Use of Saliva Biomarkers to Monitor Efficacy of Vitamin C in Exercise-Induced Oxidative Stress
Abstract
:1. Introduction
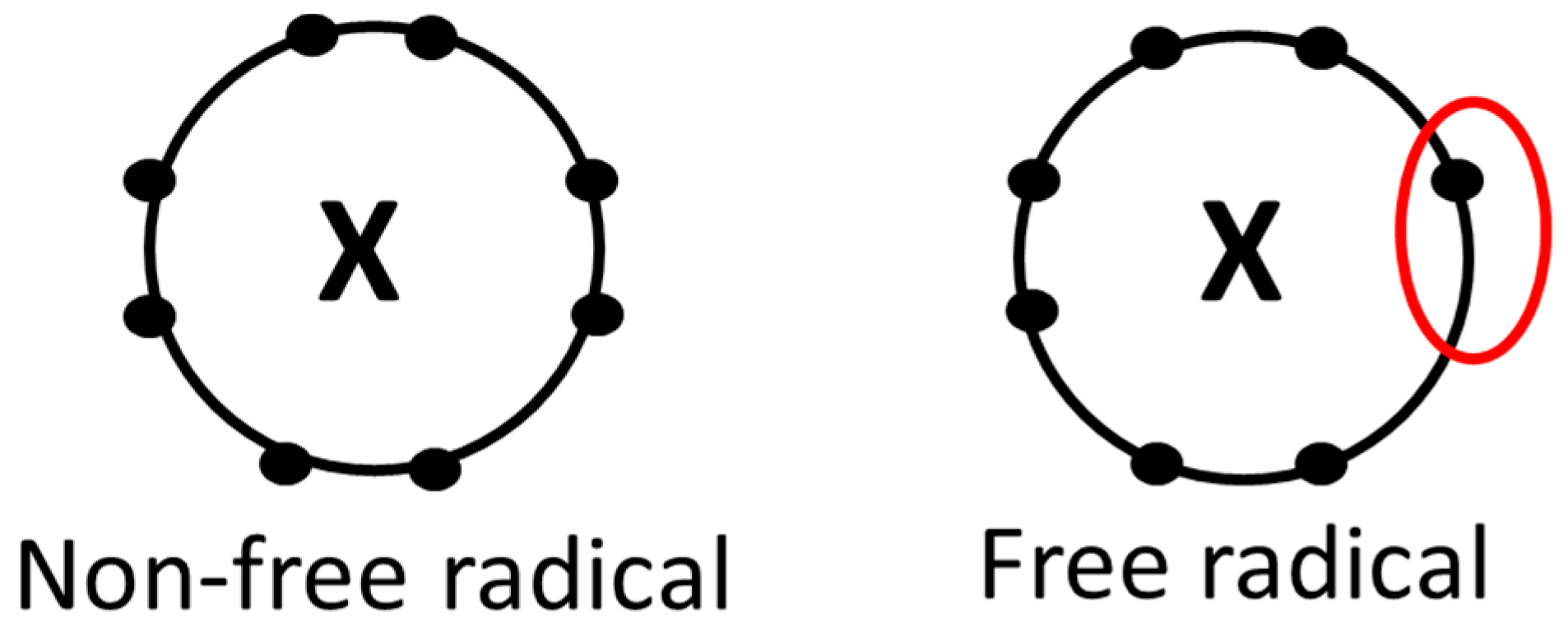
2. Oxidative Stress and Free Radicals
2.1. Basic Concepts
2.2. Health, Disease, and Disorders Associated with Oxidative Stress
2.3. The Relationship between Inflammation and Oxidative Stress
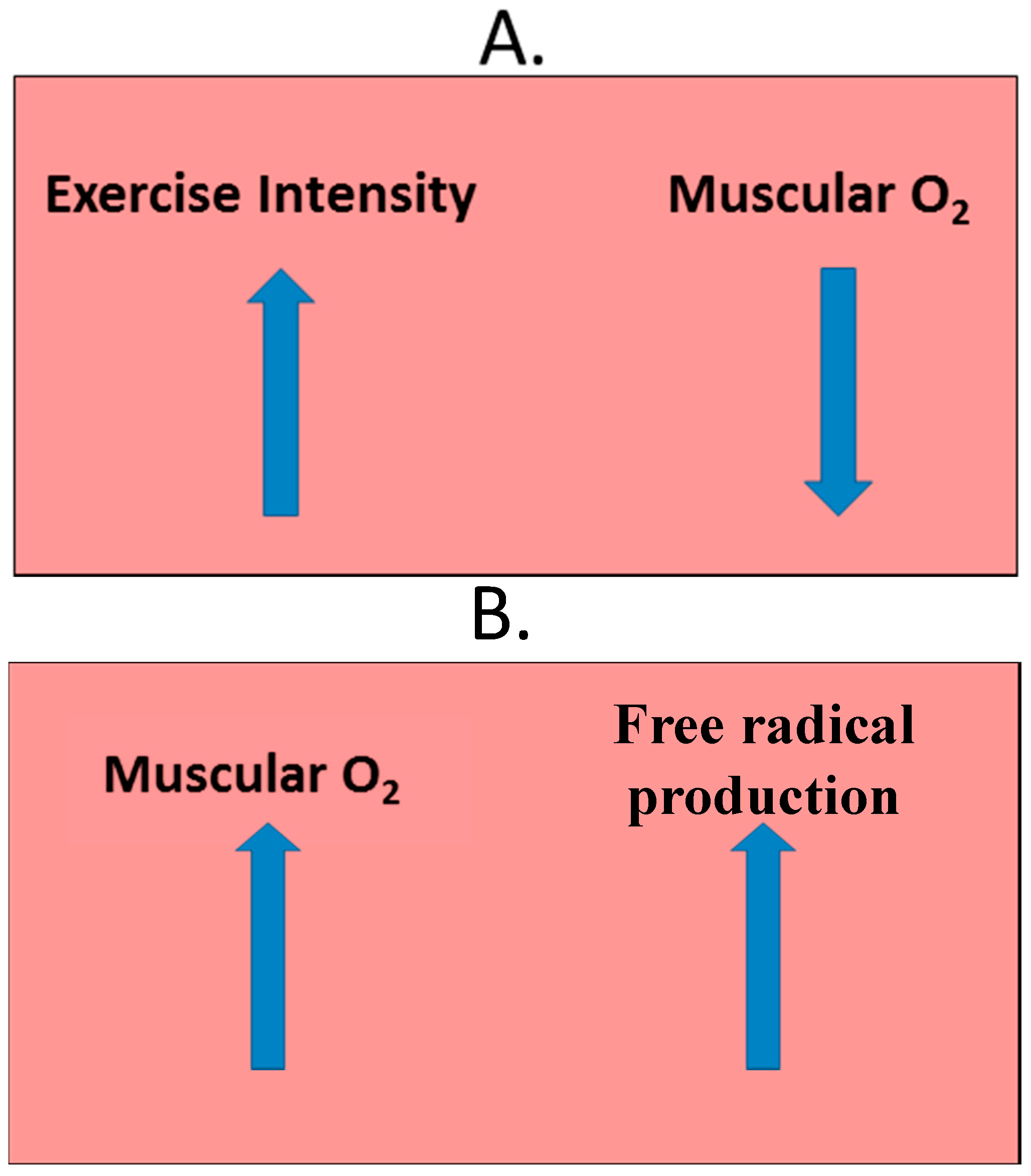
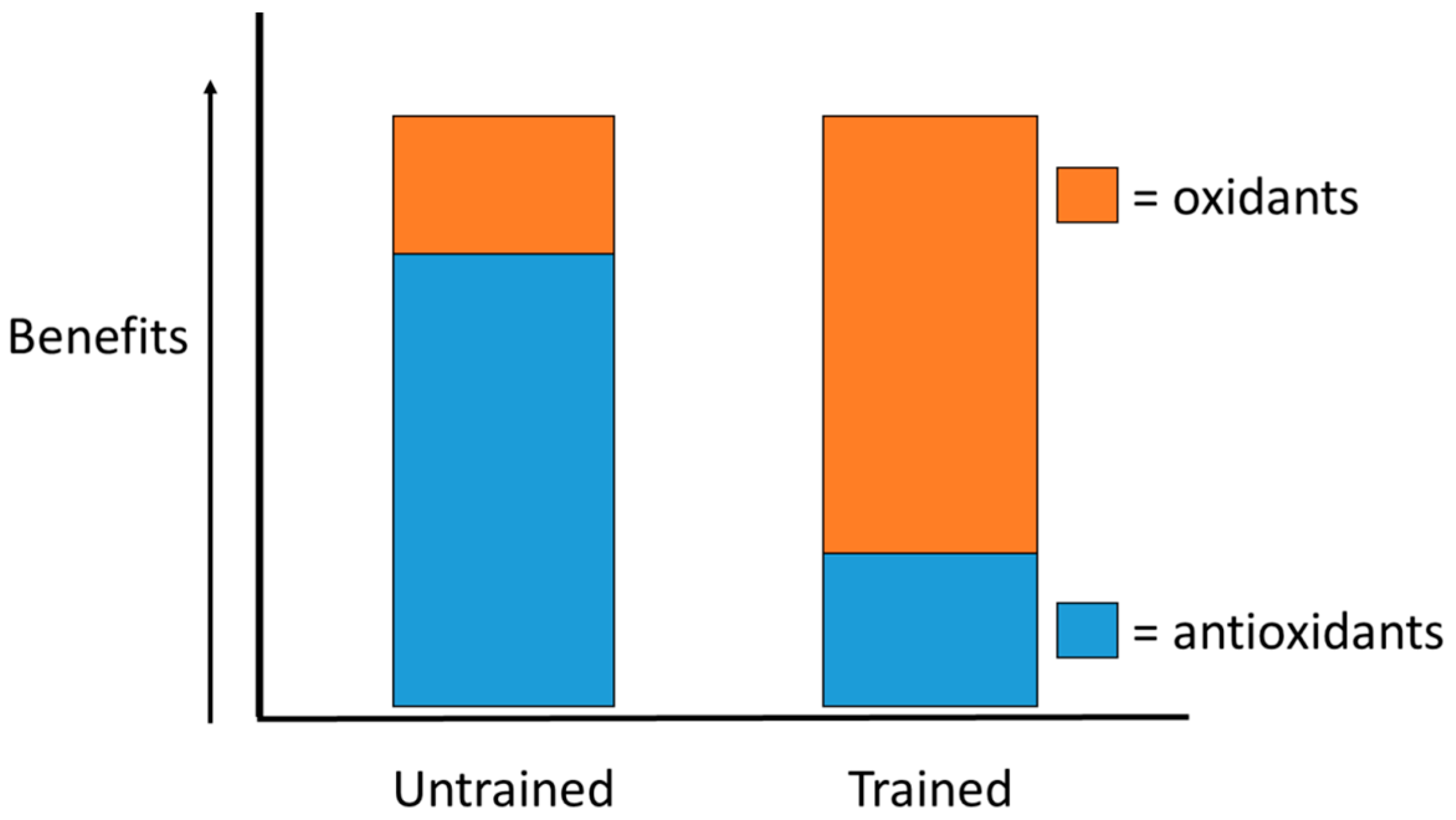
2.4. Exercise-Induced Oxidative Stress
2.5. Analyzing Exercise-Induced Oxidative Stress
3. Use of Salivary Biomarkers
3.1. The Basics of Saliva
3.2. Biomarkers of Oxidative Stress in Saliva
4. Vitamin C
4.1. Vitamin C as a Salivary Biomarker
4.2. Systemic Vitamin C
4.3. Vitamin C Supplementation for Exericse-Induced Oxidative Stress
5. Future Research
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Gomberg, M. An instance of trivalent carbon: Triphenylmethyl. J. Am. Chem. Soc. 1900, 22, 757–771. [Google Scholar] [CrossRef]
- Halliwel, B.; Getteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: Oxford, MS, USA, 2007. [Google Scholar]
- Cristiana, F.; Elena, A.; Nina, Z. Superoxide dismutase: Therapeutic targets in SOD related pathology. Health 2014, 6, 975–988. [Google Scholar] [CrossRef]
- Gerschman, R.; Gilbert, D.L.; Nye, S.W.; Dwyer, P.; Fenn, W.O. Oxygen poisoning and X-irradiation: A mechanism in common. Science 1954, 119, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Semchyshyn, H.M.; Lozinska, L.M. Fructose protects baker’s yeast against peroxide stress: Potential role of catalase and superoxide dismutase. FEMS Yeast Res. 2012, 12, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C.K.; Souto, P.C.; França, E.L.; Honorio-França, A.C. Oxidative and nitrosative stress on phagocytes’ function: From effective defense to immunity evasion mechanisms. Arch. Immunol. Ther. Exp. 2011, 59, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Bild, W.; Ciobica, A.; Padurariu, M.; Bild, V. The interdependence of the reactive species of oxygen, nitrogen, and carbon. J. Physiol. Biochem. 2013, 69, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef] [PubMed]
- Veselá, A.; Wilhelm, J. The role of carbon dioxide in free radical reactions of the organism. Physiol. Res. 2002, 51, 335–339. [Google Scholar] [PubMed]
- Lushchak, O.V.; Bayliak, M.M.; Korobova, O.V.; Levine, R.L.; Lushchak, V.I. Buffer modulation of menadione-induced oxidative stress in Saccharomyces cerevisiae. Redox Rep. 2009, 14, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C. A scent of therapy: Pharmacological implications of natural products containing redox-active sulfur atoms. Nat. Prod. Rep. 2006, 23, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Hattersley, J.G. The Negative Health Effects of Chlorine. J. Orthomol. Med. 2000, 15, 89–95. [Google Scholar]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.; Quintanilha, A.T.; Brooks, G.A.; Packer, L. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 1982, 107, 1198–1205. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Oxidants and Antioxidants. Exp. Phsiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Kasting, J.F. Earth’s early atmosphere. Science 1993, 259, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Lane, N. Oxygen, the Molecule That Made the World; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Halliwell, B. Chloroplast Metabolism; Oxford University Press: Oxford, UK, 1984. [Google Scholar]
- Belntine, J.D. Pathology of O2 Toxicity; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Bruyninckx, W.J.; Howard, M.S. Are physiological O2 concentrations mutagenic? Nature 1978, 274, 606–607. [Google Scholar] [CrossRef] [PubMed]
- Deneke, S.M.; Fanburg, B.L. Normobaric O2 toxicity of the lung. N. Engl. J. Med. 1980, 303, 76. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.S.; Valentine, J.S.; Greenberg, A.; Liebman, J.F. Active O2 in Chemistry; Blackie Academic and Professional: London, UK, 1995. [Google Scholar]
- Giles, G.I.; Jacob, C. Reactive sulfur species: An emerging concept in oxidative stress. Biol. Chem. 2002, 383, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Symons, M.C.R. Radicals generated by bone cutting and fracture. Free Radic. Biol. Med. 1996, 20, 831–835. [Google Scholar] [CrossRef]
- Davies, M.J. Singlet O2- Mediated Damage to Proteins and Its Consequences. Biochem. Biophys. Res. Commun. 2003, 305, 761. [Google Scholar] [CrossRef]
- Cox, T.M.; Jack, N.; Lofthouse, S.; Watling, J.; Haines, J.; Warren, M.J. King George III and Porphyria: An Elemental Hypothesis and Investigation. Lancet 2005, 366, 332–335. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Hazen, S.L. MPO and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Roos, R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 1993, 362, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Inoue, N.; Ohashi, Y.; Terashima, M.; Matsui, K.; Mori, T.; Fujita, H.; Awano, K.; Kobayashi, K.; Azumi, H.; et al. Interaction of oxidative stress and inflammatory response in coronary plaque instability: Important role of C-reactive protein. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Heron, M. Deaths: Leading Causes for 2013. Natl. Vital Stat. Rep. 2016, 65, 5. [Google Scholar]
- Hadjivassiliou, V.; Green, M.H.; James, R.F.; Swift, S.M.; Clayton, H.A.; Green, I.C. Insulin secretion, DNA damage, and apoptosis in human and rat islets of Langerhans following exposure to NO, ONOO− and cytokines. Nitric Oxide 1998, 2, 429–441. [Google Scholar] [CrossRef]
- Grankvist, K.; Marklund, S.L. Opposite effects of two metal-chelators on alloxan-induced diabetes in mice. Life Sci. 1983, 33, 2535–2540. [Google Scholar] [CrossRef]
- Davi, G.; Falco, A.; Patrono, C. Determinants of F2-isoprostane biosynthesis and inhibition in man. Chem. Phys. Lipids 2004, 128, 149–163. [Google Scholar] [CrossRef]
- Rehman, A.; Nourooz-Zadeh, J.; Möller, W.; Tritschler, H.; Pereira, P.; Halliwell, B. Increased oxidative damage to all DNA bases in patients with type 2 diabetes mellitus. FEBS Lett. 1999, 448, 120–122. [Google Scholar] [CrossRef]
- Kumar, B.; Koul, S.; Khandrika, L.; Meacham, R.B.; Koul, H.K. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008, 68, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [PubMed]
- Bechman, K.B.; Ames, B.N. Mitochondrial aging: Open questions. Ann. N. Y. Acad. Sci. 1998, 854, 118–127. [Google Scholar] [CrossRef]
- Bechman, K.B.; Ames, B.N. The Free Radical Theory of Aging Matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar]
- Bechman, K.B.; Ames, B.N. Endogenous Oxidative Damage of mtDNA. Mutat. Res. 1999, 424, 51–58. [Google Scholar] [CrossRef]
- Orr, W.C.; Sohal, R.S. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science 1994, 263, 1128–1130. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.F.; Reid, M.B. Muscle-derived ROS and thiol regulation in muscle fatigue. J. Appl. Physiol. 2008, 104, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Fanburg, B.L. Reactive Oxygen Species in Cell Signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, 1005–1028. [Google Scholar]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, K.; Puri, S. Free Radicals and Antioxidants in Health and Disease: A Review. EMHJ 1998, 4, 350–360. [Google Scholar]
- Ebadi, M. Antioxidants and Free Radicals in Health and Disease: An Introduction to Reactive Oxygen Species, Oxidative Injury, Neuronal Cell Death and Therapy in Neurodegenerative Diseases; Prominent Press: Scottsdale, AZ, USA, 2001. [Google Scholar]
- Saladin, K.S. Anatomy & Physiology: A Unity of Form and Function, 5th ed.; McGraw-Hill: New York, NY, USA, 2010. [Google Scholar]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Murphy, N.; Black, S.J. Infection-associated decline of Cape buffalo blood catale augments serum trypanocidal activity. Infect. Immun. 1999, 67, 2797–2803. [Google Scholar] [PubMed]
- Shapiro, S.D. Immunology: Mobilizing the army. Nature 2003, 421, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Erbay, E. Nutrient sensing and inflammation in metabolic diseases. Nat. Rev. 2008, 8, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nut. 2006, 83, S456–S460. [Google Scholar]
- Trinchieri, G. Cancer and inflammation: An old intuition with rapidly evolving new concepts. Annu. Rev. Immunol. 2012, 30, 677–706. [Google Scholar] [CrossRef] [PubMed]
- Hagen, T.M.; Huang, S.; Curnutte, J.; Fowler, P.; Martinez, V.; Wehr, C.M.; Ames, B.M.; Chisari, F.V. Extensive oxidative DNA damage in hepatocytes of transgenic mice with chronic active hepatitis destined to develop hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 1994, 91, 12808–12812. [Google Scholar] [CrossRef] [PubMed]
- Ndisang, J.F.; Vannacci, A.; Rastogi, S. Oxidative stress and inflammation in obesity, diabetes, hypertension, and related cardiometabolic complications. Oxidative Med. Cell. Longev. 2014, 2014, 506948. [Google Scholar] [CrossRef] [PubMed]
- Lugrin, J.; Rosenblatt-Belin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Brennan, M.L.; Shen, Z.; MacPherson, J.C.; Schmitt, D.; Molenda, C.E.; Hazen, S.L. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J. Biol. Chem. 2002, 277, 46116–46122. [Google Scholar] [CrossRef] [PubMed]
- Baldus, S.; Heitzer, T.; Eiserich, J.P.; Lau, D.; Mollnau, H.; Ortak, M.; Petri, S.; Goldmann, B.; Duchstein, H.J.; Berger, J.; et al. Myeloperoxidase enhances nitric oxide catabolism during myocardial ischemia and reperfusion. Free Radic. Biol. Med. 2004, 37, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Bo, L.; Jiang, S.; Kan, H.; Song, W.; Zhao, J. Effect of vitamin E and omega-3 fatty acids on protecting ambient PM2.5-induced inflammatory response and oxidative stress in vascular endothelial cells. PLoS ONE 2016, 11, e0152216. [Google Scholar] [CrossRef] [PubMed]
- Niess, A.M.; Dickhuth, H.H.; Northoff, H.; Fehrenbach, E. Free radicals and oxidative stress in exercise—Immunological aspects. Exerc. Immunol. Rev. 1999, 5, 22–56. [Google Scholar] [PubMed]
- Christian, J.; Bessesen, D.; Byers, T.; Christian, K.; Goldstein, M.; Bock, B. Clinic-based support to help overweight patients with type 2 diabetes increase physical activity and lose weight. Arch. Intern. Med. 2008, 168, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Rexrode, K.; Vandam, R.; Li, T.; Hu, F. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: Sixteen years follow-up in US women. Circulation 2008, 117, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Zuhl, M. Mechanisms of oxidative damage and their impact on contracting muscle. In Antioxidants in Sport Nutrition; Lamprecht, M., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2015. [Google Scholar]
- Muller, F. The nature and mechanism of superoxide production by the electron transport chain: Its relevance to aging. J. Am. Aging Assoc. 2000, 23, 227–253. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Zhao, Z.; Koltai, E.; Ohno, H.; Atalay, H. Oxygen consumption and usage during physical exercise: The balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid. Redox Signal. 2013, 18, 1208–1246. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.A.; Crapo, J.D. Hyperoxia Increases O2 Radical Production in Rat Lungs and Lung Mitochondria. J. Biol. Chem. 1981, 256, 10986–10992. [Google Scholar] [PubMed]
- Astrand, P.O.; Rodahl, K. Textbook of Work Physiology; McGraw Hill: New York, NY, USA, 1986. [Google Scholar]
- Keul, J.; Doll, E.; Koppler, D. Energy Metabolism of Human Muscle; Karger: Basel, Switzerland, 1972. [Google Scholar]
- St-Pierre, J.; Buckingham, J.A.; Roebuck, S.J.; Brand, M.D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002, 277, 44784–44790. [Google Scholar] [CrossRef] [PubMed]
- Kellog, E.W., III; Fridovich, I. Superoxide, Hydrogen Peroxide, and Singlet Oxygen in Lipid Peroxidation by a Xanthine Oxidase System. J. Biol. Chem. 1975, 250, 8812–8817. [Google Scholar]
- Wolbarsht, M.L.; Fridovich, I. Hyperoxia during reperfusion is a factor in reperfusion injury. Free Radic. Biol. Med. 1989, 6, 61–62. [Google Scholar] [CrossRef]
- Gourgoura, S.; Nikolaidis, M.G.; Kostaropoulos, I.A.; Jamurtas, A.Z.; Koukoulis, G.; Kouretas, D. Increased oxidative stress indices in the blood of child swimmers. Eur. J. Appl. Physiol. 2007, 100, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, Y.; Jamurtas, A.Z.; Nikolaidis, M.G.; Fatouros, I.G.; Koutedakis, Y.; Papassotiriou, I.; Kouretas, D. Sampling time is crucial for measurement of aerobic exercise-induced oxidative stress. Med. Sci. Sports Exerc. 2007, 39, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.G.; Paschalis, V.; Giakas, G.; Fatouros, I.G.; Kourtedakis, Y.; Kouretas, D.; Jamurtas, A.Z. Decreased blood oxidative stress after repeated muscle-damaging exercise. Med. Sci. Sports Exerc. 2007, 39, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.G.; Jamurtas, A.Z.; Paschailis, V.; Fatouros, I.G.; Koutedakis, Y.; Kouretas, D. The effect of muscle-damaging exercise on blood and skeletal muscle oxidative stress: Magnitude and time-course considerations. Sports Med. 2008, 38, 579–606. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Taylor, A.W.; Ohno, H.; Goto, S. Adaptation to Exercise-Induced Oxidative Stress: From Muscle to Brain. Exerc. Immunol. Rev. 2001, 7, 90–107. [Google Scholar] [PubMed]
- Powers, S.K.; Duarte, J.; Kavazis, A.N.; Talbert, E.E. Reactive oxygen species are signaling molecules for skeletal muscle adaption. Exp. Physiol. 2010, 95, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Talbert, E.E.; Adhihetty, P.J. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J. Physiol. 2011, 589, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.F.; Goodyear, L.J. Exercise, MAPK and NF-kappaB signaling in skeletal muscle. J. Appl. Physiol. 2007, 103, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Gomez-Cabrera, M.C.; Vina, J. Exercise and hormesis: Activation of cellular antioxidant signaling pathway. Ann. N. Y. Acad. Sci. 2006, 1067, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Ballmann, C.; McGinnis, G.; Peters, B.; Slivka, D.; Cuddy, J.; Hailes, W.; Dumke, C.; Ruby, B.; Quindry, J. Exercise-induced oxidative stress and hypoxic exercise recovery. Eur. J. Appl. Physiol. 2014, 114, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Bogdanis, G.C.; Stavrinou, P.; Fatouros, I.G.; Philippou, A.; Chatzinikolaou, A.; Draganidis, D.; Ermidis, G.; Maridaki, M. Short-term high-intensity interval exercise training attenuates oxidative stress responses and improves antioxidant status in healthy humans. Food Chem. Toxicol. 2013, 61, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Quindry, J.; Miller, L.; Mcginnis, G.; Kliszczewiscz, B.; Slivka, D.; Dumke, C.; Cuddy, J.; Ruby, B. Environmental temperature and exercise-induced blood oxidative stress. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, M.; Oettl, K.; Schwaberger, G.; Hofmann, P.; Gerilberger, J.F. Protein modification responds to exercise intensity and antioxidant supplementation. Med. Sci. Sports Exerc. 2009, 41, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Leelarungrayub, D.; Khansuwan, R.; Pothongsunun, P.; Klaphajone, J. N-acetylcysteine supplementation controls total antioxidant capacity, creatine kinase, lactate, and tumor necrotic factor-alpha against oxidative stress induced by graded exercise in sedentary men. Oxidative Med. Cell. Longev. 2011. [Google Scholar] [CrossRef] [PubMed]
- Wadley, A.J.; Killer, S.C.; Svendsen, I.S.; Gleeson, M. The impact of intensified training with a high or moderate carbohydrate feeding strategy on resting and exercise-induced oxidative stress. Eur. J. Appl. Physiol. 2015, 115, 1757–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacheck, J.M.; Milbury, P.E.; Cannon, J.G.; Roubenoff, R.; Blumberg, J.B. Effect of Vitamin E and Eccentric Exercise on Selected Biomarkers of Oxidative Stress in Young and Elderly Men. Free Radic. Biol. Med. 2003, 34, 1575–1588. [Google Scholar] [CrossRef]
- McAnulty, L.S.; Miller, L.E.; Hosick, P.A.; Utter, A.C.; Quindry, J.C.; McAnulty, S.R. Effect of resveratrol and quercetin supplementation on redox status and inflammation after exercise. Appl. Physiol. Nutr. Metab. 2013, 38, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Fisher-Wellman, K.H. Blood oxidative stress biomarkers: Influence of sex, exercise training status and dietary intake. Gend. Med. 2008, 5, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Gwozdzinski, K.; Pieniazek, A.; Tabaczar, S.; Jequer, A.; Brzeszczynska, J. Investigation of oxidative stress parameters in different life span erythrocyte fractions in young untrained men after an acute exercise. Exp. Physiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Margaritis, M.; Channon, K.M.; Antoniades, C. Evaluating oxidative stress in human cardiovascular disease: Methodological aspects and considerations. Curr. Med. Chem. 2012, 19, 2504–2520. [Google Scholar] [CrossRef] [PubMed]
- Cavas, L.; Arpinar, P.; Yurdakoc, K. Possible interactions between antioxidant enzymes and free sialic acids in saliva: A preliminary study on elite judoists. Int. J. Sports Med. 2005, 26, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Chicharro, J.L.; Lucía, A.; Pérez, M.; Vaquero, A.F.; Ureña, R. Saliva composition and exercise. Sports Med. 1998, 26, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Deminice, R.; Sicchieri, T.; Payão, P.O.; Jordão, A.A. Blood and salivary oxidative stress biomarkers following an acute session of resistance exercise in humans. Int. J. Sports Med. 2010, 31, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Sant’anna, M.; Casimiro-Lopes, G.; Boaventura, G.; Marques, S.T.; Sorenson, M.M.; Simão, R.; Pinto, V.S. Anaerobic exercise affects the saliva antioxidant/oxidant balance in high-performance pentathlon athletes. Hum. Mov. 2016, 17, 50–55. [Google Scholar] [CrossRef]
- González, D.; Marquina, R.; Rondόn, N.; Rodriguez-Malaver, A.J.; Reyes, R. Effects of aerobic exercise on uric acid, total antioxidant activity, oxidative stress, and nitric oxide in human saliva. Res. Sports Med. 2008, 16, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Sariri, R.; Damirchi, A.; Nazari, Y. Salivary Antioxidant Variations in Athletes after Intense Exercise. Medicina Sportiva. 2013, 9, 2043–2050. [Google Scholar]
- Edgar, W.M. Saliva: Its secretion, composition, and functions. Br. Dent. J. 1992, 172, 305–312. [Google Scholar] [CrossRef]
- Van Nieuw Amerongen, A.; Bolscher, J.G.; Veerman, E.C. Salivary proteins: Protective and diagnostic value in cariology? Caries Res. 2004, 38, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, A.; Zwierz, K.; Zόlkowski, K.; Gindzienski, A. Structure and Biosynthesis of Human Salivary Mucins. Acta. Biochim. Pol. 2000, 47, 1067–1079. [Google Scholar] [PubMed]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Berkovitz, B.K.B.; Holland, G.R.; Moxham, B.J. Oral Anatomy, Histology and Embryology, 3rd ed.; Mosby: New York, NY, USA, 2002. [Google Scholar]
- Ahmadi-Motamayel, F.; Falsafi, P.; Goodarzi, M.T.; Poorolajal, J. Evaluation of salivary catalase, vitamin C, and alpha-amylase in smokers and non-smokers: A retrospective cohort study. J. Oral Pathol. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Holsinger, F.; Bui, D. Salivary Gland Disorders; Springer: Berlin, Germany, 2007. [Google Scholar]
- Grant, D.A.; Stern, I.B.; Listgarten, M.A. Periodontics, 6th ed.; CV Mosby: St. Louis, MO, USA, 1988; pp. 135–146. [Google Scholar]
- Forde, M.D.; Koka, S.; Eckert, S.E.; Carr, A.B.; Wong, D.T. Systemic assessments utilizing saliva: Part 1 general consideration and current assessments. Int. J. Prosthodont. 2006, 19, 43–52. [Google Scholar] [PubMed]
- Holmberg, K.V.; Hoffman, M.P. Anatomy, biogenesis and regeneration of salivary glands. Monogr. Oral Sci. 2014, 24, 1–13. [Google Scholar] [PubMed]
- De Almedia Pdel, V.; Gregio, A.M.; Machado, M.A.; de Lima, A.A.; Azevedo, L.R. Saliva Composition and Functions: A Comprehensive Review. J. Contemp. Dent. Pract. 2008, 9, 72–80. [Google Scholar]
- Pedersen, A.M.; Bardo, A.; Jensen, S.B.; Nauntofte, B. Saliva and gastrointestinal functions of taste, mastication, swallowing, and digestion. Oral Dis. 2002, 8, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Mese, H.; Matsuo, R. Salivary secretion, taste and hyposalivation. J. Oral Rehabil. 2007, 34, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Edgar, W. Saliva and dental health. Clinical implications of saliva: Report of a consensus meeting. Br. Dent. J. 1990, 169, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Delaney, K.P.; Branson, B.M.; Uniyal, A.; Kerndt, P.R.; Keenan, P.A.; Jafa, K.; Gardner, A.D.; Jamieson, D.J.; Bulterys, M. Performance of an oral fluid rapid HIV-1/2 test: Experience from four CDC studies. AIDS 2006, 20, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Amado, L.A.; Villar, L.M.; de Paula, V.S.; de Almeida, A.J.; Gaspar, A.M. Detection of hepatitis A, B, and C virus-specific antibodies using oral fluid for epidemiological studies. Mem. Inst. Oswaldo Cruz 2006, 101, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Mager, D.L.; Haffajee, A.D.; Devlin, P.M.; Norris, C.M.; Posner, M.R.; Goodson, J.M. The salivary microbiota as a diagnostic indicator of oral cancer: A descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J. Transl. Med. 2005, 3, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paju, S.; Pussinen, P.J.; Suominen-Taipale, L.; Hyvönen, M.; Knuuttila, M.; Könönen, E. Detection of multiple pathogenic species in saliva is associated with periodontal infection in adults. J. Clin. Microbiol. 2009, 47, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Paster, B.J.; Dewhirst, F.E. Molecular microbial diagnosis. Periodontology 2000 2009, 51, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Goodson, J.M.; Groppo, D.; Halem, S.; Carpino, E. Is obesity an oral bacterial disease? J. Dent. Res. 2009, 88, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Schipper, H.M.; Velly, A.M.; Mohit, S.; Gornitsky, M. Salivary biomarkers of oxidative stress: A critical review. Free Radic. Biol. Med. 2015, 85, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Saral, Y.; Coskun, B.K.; Ozturk, P.; Karatas, F.; Ayar, A. Assessment of salivary and serum antioxidant vitamins and lipid peroxidation in patients with recurrent aphthous ulceration. Tohoku J. Exp. Med. 2005, 206, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Khademi, H.; Khozeimeh, F.; Tavangar, A.; Amini, S.; Parichehr, G. The serum and salivary level of malondialdehyde, vitamins A, E, and C in patient with recurrent aphthous stomatitis. Adv. Biomed. Res. 2014, 3, 246. [Google Scholar] [PubMed]
- Al-Rawi, N.H. Oxidative stress, antioxidant status and lipid profile in the saliva of type 2 diabetics. Diabetes Vasc. Dis. Res. 2011, 8, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Major, R.H. A History of Medicine; Blackwell: Springfield, IL, USA, 1954; p. 51. [Google Scholar]
- Stuteville, O.H. Presence of Vitamin C in Saliva. Proc. Soc. Exp. Biol. 1935, 32, 1454–1455. [Google Scholar] [CrossRef]
- Mäkilä, E.; Kirveskari, P. A study of ascorbic acid in human saliva. Arch. Oral Biol. 1969, 14, 1285–1292. [Google Scholar] [CrossRef]
- Dessy, J.; Doneddu, K. Einfluss des insulins auf die vitamin C- Ausscheidung im Harn und Speichel nach intravenöser Ascorbinsäure-Belastung bei Normalen und Diabetkern. Endokrinologie 1940, 23, 165–175. [Google Scholar]
- Dreizen, S.; Gilley, E.J.; Mosny, J.J.; Spies, T.D. The distribution of selected vitamins in human whole saliva. Int. Z. Vitaminforsch. Beih. 1955, 26, 257–262. [Google Scholar] [PubMed]
- Freeman, J.T.; Hafkesbring, R. Comparative study of ascorbic acid levels in gastric secretion, blood, urine and saliva. Gastroenterology 1951, 18, 224–229. [Google Scholar] [PubMed]
- Glavind, J.; Grandados, H.; Hansen, L.A.; Schilling, K.; Kruse, I.; Dam, H. The presence of vitamins in the saliva. Int. Z. Vitam. Forsch. 1948, 20, 234–238. [Google Scholar]
- Hess, W.C.; Smith, B.T. The ascorbic acid content of the saliva of carious and noncarious individuals. J. Dent. Res. 1949, 28, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, D.; Dubois-Ferrière, H. Vitamine C dans la salive humaine et paradentoses. C. R. Sèanc. Soc. Phys. Hist. Nat. Genève 1936, 53, 151–154. [Google Scholar]
- Leggott, P.J.; Robertson, P.B.; Rothman, D.L.; Murrary, P.A.; Jacob, R.A. Response of lingual ascorbic acid test and salivary ascorbate levels to changes in ascorbic acid intake. J. Dent. Res. 1986, 65, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef] [PubMed]
- Bijos, G.M. Determination of ascorbic acid in the saliva of tuberculous subjects. Chem. Abstr. 1948, 42, 5548. [Google Scholar]
- Abdolsamadi, H.; Rafieian, N.; Goodarzi, M.T.; Feradmal, J.; Davoodi, P.; Jazayeri, M.; Taghavi, Z.; Hoseyni, S.M.; Ahmadi-Motamayel, F. Levels of salivary antioxidant vitamins and lipid peroxidation in patients with oral lichen planus and healthy individuals. Chonnam Med. J. 2014, 50, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Politis, C.; Jacobs, R. Salivary 8-hydroxy-2-deoxyguanosine, malondialdehyde, vitamin C, and vitamin E in oral pre-cancer and cancer: Diagnostic value and free radical mechanism of action. Clin. Oral Investig. 2016, 20, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Patni, V.; Baliga, S.; Sawal, S. Saliva as a diagnostic tool for measurement of total antioxidant capacity in children with leprosy and born to leprosy parent. Indian J. Lepr. 2015, 87, 17–21. [Google Scholar] [PubMed]
- Shetty, S.R.; Babu, S.; Kumari, S.; Shetty, P.; Vijay, R.; Karikal, A. Evaluation of micronutrient status in serum and saliva of oral submucous fibrosis patients: A clinicopathological study. Indian J. Med. Paediatr. Oncol. 2012, 33, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Omaye, S.T.; Turnbull, J.D.; Sauberlich, H.E. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Methods Enzymol. 1979, 62, 3–11. [Google Scholar] [PubMed]
- Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academy Press: Washington, DC, USA, 2000; pp. 95–185. [Google Scholar]
- Levine, M.; Wang, Y.; Padayatty, S.; Morrow, J. A new recommended dietary allowance of Vitamin C for healthy young women. Proc. Natl. Acad. Sci. USA 2001, 98, 9842–9846. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.A.; Sotoudeh, G. Vitamin C function and status in chronic disease. Nutr. Clin. Care 2002, 5, 66–74. [Google Scholar] [CrossRef]
- Food Surveys Research Group. What We Eat in America, NHANES 2007–2010; US Department of Agriculture, Beltsville Human Nutrition Research Center: Beltsville, MD, USA, 2013.
- Padayatty, S.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Combs, G.F., Jr. The Vitamins: Fundamental Aspects in Nutrition and Health, 3rd ed.; Elsevier Academic Press: Burlington, VT, USA, 2008. [Google Scholar]
- Kittisakmontri, K.; Swangtrakul, N.; Padungmaneesub, W.; Charoenkwan, P. Gingival bleeding and bloody dialysate: A case report of scurvy in a child with end-stage renal disease receiving peritoneal dialysis. J. Ren. Nutr. 2016, 26, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Shaath, T.; Fischer, R.; Goeser, M.; Rajpara, A.; Aires, D. Scurvy in the present times: Vitamin C allergy leading to strict fast food diet. Dermatol. Online 2016, 22, 10. [Google Scholar]
- Vitoria, I.; Lόpez, B.; Gόmez, J.; Torres, C.; Calvo, I.; Dalmau, J. Improper use of a plant-based vitamin C-deficient beverage causes scurvy in an infant. Pediatrics 2016, 137, e20152781. [Google Scholar] [CrossRef] [PubMed]
- Kallner, A.B.; Hartmann, D.; Hornig, D.H. On the requirements of ascorbic acid in man: Steady-state turnover and body pool in smokers. Am. J. Clin. Nutr. 1981, 34, 1347–1355. [Google Scholar] [PubMed]
- Lykkesfeldt, J.; Christen, S.; Wallock, L.M.; Chang, H.H.; Jacob, R.A.; Ames, B.N. Ascorbate is depleted by smoking and repleted by moderate supplementation: A study in male smokers and nonsmokers with matched dietary antioxidant intakes. Am. J. Clin. Nutr. 2000, 71, 530–536. [Google Scholar] [PubMed]
- Schectman, G.; Byrd, J.C.; Hoffman, R. Ascorbic acid requirements for smokers: Analysis of a population survey. Am. J. Clin. Nutr. 1991, 53, 1466–1470. [Google Scholar] [PubMed]
- Schectman, G.; Byrd, J.C.; Gruchow, H.W. The influence of smoking on vitamin C status in adults. Am. J. Public Health 1989, 79, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Dorchy, H. Lower plasma vitamin C levels in young type 1 diabetic patients with microalbuminuria. J. Diabetes Complicat. 1999, 13, 119. [Google Scholar] [PubMed]
- Kaviarasan, K.; Arjunan, M.M.; Pugalendi, K.V. Lipid profile, oxidant-antioxidant status and glycoprotein components in hyperlipidemic patients with/without diabetes. Clin. Chim. Acta 2005, 362, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Pekkala, M.; Hieta, R.; Kursula, P.; Kivirikko, K.I.; Wierenga, R.K.; Myllyharju, J. Crystallization of the proline-rich-pepride binding domain of hyman type 1 collagen prolyl 4-hydroxylase. Acta. Crystallogr. D Biol. Crystallogr. 2003, 59, 940–942. [Google Scholar] [CrossRef] [PubMed]
- Peterkofsky, B. Ascorbate requirement for hydroxylation and secretion of procollagen: Relationship to inhibition of collagen synthesis in scurvy. Am. J. Clin. Nutr. 1991, 54, S1135–S1140. [Google Scholar]
- Prockop, D.J.; Kivirikko, K.I. Collagens: Molecular biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 1995, 64, 403–434. [Google Scholar] [CrossRef] [PubMed]
- Rebouche, C.J. Ascorbic acid and carnitine biosynthesis. Am. J. Clin. Nutr. 1991, 54, S1147–S1152. [Google Scholar]
- Levine, M.; Dhariwal, K.R.; Washko, P.W.; Butler, J.D.; Welch, R.W.; Wang, Y.H.; Bergsten, P. Ascorbic acid and in situ kinetics: A new approach to vitamin requirements. Am. J. Clin. Nutr. 1991, 54, S1157–S1162. [Google Scholar] [CrossRef]
- Lindblad, B.; Lindstedt, G.; Lindstedt, S. The mechanism of enzymic formation of homogentisate from p-hydroxyphenylpyruvate. J. Am. Chem. Soc. 1970, 92, 7446–7449. [Google Scholar] [CrossRef] [PubMed]
- Sieburg, H. Redoxon as a tonic for sportsmen. Dtsch. Med. Wochenschr. 1937, 63, 11–12. [Google Scholar]
- Jetzler, A.; Haffler, C. Vitamin C- Bedarf bei einmaligar sportlicher Dauerleistung. Wein. Med. Wochenschr. 1939, 89, 332. [Google Scholar]
- Sauberlich, H.E. Pharmacology of vitamin-C. Ann. Rev. Nutr. 1994, 14, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.K.; Thacker, R.R.; Changchit, C.; Bridges, R.B.; Rehm, S.R.; Humble, J.; Turbek, J. Lower levels of vitamin C and carotenes in plasma of cigarette smokers. J. Am. Coll. Nutr. 1986, 5, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Namyslowski, L.; Desperak-Secomska, B. The vitamin C content of the blood in a selected group of students during 1952 and 1953. Rocz. Panstw. Zakl. Hig. 1955, 6, 289. [Google Scholar]
- Namyslowski, L. Investigations of the vitamin C requirements of athletes during physical exertion. Rocz. Panstw. Zakl. Hig. 1956, 7, 97–122. [Google Scholar]
- Ferrandez, M.D.; Maynar, M.; De la Fuente, M. Effects of a long-term training program of increasing intensity on the immune function of indoor Olympic cyclists. Int. J. Sports Med. 1996, 17, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Robertson, J.D.; Maughan, R.J. Influence of exercise on ascorbic acid status in man. Clin. Sci. 1987, 73, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S.R.; Jakeman, P.; Thomason, H.; Leguen, C.; Thorpe, G.H. Changes in plasma antioxidant status during eccentric exercise and the effect of vitamin supplementation. Free Radic. Res. Commun. 1993, 19, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.W.; Ostrowski, K.; Ibfelt, T.; Richelle, M.; Offord, E.; Halkjaer-Kristensen, J.; Pedersen, B.K. Effect of vitamin supplementation on cytokine response and on muscle damage after strenuous exercise. Am. J. Physiol. Cell Physiol. 2001, 280, C1570–C1575. [Google Scholar] [PubMed]
- Peake, J.M. Vitamin C: Effects of exercise and requirements with training. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 125–151. [Google Scholar] [CrossRef] [PubMed]
- Vasankari, T.; Kujala, U.; Sarna, S.; Ahotupa, M. Effects of ascorbic acid and carbohydrate ingestion on exercise induced oxidative stress. J. Sports Med. Phys. Fit. 1998, 38, 281–285. [Google Scholar]
- Ashton, T.; Young, I.S.; Peters, J.R.; Jones, E.; Jackson, S.K.; Davies, B.; Rowlands, C.C. Electron spine resonance spectroscopy, exercise, and oxidative stress: An ascorbic acid intervention study. J. Appl. Physiol. 1999, 87, 2032–2036. [Google Scholar] [PubMed]
- Popovic, L.M.; Mitic, N.R.; Miric, D.; Bisevac, B.; Miric, M.; Popovic, B. Influence of vitamin C supplementation on oxidative stress and neutrophil inflammatory response in acute and regular exercise. Oxidative Med. Cell. Longev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N.; McHardy, H.; Morton, J.P.; Nikolaidis, M.G.; Close, G.L. Influence of vitamin C and vitamin E on redox signaling: Implications for exercise adaptations. Free Radic. Biol. Med. 2015, 84, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Cumming, K.T.; Holden, G.; Hallén, J.; Rønnestad, B.R.; Sveen, O.; Skaug, A.; Paur, I.; Bastani, N.E.; Østgaard, H.N.; et al. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: A double-blind, randomized, controlled trial. J. Physiol. 2014, 592, 1887–1901. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.; Hughes, J.; Della Gatta, P.A.; Mason, S.; Lamon, S.; Russell, A.P.; Wadley, G.D. Vitamin C and E supplementation prevents some of the cellular adaptation to endurance-training in humans. Free Radic. Biol. Med. 2015, 89, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Viña, J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [PubMed]
- Meyer, B.J.; deBruin, E.J.; Brown, J.M.; Bieler, E.U.; Meyer, A.C.; Grey, P.C. The effect of a predominately fruit diet on athletic performance. Plant Foods Man 1975, 1, 223. [Google Scholar]
- Thompson, D.; Williams, C.; Kingsley, M.; Nicholas, C.W.; Lakomy, H.K.; McArdle, F.; Jackson, M.J. Muscle soreness and damage parameters after prolonged intermittent shuttle-running following acute vitamin C supplementation. Int. J. Sports Med. 2001, 22, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; McAnulty, S.R.; McAnulty, L.; Swick, N.S.; Utter, A.C.; Vinci, D.M.; Opiela, S.J.; Morrow, J.D. Influence of vitamin C supplementation on oxidative and immune changes after an ultramarathon. J. Appl. Physiol. 2002, 92, 1970–1977. [Google Scholar] [CrossRef] [PubMed]
- Howald, H.; Segesser, B.; Körner, W.F. Ascorbic acid and athletic performance. Ann. N. Y. Acad. Sci. 1975, 258, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Samata, S.C.; Biswas, K. Effect of supplementation of vitamin C on the cardiorespiratory endurance capacity of college women. Snipes J. 1985, 8, 55. [Google Scholar]
- Thompson, D.; Williams, C.; McGregor, S.J.; Nicholas, C.W.; McArdle, F.; Jackson, M.J.; Powell, J.R. Prolonged vitamin C supplementation and recovery from demanding exercise. Int. J. Sports Nutr. Exerc. Metab. 2001, 11, 466–481. [Google Scholar] [CrossRef]
- Roberts, L.A.; Beattie, K.; Close, G.L.; Morton, J.P. Vitamin C consumption does not impair training-induced improvements in exercise performance. Int. J. Sports Physiol. Perform. 2011, 6, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Gey, G.O.; Cooper, K.H.; Bottenberg, R.A. Effect of ascorbic acid on endurance performance and athletic injury. JAMA 1970, 211, 105. [Google Scholar] [CrossRef] [PubMed]
- Wacholder, K. Rise in the turnover and destruction of ascorbic acid (vitamin C) during muscle work. Arbeisphysiologie 1951, 14, 342. [Google Scholar]
- Lemmel, G. Vitamin C deficiency and general capacity for work. Munchener Medizinische Wochenschrift 1938, 85, 1381. [Google Scholar]
- Buzina, R.; Suboticanec, K. Vitamin C and physical working capacity. Int. J. Vitam. Nutr. Res. Suppl. 1985, 27, 157–166. [Google Scholar] [PubMed]
- Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Dipla, K.; Zafeiridis, A.; Panayiotou, G.; Vrabas, I.S.; Nikolaidis, M.G. Low vitamin C values are linked with decreased physical performance and increased oxidative stress: Reversal by vitamin C supplementation. Eur. J. Nutr. 2016, 55, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Gerster, H. The role of vitamin C in athletic performance. J. Am. Coll. Nutr. 1989, 8, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, K.J. The History of Scurvy & Vitamin C; Cambridge Unniversity Press: London, UK, 1986. [Google Scholar]
- Bates, J.F.; Hughes, R.E.; Hurley, R.J. Ascorbic acid status in man: Measurement of salivary, plasma, and white blood cell concentration. Arch. Oral Biol. 1972, 17, 1017–1020. [Google Scholar] [CrossRef]
- Paulsen, G.; Hamarsland, H.; Cumming, K.T.; Johansen, R.E.; Hulmi, J.J.; Børsheim, E.; Wiig, H.; Garthe, I.; Raastad, T. Vitamin C and E supplementation alters protein signaling after a strength training session, but not muscle growth during 10 weeks of training. J. Physiol. 2014, 592, 5391–5408. [Google Scholar] [CrossRef] [PubMed]
- Bobeuf, F.; Labonte, M.; Dionne, I.J.; Khalil, A. Combined effect of antioxidant supplementation and resistance training on oxidative stress markers, muscle and body composition in an elderly population. J. Nutr. Health Aging 2011, 15, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Wolff, C.; Musci, R.; Whedbee, M. Vitamin supplementation and resistance exercise-induced muscle hypertrophy: Shifting the redox balance scale? J. Physiol. 2015, 593, 2991–2992. [Google Scholar] [CrossRef] [PubMed]
- Bryer, S.C.; Goldfarb, A.H. Effect of high dose vitamin C supplementation on muscle soreness, damage, function, and oxidative stress to eccentric exercise. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Lamb, G.D.; Westerblad, H. Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J. Physiol. 2011, 589, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.H.; Redi, M.B.; Allen, D.G.; Westerblad, H. Effect of hydrogen peroxide and dithothreitol on contractile function of single skeletal muscle fibres from the mouse. J. Physiol. 1998, 509, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Steinbacher, P.; Eckl, P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, L.W.; Omaye, S.T. Use of Saliva Biomarkers to Monitor Efficacy of Vitamin C in Exercise-Induced Oxidative Stress. Antioxidants 2017, 6, 5. https://doi.org/10.3390/antiox6010005
Evans LW, Omaye ST. Use of Saliva Biomarkers to Monitor Efficacy of Vitamin C in Exercise-Induced Oxidative Stress. Antioxidants. 2017; 6(1):5. https://doi.org/10.3390/antiox6010005
Chicago/Turabian StyleEvans, Levi W., and Stanley T. Omaye. 2017. "Use of Saliva Biomarkers to Monitor Efficacy of Vitamin C in Exercise-Induced Oxidative Stress" Antioxidants 6, no. 1: 5. https://doi.org/10.3390/antiox6010005




