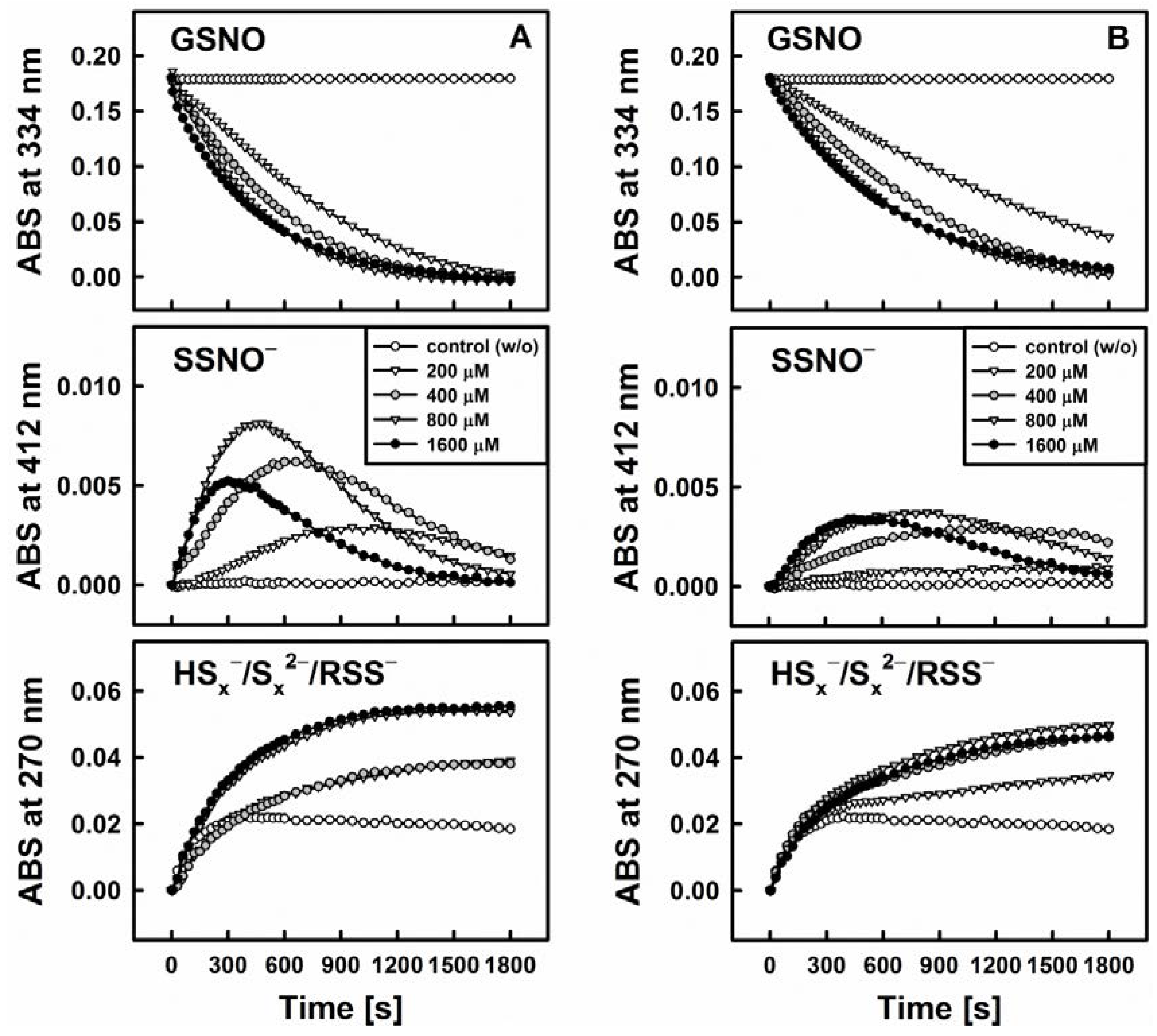
3.2. Nitric Oxide Release from S-Nitrosoglutathione Is Triggered by Reduction of Polysulfanes and Hydrogen Sulfide Formation
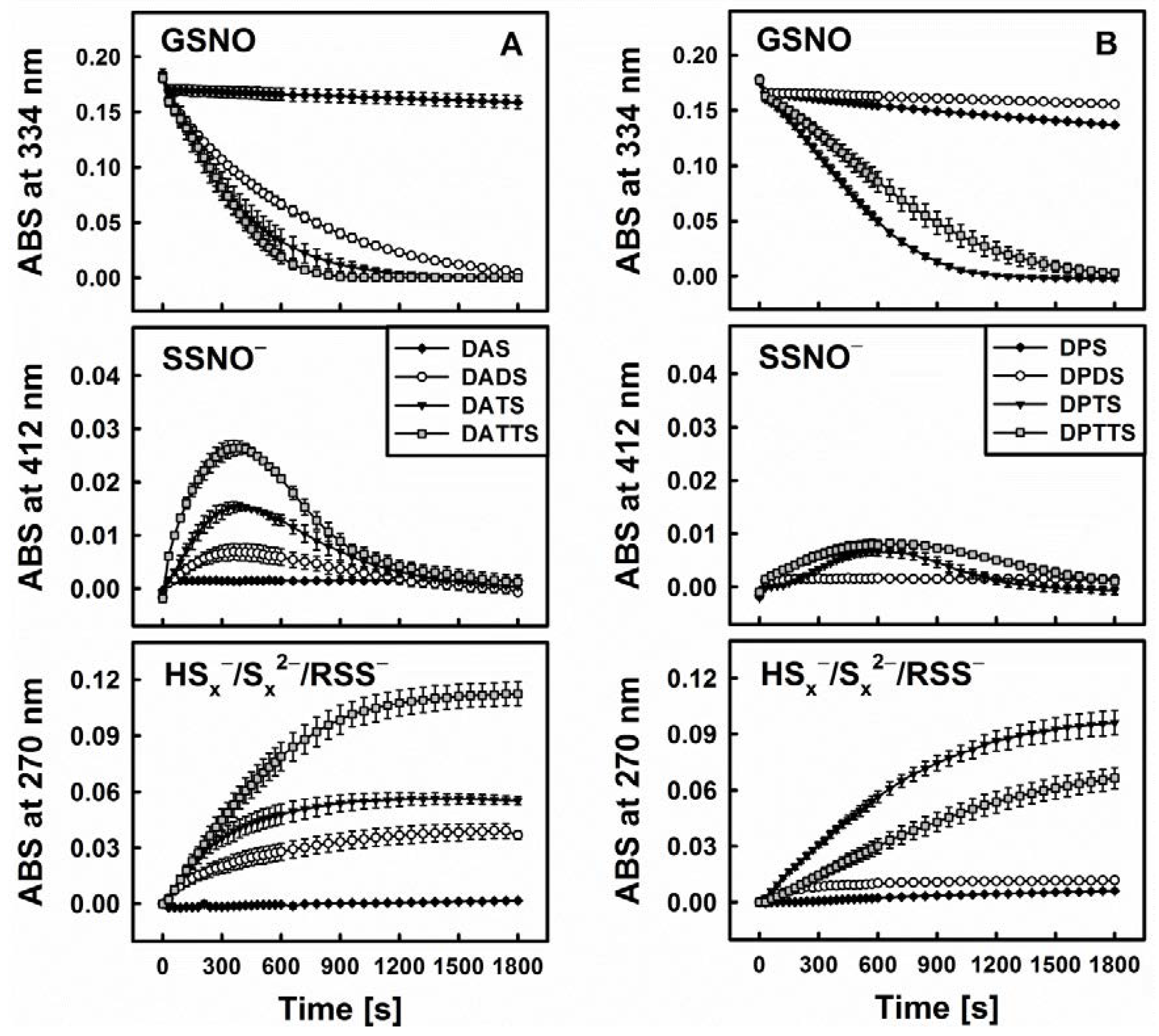
3.3. Smell Another Day: Synthetic Polysulfanes as a more Practical Alternative?
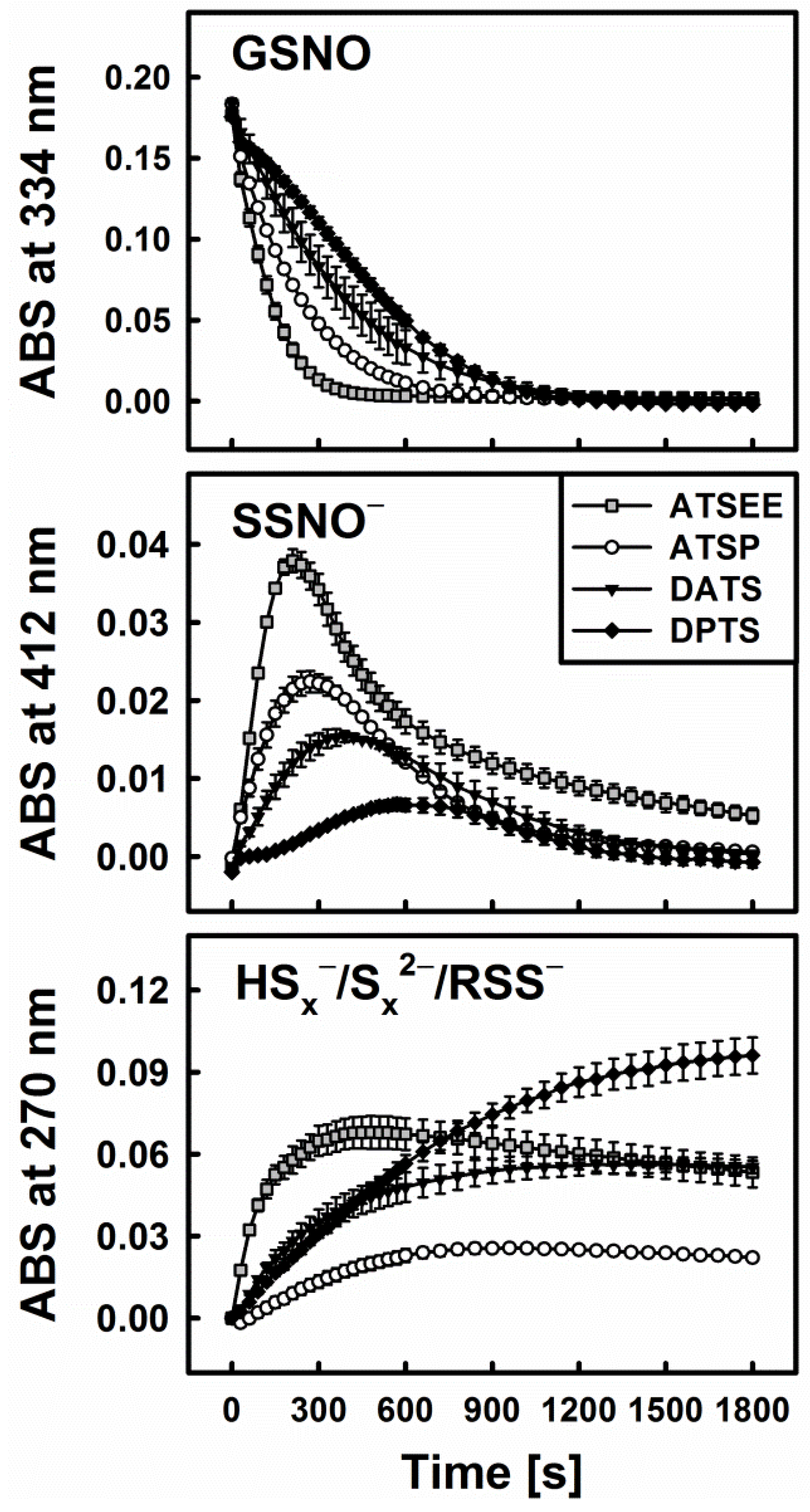
3.4. Polysulfanes, H2S, H2Sx, GSNO and •NO: Complex Interactions or Cacophony?
3.5. Biological Relevance of the Diallyltrisulfane, Thiol and GSNO Ménage à Trois: Hard to Get or Getting Hard?
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Leonard, S.E.; Reddie, K.G.; Carroll, K.S. Mining the thiol proteome for sulfenic acid modifications reveals new targets for oxidation in cells. ACS Chem. Biol. 2009, 4, 783–799. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C. Redox signalling via the cellular thiolstat. Biochem. Soc. Trans. 2011, 39, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Milligan, B.; Saville, B.; Swan, J.M. New syntheses of trisulphides. J. Chem. Soc. 1961, 954, 4850–4853. [Google Scholar] [CrossRef]
- Derbesy, G.; Harpp, D.N. A simple method to prepare unsymmetrical di- tri- and tetrasulfides. Tetrahedron Lett. 1994, 35, 5381–5384. [Google Scholar] [CrossRef]
- Fogo, J.K.; Popowsky, M. Spectrophotometric determination of hydrogen sulfide: Methylene blue method. Anal. Chem. 1949, 21, 732–734. [Google Scholar] [CrossRef]
- Allah, D.R.; Schwind, L.; Asali, I.A.; Nasim, J.; Jacob, C.; Götz, C.; Montenarh, M. A scent of therapy: Synthetic polysulfanes with improved physico-chemical properties induce apoptosis in human cancer cells. Int. J. Oncol. 2015, 47, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Czepukojc, B.; Baltes, A.K.; Cerella, C.; Kelkel, M.; Viswanathan, U.M.; Salm, F.; Burkholz, T.; Schneider, C.; Dicato, M.; Montenarh, M.; et al. Synthetic polysulfane derivatives induce cell cycle arrest and apoptotic cell death in human hematopoietic cancer cells. Food Chem. Toxicol. 2014, 64, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.P.; Williams, D.L.H. Reactivity of sulfur nucleophiles towards S-nitrosothiols. J. Chem. Soc. Perkin 2000, 2, 1794–1797. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Miljkovic, J.L.; Nauser, T.; Royzen, M.; Klos, K.; Shubina, T.; Koppenol, W.H.; Lippard, S.J.; Ivanović-Burmazović, I. Chemical characterization of the smallest S-nitrosothiol, HSNO; Cellular cross-talk of H2S and S-nitrosothiols. J. Am. Chem. Soc. 2012, 134, 12016–12027. [Google Scholar] [CrossRef] [PubMed]
- Cortese-Krott, M.M.; Kuhnle, G.G.C.; Dyson, A.; Fernandez, B.O.; Grman, M.; DuMond, J.F.; Barrow, M.P.; McLeod, G.; Nakagawa, H.; Ondrias, K.; et al. Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl. Proc. Natl. Acad. Sci. USA 2015, 112, E4651–E4660. [Google Scholar] [CrossRef] [PubMed]
- Ondrias, K.; Stasko, A.; Cacanyiova, S.; Sulova, Z.; Krizanova, O.; Kristek, F.; Malekova, L.; Knezl, V.; Breier, A. H2S and HS− donor NaHS releases nitric oxide from nitrosothiols, metal nitrosyl complex, brain homogenate and murine L1210 leukaemia cells. Pflugers Arch. 2008, 457, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Grman, M.; Misak, A.; Jacob, C.; Tomaskova, Z.; Bertova, A.; Burkholz, T.; Docolomansky, P.; Habala, L.; Ondrias, K. Low molecular thiols, pH and O2 modulate H2S-induced S-nitrosoglutathione decomposition—•NO release. Gen. Physiol. Biophys. 2013, 32, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Cortese-Krott, M.M.; Fernandez, B.O.; Santos, J.L.T.; Mergia, E.; Grman, M.; Nagy, P.; Kelm, M.; Butler, A.; Feelisch, M. Nitrosopersulfide (SSNO−) accounts for sustained NO bioactivity of S-nitrosothiols following reaction with sulfide. Redox Biol. 2014, 2, 234–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.P.; Wishnok, J.S.; Keshive, M.; Deen, W.M.; Tannenbaum, S.R. The chemistry of the S-nitrosoglutathione/glutathione system. Proc. Natl. Acad. Sci. USA 1996, 93, 14428–14433. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, G.; Fischer, A. Die Acidität der Sulfane und die Zusammensetzung wässeriger Polysulfidlösungen. Helv. Chim. Acta 1960, 43, 1365–1390. [Google Scholar] [CrossRef]
- Bari, S.E.; Olabe, J.A. New Features of the NO/H2S Cross Talk: A Chemical Basis. In Gasotransmitters in Plants: The Rise of a New Paradigm in Cell Signaling; Lamattina, L., García-Mata, C., Eds.; Springer: Cham, Switzerland, 2016; pp. 289–327. [Google Scholar]
- Cortese-Krott, M.M.; Butler, A.R.; Woollins, J.D.; Feelisch, M. Inorganic sulfur-nitrogen compounds: From gunpowder chemistry to the forefront of biological signaling. Dalton Trans. 2016, 45, 5908–5919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seel, F.; Kuhn, R.; Simon, G.; Wagner, M.; Krebs, B.; Dartmann, M. PNP-Perthionitrit und PNP-Monothionitrit. Zeitschrift Naturforschung Teil B 1985, 40, 1607–1617. [Google Scholar]
- Seel, F.; Wagner, M. Über die Umsetzung von Polysulfiden mit Stickstoffmonoxid in nichtwäßrigen Lösungsmitteln—Nitrosodisulfide. Zeitschrift Naturforschung. Teil B 1985, 40, 762–764. [Google Scholar]
- Seel, F.; Simon, G.; Schuh, J.; Wagner, M.; Wolf, B.; Ruppert, I.; Wieckowski, A.B. Untersuchung der Umsetzung von Schwefel mit Natriumnitrit in DMF, DMSO und HMPT. Zeitschrift Anorganische Allgemeine Chemie 1986, 538, 177–190. [Google Scholar] [CrossRef]
- Seel, F.; Wagner, M. Über die Umsetzung von Sulfiden mit Stickstoffmonoxid in Wäßrigen Lösungen. Zeitschrift Anorganische Allgemeine Chemie 1988, 558, 189–192. [Google Scholar] [CrossRef]
- Wedmann, R.; Zahl, A.; Shubina, T.E.; Dürr, M.; Heinemann, F.W.; Bugenhagen, B.E.C.; Burger, P.; Ivanovic-Burmazovic, I.; Filipovic, M.R. Does Perthionitrite (SSNO−) Account for Sustained Bioactivity of NO? A (Bio)chemical Characterization. Inorg. Chem. 2015, 54, 9367–9380. [Google Scholar] [CrossRef] [PubMed]
- Bianco, C.L.; Chavez, T.A.; Sosa, V.; Saund, S.S.; Nguyen, Q.N.N.; Tantillo, D.J.; Ichimura, A.S.; Toscano, J.P.; Fukuto, J.M. The chemical biology of the persulfide (RSSH)/perthiyl (RSS·) redox couple and possible role in biological redox signaling. Free Radic. Biol. Med. 2016, 101, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Marcolongo, J.P.; Morzan, U.N.; Zeida, A.; Scherlis, D.A.; Olabe, J.A. Nitrosodisulfide [S2NO]− (perthionitrite) is a true intermediate during the “cross-talk” of nitrosyl and sulfide. Phys. Chem. Chem. Phys. 2016, 18, 30047–30052. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.S.; Henthorn, H.A.; Pluth, M.D. The Intersection of NO and H2S: Persulfides Generate NO from Nitrite through Polysulfide Formation. Inorg. Chem. 2016, 55, 12618–12625. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P. Mechanistic chemical perspective of hydrogen sulfide signaling. Methods Enzymol. 2015, 554, 3–29. [Google Scholar] [PubMed]
- Steudel, R. Mechanism for the Formation of Elemental Sulfur from Aqueous Sulfide in Chemical and Microbiological Desulfurization Processes. Ind. Eng. Chem. Res. 1996, 35, 1417–1423. [Google Scholar] [CrossRef]
- Giggenbach, W. Optical spectra and equilibrium distribution of polysulfide ions in aqueous solution at 20°. Inorg. Chem. 1972, 11, 1201–1207. [Google Scholar] [CrossRef]
- Kamyshny, A., Jr.; Goifman, A.; Rizkov, D.; Lev, O. Kinetics of disproportionation of inorganic polysulfides in undersaturated aqueous solutions at environmentally relevant conditions. Aquat. Geochem. 2003, 9, 291–304. [Google Scholar] [CrossRef]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.H. The chemistry of S-nitrosothiols. Acc. Chem. Res. 1999, 32, 869–876. [Google Scholar] [CrossRef]
- Saund, S.S.; Sosa, V.; Henriquez, S.; Nguyen, Q.N.N.; Bianco, C.L.; Soeda, S.; Millikin, R.; White, C.; Le, H.; Ono, K.; et al. The chemical biology of hydropersulfides (RSSH): Chemical stability, reactivity and redox roles. Arch. Biochem. Biophys. 2015, 588, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Grman, M.; Misak, A.; Cacanyiova, S.; Kristek, F.; Tomaskova, Z.; Bertova, A.; Ondrias, K. The aqueous garlic, onion and leek extracts release nitric oxide from S-nitrosoglutathione and prolong relaxation of aortic rings. Gen. Physiol. Biophys. 2011, 30, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.Z.; Hussain, M.E.; Fahim, M. Endothelium mediated vasorelaxant response of garlic in isolated rat aorta: Role of nitric oxide. J. Ethnopharmacol. 2004, 90, 5–9. [Google Scholar] [CrossRef] [PubMed]
- DeLeon, E.R.; Gao, Y.; Huang, E.; Olson, K.R. Garlic oil polysulfides: H2S-and O2-independent prooxidants in buffer and antioxidants in cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R1212–R1225. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Wu, H.; Wong, M.W.; Huang, D. Diallyl Trisulfide Is a Fast H2S Donor, but Diallyl Disulfide Is a Slow One: The Reaction Pathways and Intermediates of Glutathione with Polysulfides. Org. Lett. 2015, 17, 4196–4199. [Google Scholar] [CrossRef] [PubMed]
- Berenyiova, A.; Grman, M.; Mijuskovic, A.; Stasko, A.; Misak, A.; Nagy, P.; Ondriasova, E.; Cacanyiova, S.; Brezova, V.; Feelisch, M.; et al. The reaction products of sulfide and S-nitrosoglutathione are potent vasorelaxants. Nitric Oxide Biol. Chem. 2015, 46, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Cortese-Krott, M.M.; Pullmann, D.; Feelisch, M. Nitrosopersulfide (SSNO−) targets the Keap-1/Nrf2 redox system. Pharmacol. Res. 2016, 113, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Ried, K.; Fakler, P. Potential of garlic (Allium sativum) in lowering high blood pressure: Mechanisms of action and clinical relevance. Integr. Blood Press. Control. 2014, 7, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochimica Biophysica Acta Bioenergetics 2009, 1787, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Kabil, O.; Banerjee, R. Redox biochemistry of hydrogen sulfide. J. Biol. Chem. 2010, 285, 21903–21907. [Google Scholar] [CrossRef] [PubMed]
- Tomasova, L.; Konopelski, P.; Ufnal, M. Gut bacteria and hydrogen sulfide: The new old players in circulatory system homeostasis. Molecules 2016, 21, 1558. [Google Scholar] [CrossRef] [PubMed]
- Tomasova, L.; Dobrowolski, L.; Jurkowska, H.; Wróbel, M.; Huc, T.; Ondrias, K.; Ostaszewski, R.; Ufnal, M. Intracolonic hydrogen sulfide lowers blood pressure in rats. Nitric Oxide Biol. Chem. 2016, 60, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Broniowska, K.A.; Hogg, N. The chemical biology of S-nitrosothiols. Antioxid. Redox Signal. 2012, 17, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Bolden, C.; King, S.B.; Kim-Shapiro, D.B. Reactions between nitrosopersulfide and heme proteins. Free Radic. Biol. Med. 2016, 99, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, S.L.; Almaraz, A.E.; Amorebieta, V.T.; Perissinotti, L.L.; Olabe, J.A. Addition and redox reactivity of hydrogen sulfides (H2S/HS−) with nitroprusside: New chemistry of nitrososulfide ligands. Chem. Eur. J. 2011, 17, 4145–4156. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H.; Bounds, P.L. Signaling by sulfur-containing molecules. Quantitative aspects. Arch. Biochem. Biophys. 2016. [Google Scholar] [CrossRef] [PubMed]
- Richardson, G.; Hicks, S.L.; O’Byrne, S.; Frost, M.T.; Moore, K.; Benjamin, N.; McKnight, G.M. The ingestion of inorganic nitrate increases gastric S-nitrosothiol levels and inhibits platelet function in humans. Nitric Oxide Biol. Chem. 2002, 7, 24–29. [Google Scholar] [CrossRef]
- Nava, M.; Martin-Drumel, M.A.; Lopez, C.A.; Crabtree, K.N.; Womack, C.C.; Nguyen, T.L.; Thorwirth, S.; Cummins, C.C.; Stanton, J.F.; McCarthy, M.C. Spontaneous and Selective Formation of HSNO, a Crucial Intermediate Linking H2S and Nitroso Chemistries. J. Am. Chem. Soc. 2016, 138, 11441–11444. [Google Scholar] [CrossRef] [PubMed]
- Cerella, C.; Dicato, M.; Jacob, C.; Diederich, M. Chemical properties and mechanisms determining the anti-cancer action of garlic-derived organic sulfur compounds. Anticancer Agents Med. Chem. 2011, 11, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Ba, L.A.; Khairan, K.; Zwergel, C.; Bach, N.D.; Bernhardt, I.; Brandt, W.; Wessjohann, L.; Diederich, M.; Jacob, C. Interactions of polysulfanes with components of red blood cells. MedChemComm 2011, 2, 196–200. [Google Scholar] [CrossRef]
- Gruhlke, M.C.H.; Slusarenko, A.J. The biology of reactive sulfur species (RSS). Plant Physiol. Biochem. 2012, 59, 98–107. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grman, M.; Nasim, M.J.; Leontiev, R.; Misak, A.; Jakusova, V.; Ondrias, K.; Jacob, C. Inorganic Reactive Sulfur-Nitrogen Species: Intricate Release Mechanisms or Cacophony in Yellow, Blue and Red? Antioxidants 2017, 6, 14. https://doi.org/10.3390/antiox6010014
Grman M, Nasim MJ, Leontiev R, Misak A, Jakusova V, Ondrias K, Jacob C. Inorganic Reactive Sulfur-Nitrogen Species: Intricate Release Mechanisms or Cacophony in Yellow, Blue and Red? Antioxidants. 2017; 6(1):14. https://doi.org/10.3390/antiox6010014
Chicago/Turabian StyleGrman, Marian, Muhammad Jawad Nasim, Roman Leontiev, Anton Misak, Veronika Jakusova, Karol Ondrias, and Claus Jacob. 2017. "Inorganic Reactive Sulfur-Nitrogen Species: Intricate Release Mechanisms or Cacophony in Yellow, Blue and Red?" Antioxidants 6, no. 1: 14. https://doi.org/10.3390/antiox6010014





