Is Root Catalase a Bifunctional Catalase-Peroxidase?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Catalase Activity and Protein Concentration
2.3. Data Analysis
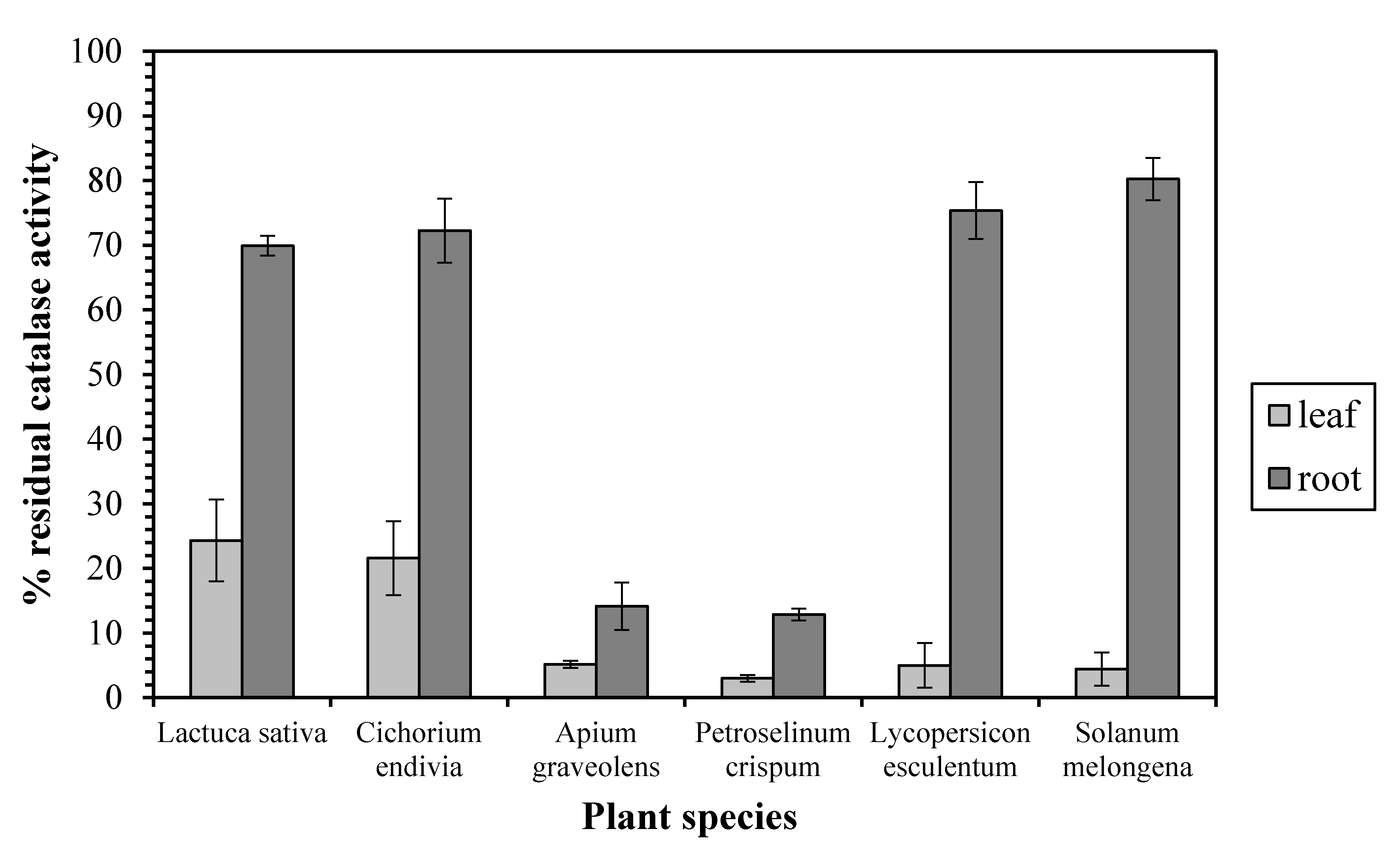
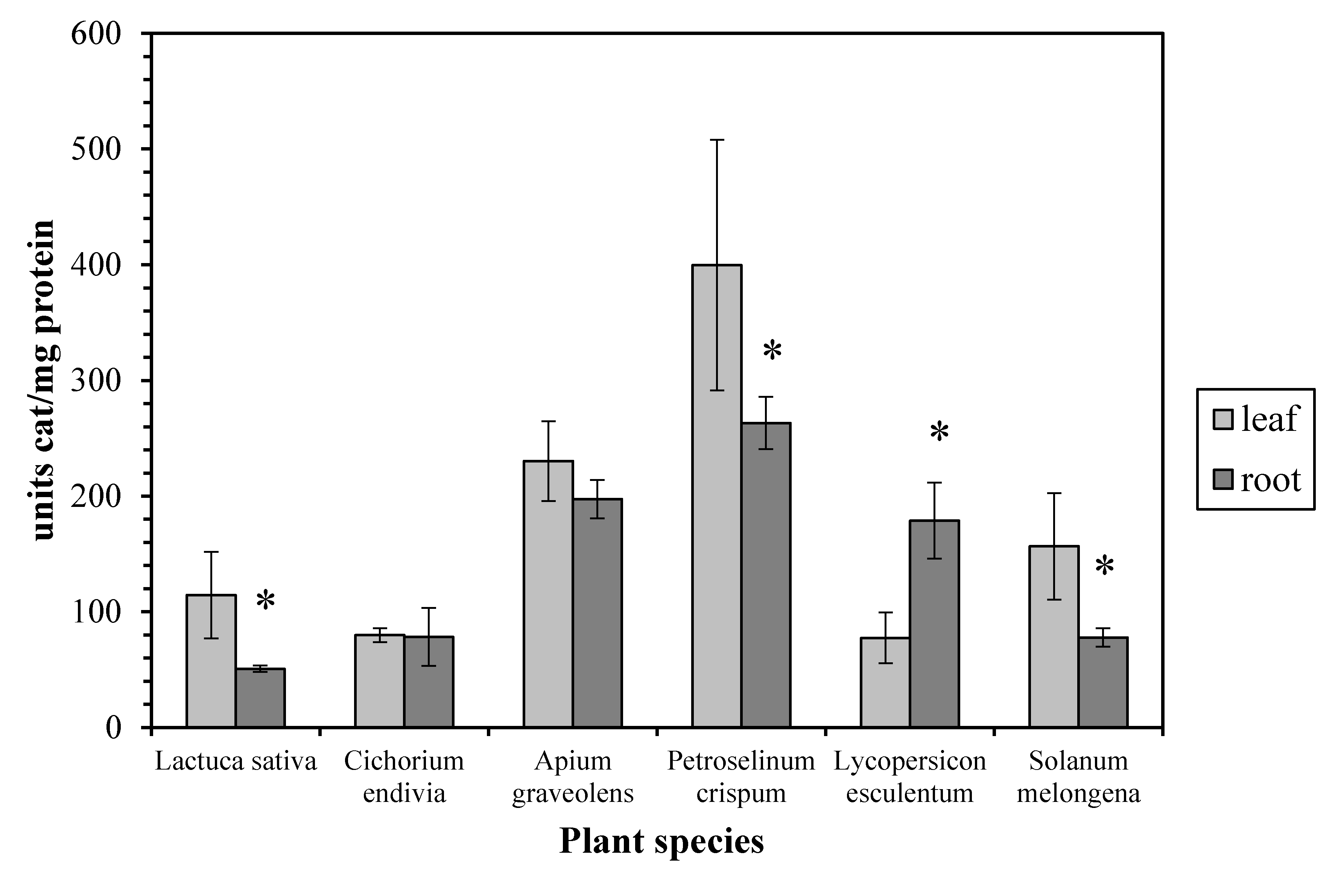
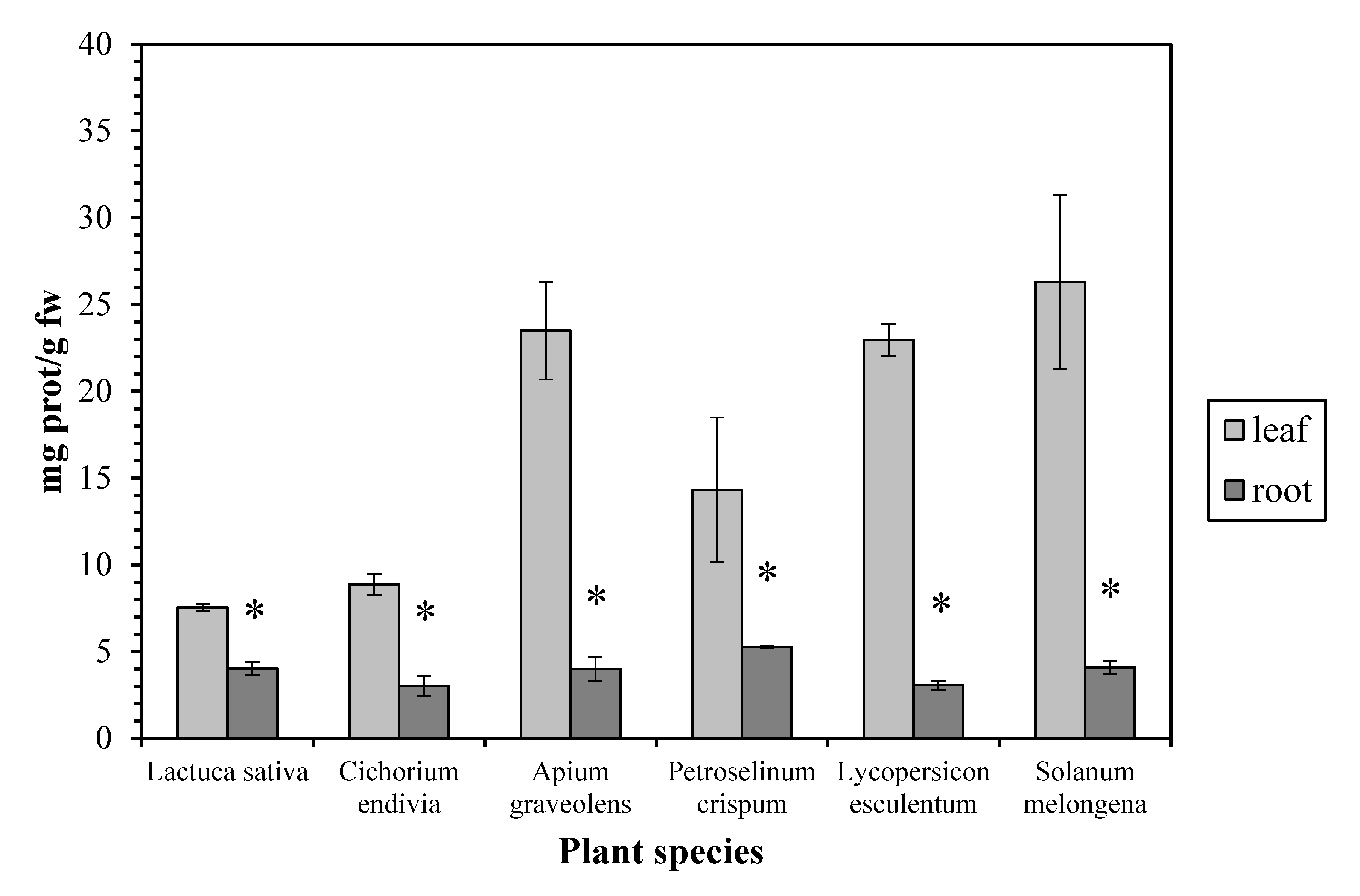
3. Results and Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Hameed, A.; Sheikh, M.A. Changes in catalase, peroxidase activities and soluble proteins in wheat leaves on thiourea and H2O2 treatments. Biosci. Res. 2007, 4, 21–27. [Google Scholar]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Redinbaugh, M.G.; Sabre, M.; Scandalios, J.G. The distribution of catalase activity, isozyme protein, and transcript in the tissues of the developing maize seedling. Plant Physiol. 1990, 92, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Noctor, G.; Baker, A. Plant catalases: Peroxisomal redox guardians. Arch. Biochem. Biophys. 2012, 525, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Zervoudakis, G.; Salahas, G.; Rodi, M. Nitrogen nutrition effect on aeroponic basil (Ocimum basilicum L.) catalase and lipid peroxidation. Not. Bot. HortiAgrobot. 2015, 43, 561–567. [Google Scholar]
- Chandlee, J.M.; Tsaftaris, A.S.; Scandalios, J.G. Purification and partial characterization of three genetically defined catalases of maize. Plant Sci. Lett. 1983, 29, 117–131. [Google Scholar] [CrossRef]
- Havir, E.A.; McHale, N.A. Purification and characterization of an isozyme of catalase with enhanced-peroxidatic activity from leaves of Nicotiana sylvestris. Arch. Biochem. Biophys. 1990, 283, 491–495. [Google Scholar] [CrossRef]
- Havir, E.A. The in vivo and in vitro inhibition of catalase from leaves of Nicotiana sylvestris by 3-amino-1,2,4-triazole. Plant Physiol. 1992, 99, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Willekens, H.; Inzé, D.; van Montagu, M.; van Camp, W. Catalases in plants. Mol. Breed. 1995, 1, 207–228. [Google Scholar] [CrossRef]
- Havir, E.A.; McHale, N.A. Enhanced-peroxidatic activity in specific catalase isozymes of tobacco, barley, and maize. Plant Physiol. 1989, 91, 812–815. [Google Scholar] [CrossRef]
- Scandalios, J.G.; Guan, L.; Polidoros, A.N. Catalases in plants: Gene structure, properties, regulation, and expression. In Oxidative Stress and the Molecular Biology of Antioxidant Defenses; Scandalios, J.G., Ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1997; pp. 343–406. [Google Scholar]
- Giorgio, M.; Trinei, M.; Migliaccio, E.; Pelicci, P.G. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007, 8, 722–728. [Google Scholar] [CrossRef]
- Jia, S.; McLaughlin, N.B.; Gu, J.; Li, X.; Wang, Z. Relationships between root respiration rate and root morphology, chemistry and anatomy in Larix gmelinii and Fraxinus mandshurica. Tree Physiol. 2013, 33, 579–589. [Google Scholar] [CrossRef]
- Atkin, O.K.; Edwards, E.J.; Loveys, B.R. Response of root respiration to changes in temperature and its relevance to global warming. New Phytol. 2000, 147, 141–154. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Tjoelker, M.G.; Vanderklein, D.; Buschena, C. Photosynthesis and respiration rates depend on leaf and root morphology and nitrogen concentration in nine boreal tree species differing in relative growth rate. Funct. Ecol. 1998, 12, 395–405. [Google Scholar] [CrossRef]
- Azevedo, R.A.; Alas, R.M.; Smith, R.J.; Lea, P.J. Response of antioxidant enzymes to transfer from elevated carbon dioxide to air and ozone fumigation, in the leaves and roots of wild-type and a catalase-deficient mutant of barley. Physiol. Plant. 1998, 104, 280–292. [Google Scholar] [CrossRef]
- Chouliaras, V.; Dimassi, K.; Therios, I.; Molassiotis, A.; Diamantidis, G. Root-reducing capacity, rhizosphere acidification, peroxidase and catalase activities and nutrient levels of Citrus taiwanica and C. volkameriana seedlings, under Fe deprivation conditions. Agronomie 2004, 24, 1–6. [Google Scholar] [CrossRef]
- Ge, T.-D.; Sui, F.-G.; Bai, L.-P.; Lu, Y.-Y.; Zhou, G.-S. Effects of water stress on the protective enzyme activities and lipid peroxidation in roots and leaves of summer maize. Agr. Sci. China 2006, 5, 291–298. [Google Scholar] [CrossRef]
- Duman, F.; Ozturk, F. Nickel accumulation and its effect on biomass, protein content and antioxidative enzymes in roots and leaves of watercress (Nasturtium officinale R. Br.). J. Environ. Sci. 2010, 22, 526–532. [Google Scholar] [CrossRef]
- Sánchez, E.; Rivero, R.M.; Ruiz, J.M.; Romero, L. Changes in biomass, enzymatic activity and protein concentration in roots and leaves of green bean plants (Phaseolus vulgaris L. cv. Strike) under high NH4NO3 application rates. Sci. Hortic. 2004, 99, 237–248. [Google Scholar] [CrossRef]
- Sinha, S.; Saxena, R.; Singh, S. Chromium induced lipid peroxidation in the plants of Pistia stratiotes L.: Role of antioxidants and antioxidant enzymes. Chemosphere 2005, 58, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.; Sprent, J.I.; Raven, J.A.; Eady, P.E. Relationships between shoot to root ratio, growth and leaf soluble protein concentration of Pisum sativum, Phaseolus vulgaris and Triticum aestivum under different nutrient deficiencies. Plant Cell Environ. 1999, 22, 949–958. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chioti, V.; Zervoudakis, G. Is Root Catalase a Bifunctional Catalase-Peroxidase? Antioxidants 2017, 6, 39. https://doi.org/10.3390/antiox6020039
Chioti V, Zervoudakis G. Is Root Catalase a Bifunctional Catalase-Peroxidase? Antioxidants. 2017; 6(2):39. https://doi.org/10.3390/antiox6020039
Chicago/Turabian StyleChioti, Vasileia, and George Zervoudakis. 2017. "Is Root Catalase a Bifunctional Catalase-Peroxidase?" Antioxidants 6, no. 2: 39. https://doi.org/10.3390/antiox6020039





