Acute Pre-/Post-Treatment with 8th Day SOD-Like Supreme (a Free Radical Scavenging Health Product) Protects against Oxidant-Induced Injury in Cultured Cardiomyocytes and Hepatocytes In Vitro as Well as in Mouse Myocardium and Liver In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. ORAC Assay
2.3. Cell Culture
2.4. SOD-Like Supreme Incubation and Menadione Cytotoxicity
2.5. Animal Care
2.6. SOD-Like Supreme Treatment and Isoproterenol (ISO)-Induced Myocardial Injury
2.7. SOD-Like Supreme Treatment and CCl4 Hepatotoxicity
2.8. Preparation of Plasma and Mitochondrial Samples
2.9. Biochemical Analyses
2.10. Statistical Analysis
3. Results
3.1. SOD-Like Supreme Displays Oxygen Radical Scavenging Activity In Vitro
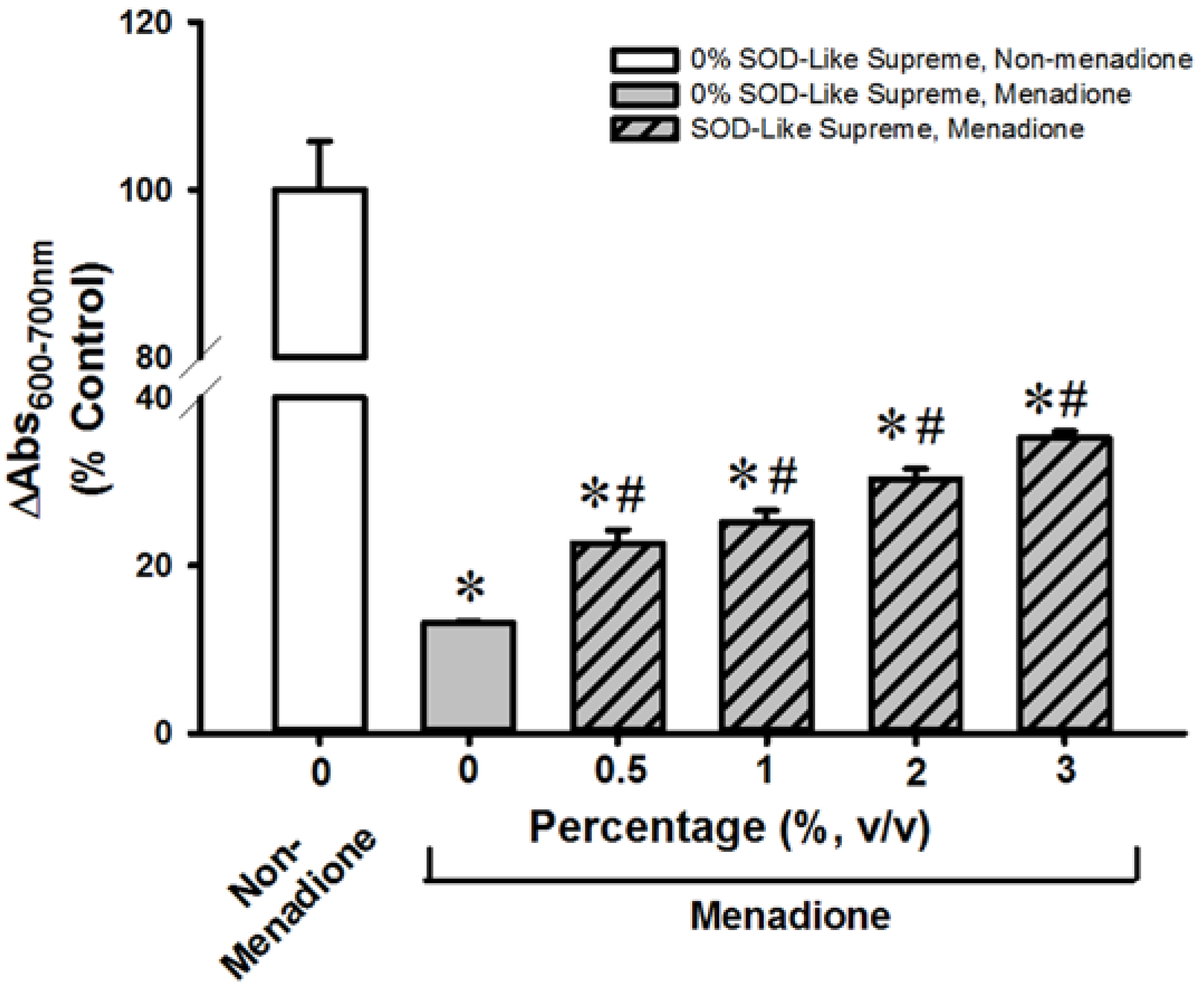
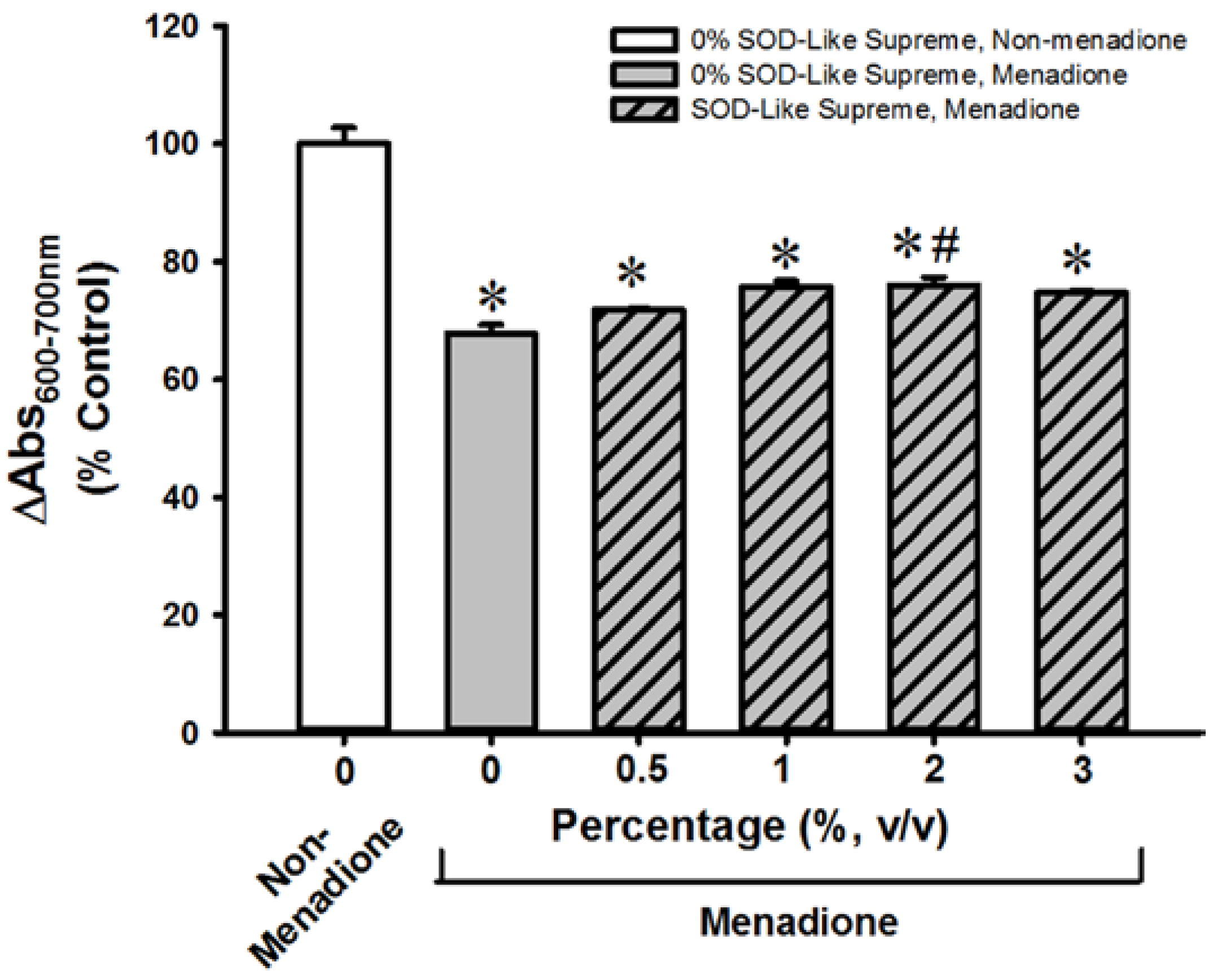
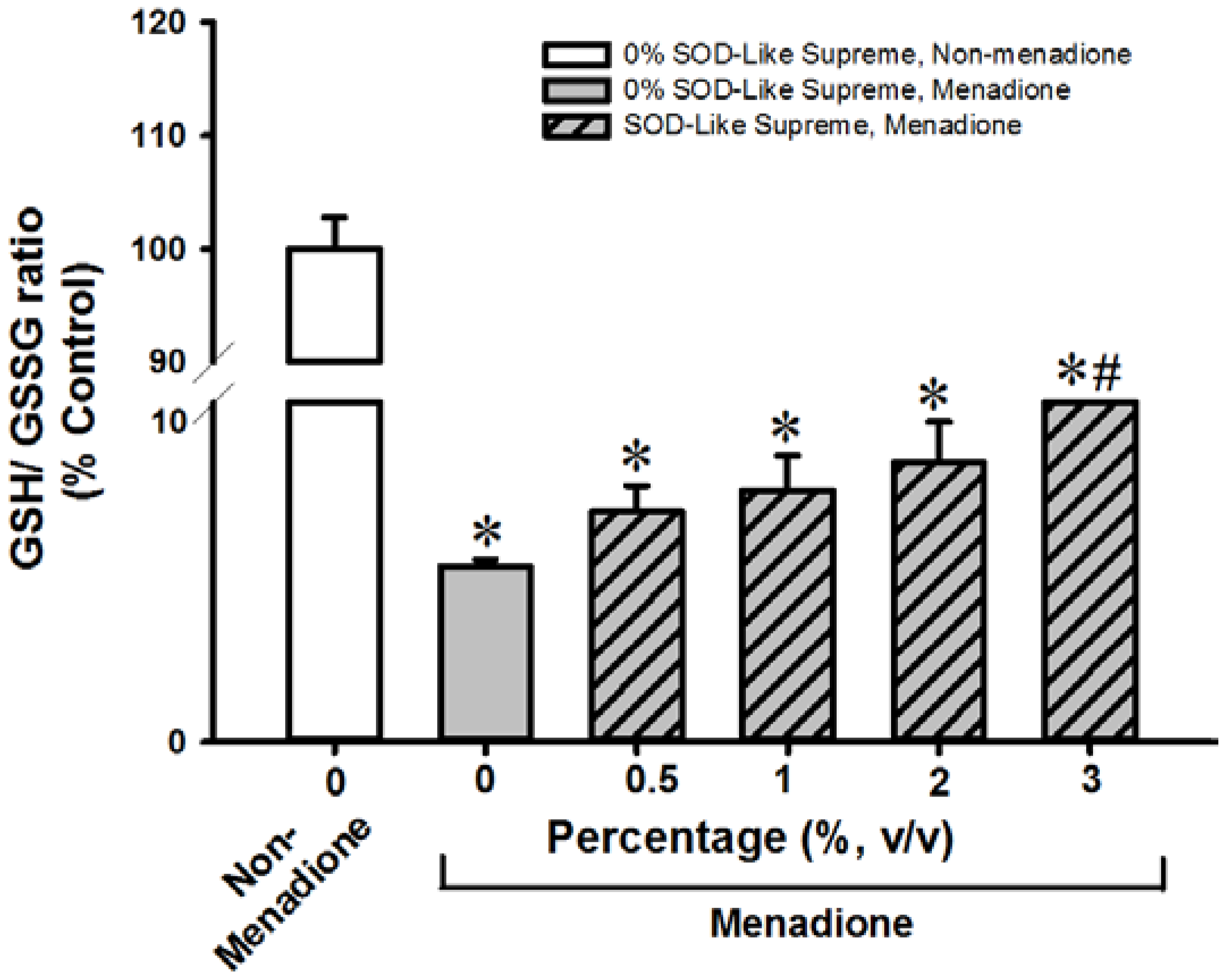
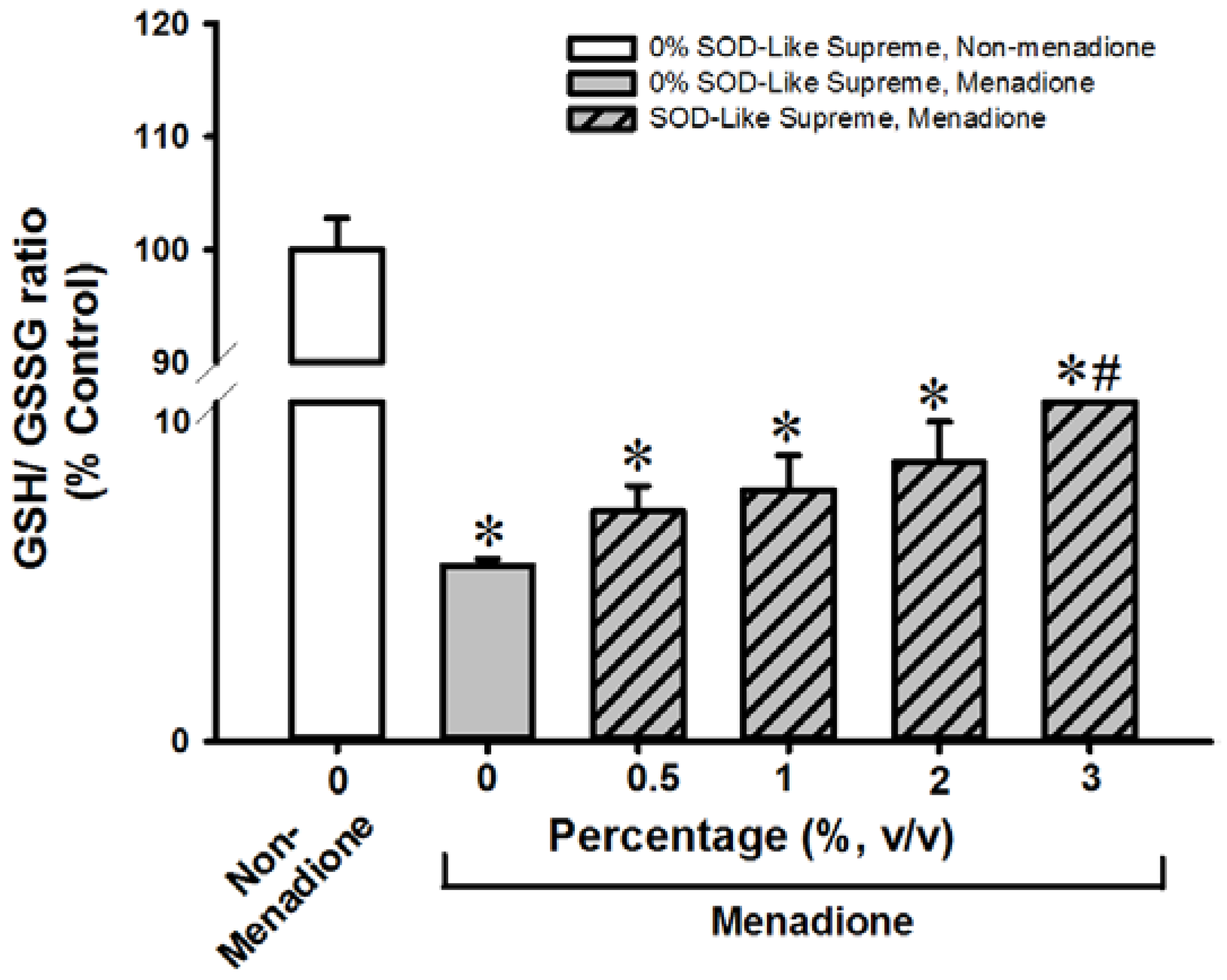
3.2. SOD-Like Supreme Protects against Menadione Toxicity in Cultured Cardiomyocytes and Hepatocytes
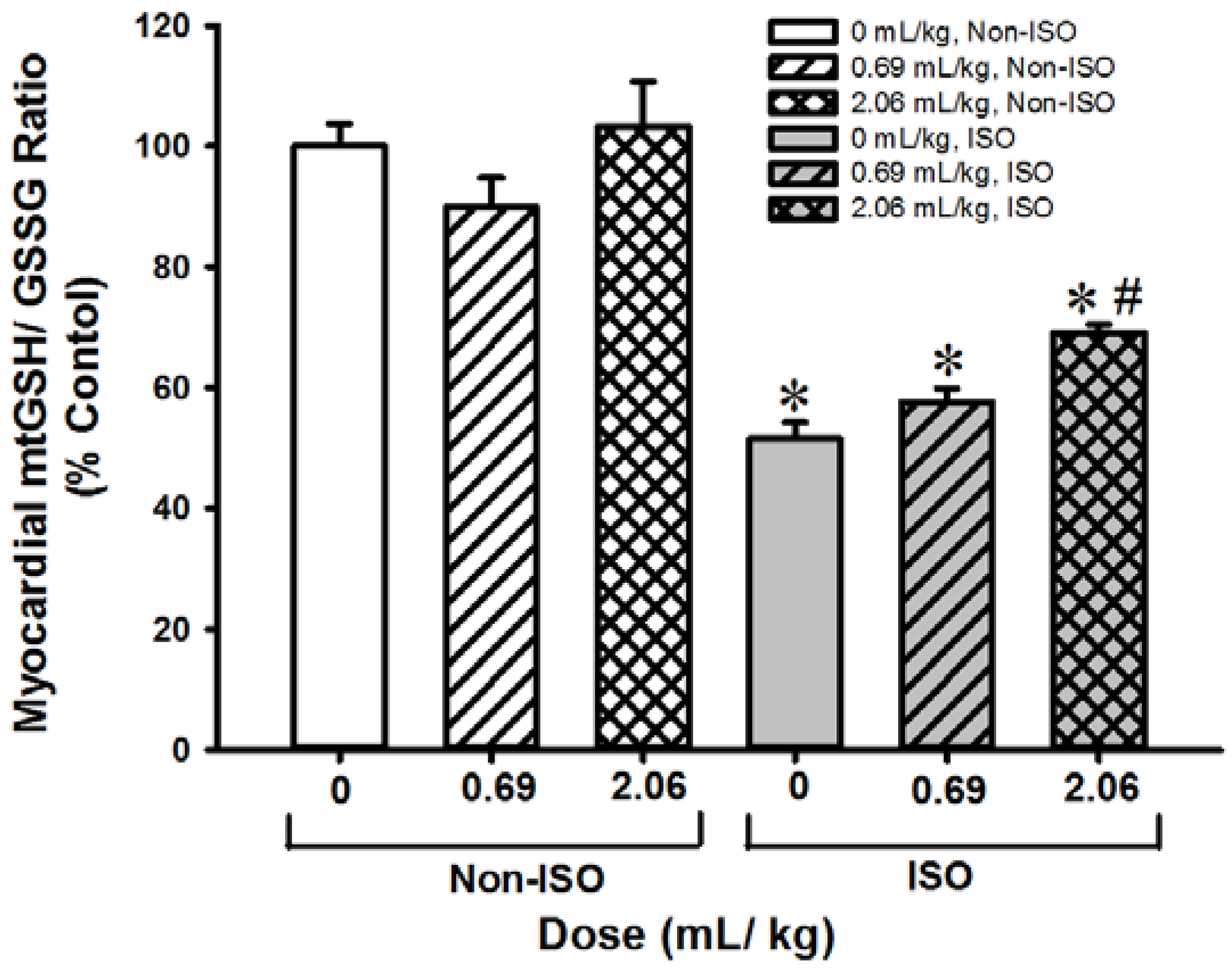
3.3. SOD-Like Supreme Protects against ISO-Induced Myocardial Injury in Mice
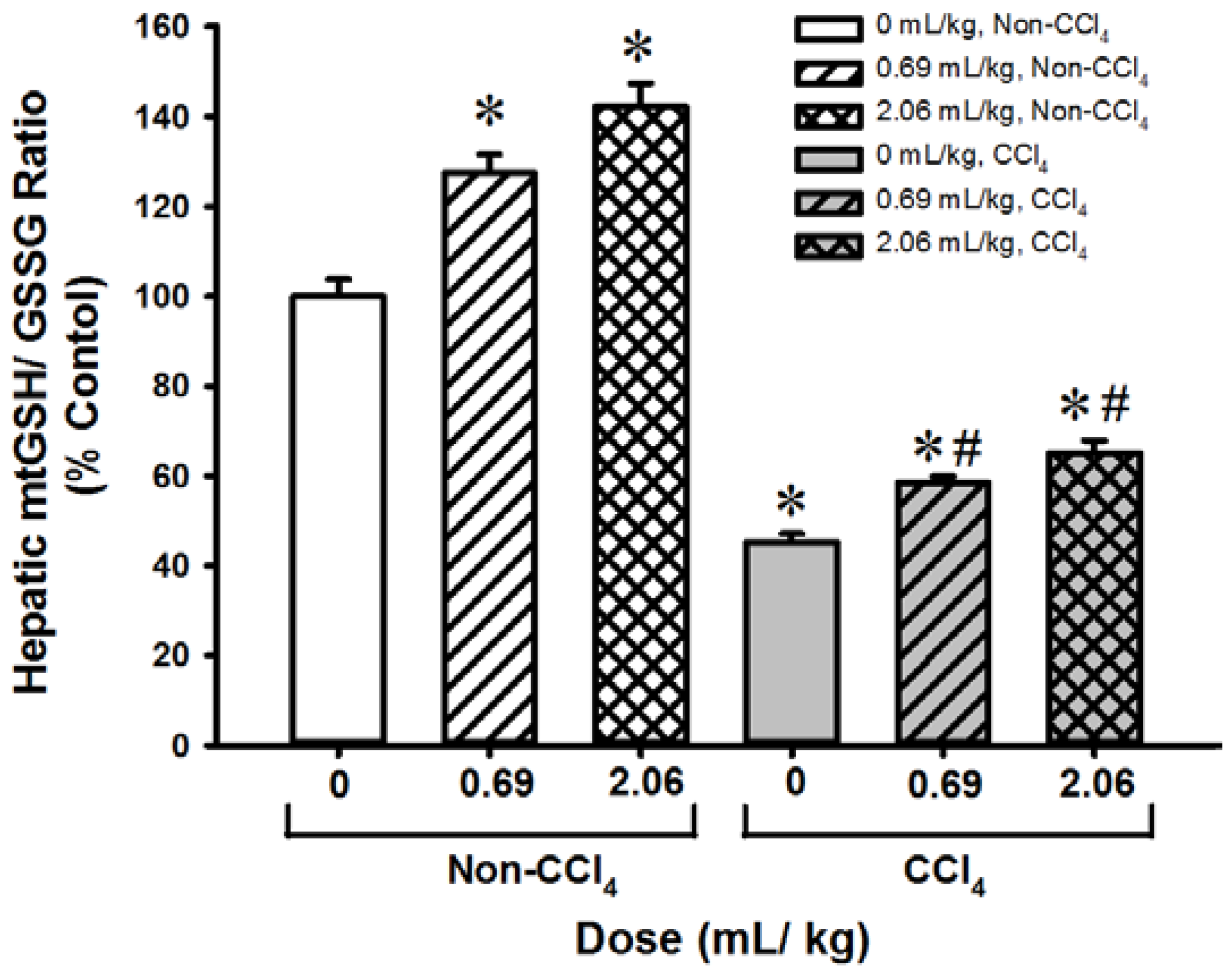
3.4. SOD-Like Supreme Protects against CCl4 Hepatotoxicity in Mice
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jang, Y.C.; Van Remmen, H. The mitochondrial theory of aging: Insight from transgenic and knockout mouse models. Exp. Gerontol. 2009, 44, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Larsson, N.G. The role of mitochondrial DNA mutations and free radicals in disease and ageing. J. Intern. Med. 2013, 273, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.J. Role of cytochromes P450 in chemical toxicity and oxidative stress: Studies with CYP2E1. Mutat. Res. 2005, 569, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Chaudière, J.; Ferrari-Iliou, R. Intracellular antioxidants: From chemical to biochemical mechanisms. Food Chem. Toxicol. 1999, 37, 949–962. [Google Scholar] [CrossRef]
- Quintana-Cabrera, R.; Bolaños, J.P. Glutathione and γ-glutamylcysteine in the antioxidant and survival functions of mitochondria. Biochem. Soc. Trans. 2013, 41, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Lin, J.M.; Lin, C.C. Raffinee, a free radical scavenger, in the treatment of subacute stage brain and spinal cord lesions: A case report. Am. J. Chin. Med. 1998, 26, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Naito, M.; Wu, X.; Lin, J.M.; Kimura, A.; Kodama, M.; Takada, A.; Okada, T.; Osawa, T. Anti-Atherogenic Effects of Fermented Fresh Coffee Bean, Soybean and Rice Bran Extracts. Food Sci. Technol. Res. 2003, 9, 170–175. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Chiu, P.Y.; Tang, M.H.; Mak, D.H.; Poon, M.K.; Ko, K.M. Hepatoprotective mechanism of schisandrin B: Role of mitochondrial glutathione antioxidant status and heat shock proteins. Free Radic. Biol. Med. 2003, 35, 368–380. [Google Scholar] [CrossRef]
- Loor, G.; Kondapalli, J.; Schriewer, J.M.; Chandel, N.S.; Vanden Hoek, T.L.; Schumacker, P.T. Menadione triggers cell death through ROS-dependent mechanisms involving PARP activation without requiring apoptosis. Free Radic. Biol. Med. 2010, 49, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Lindstad, R.I.; Hermansen, L.F.; McKinley-McKee, J.S. Inhibition and activation studies on sheep liver sorbitol dehydrogenase. Eur. J. Biochem. 1994, 221, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.C.; Fauconnier, J.; Yamada, T.; Lacampagne, A.; Zhang, S.; Katz, A.; Westerblad, H. Mitochondrial production of reactive oxygen species contributes to the β-adrenergic stimulation of mouse cardiomycytes. J. Physiol. 2011, 589, 1791–1801. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.A.; Teel, A.L.; Watts, R.J. Identification of the reactive oxygen species responsible for carbon tetrachloride degradation in modified Fenton’s systems. Environ. Sci. Technol. 2004, 38, 5465–5469. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, J.F.; Wen, X.Y.; Kan, J.; Jin, C.H. Antioxidant and protective effect of inulin and catechin grafted inulin against CCl4-induced liver injury. Int. J. Biol. Macromol. 2015, 72, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yang, X.; Lu, X.; Chen, J.; Zhao, Y. Protective effects of polyphenols-enriched extract from Huangshan Maofeng green tea against CCl4-induced liver injury in mice. Chem. Biol. Interact. 2014, 220, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Prince, P.S. (−) Epicatechin Prevents Alterations in Lysosomal Glycohydrolases, Cathepsins and Reduces Myocardial Infarct Size in Isoproterenol-Induced Myocardial Infarcted Rats. Eur. J. Pharmacol. 2013, 706, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Stanely Mainzen Prince, P. (−) Epicatechin attenuates mitochondrial damage by enhancing mitochondrial multi-marker enzymes, adenosine triphosphate and lowering calcium in isoproterenol-induced myocardial infarcted rats. Food Chem. Toxicol. 2013, 53, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Krupkova, O.; Ferguson, S.J.; Wuertz-Kozak, K. Stability of (−)-epigallocatechin gallate and its activity in liquid formulations and delivery systems. J. Nutr. Biochem. 2016, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
| Chemical Constituents | Percentage |
|---|---|
| Germ extract (an extract of fermented fresh coffee beans, soybeans and rice bran) | 60% |
| Oligosaccharides | 14% |
| DL-Alanine | 2.5% |
| Green tea extract | 1.2% |
| Dietary fiber (Pineapple fiber) | 1.0% |
| Citric acid | 0.6% |
| Vitamin C | 0.5% |
| Antioxidant | Trolox Equivalents |
|---|---|
| (mean ± SD) | |
| Trolox | 1.00 ± 0.12 |
| Ascorbic acid | 0.48 ± 0.12 |
| SOD-Like Supreme | 1.02 ± 0.06 |
| Group | % Control | |
|---|---|---|
| (mean ± SEM) | ||
| Non-ISO | Control | 100 ± 5.44 |
| 0.69 mL/kg | 109 ± 3.15 | |
| 2.06 mL/kg | 109 ± 6.30 | |
| ISO | Control | 190 ± 6.67 * |
| 0.69 mL/kg | 184 ± 6.96 * | |
| 2.06 mL/kg | 153 ± 8.65 *# | |
| Group | % Control | |
|---|---|---|
| (mean ± SEM) | ||
| Non-ISO | Control | 100 ± 3.33 |
| 0.69 mL/kg | 101 ± 4.97 | |
| 2.06 mL/ kg | 94.3 ± 3.47 | |
| ISO | Control | 136 ± 2.08 * |
| 0.69 mL/kg | 143 ± 5.33 * | |
| 2.06 mL/kg | 110 ± 4.35 # | |
| Group | % Control | |
|---|---|---|
| (mean ± SEM) | ||
| Non-CCl4 | Control | 100.00 ± 3.75 |
| 0.69 mL/kg | 141.96 ± 6.75 * | |
| 2.06 mL/kg | 182.66 ± 13.23 * | |
| CCl4 | Control | 271.75 ± 20.53 * |
| 0.69 mL/kg | 209.32 ± 4.43 * | |
| 2.06 mL/kg | 234.25 ± 20.80 * | |
| Group | % Control | |
|---|---|---|
| (mean ± SEM) | ||
| Non-CCl4 | Control | 100.00 ± 4.39 |
| 0.69 mL/kg | 160.20 ± 11.82 * | |
| 2.06 mL/kg | 160.53 ± 10.22 * | |
| CCl4 | Control | 210.29 ± 12.41 * |
| 0.69 mL/kg | 226.33 ± 16.31 * | |
| 2.06 mL/kg | 207.33 ± 8.09 * | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leong, P.K.; Chen, J.; Chan, W.M.; Leung, H.Y.; Chan, L.; Ko, K.M. Acute Pre-/Post-Treatment with 8th Day SOD-Like Supreme (a Free Radical Scavenging Health Product) Protects against Oxidant-Induced Injury in Cultured Cardiomyocytes and Hepatocytes In Vitro as Well as in Mouse Myocardium and Liver In Vivo. Antioxidants 2017, 6, 28. https://doi.org/10.3390/antiox6020028
Leong PK, Chen J, Chan WM, Leung HY, Chan L, Ko KM. Acute Pre-/Post-Treatment with 8th Day SOD-Like Supreme (a Free Radical Scavenging Health Product) Protects against Oxidant-Induced Injury in Cultured Cardiomyocytes and Hepatocytes In Vitro as Well as in Mouse Myocardium and Liver In Vivo. Antioxidants. 2017; 6(2):28. https://doi.org/10.3390/antiox6020028
Chicago/Turabian StyleLeong, Pou Kuan, Jihang Chen, Wing Man Chan, Hoi Yan Leung, Lincoln Chan, and Kam Ming Ko. 2017. "Acute Pre-/Post-Treatment with 8th Day SOD-Like Supreme (a Free Radical Scavenging Health Product) Protects against Oxidant-Induced Injury in Cultured Cardiomyocytes and Hepatocytes In Vitro as Well as in Mouse Myocardium and Liver In Vivo" Antioxidants 6, no. 2: 28. https://doi.org/10.3390/antiox6020028





