In Vitro and In Vivo Antioxidant and Anti-Hyperglycemic Activities of Moroccan Oat Cultivars
Abstract
:1. Introduction
2. Material and Methods
2.1. Oat Material Hybridization and Extraction
2.2. Chromatographic Analysis
2.3. Antioxidant Effect in Tetrahymena pyriformis Cell Culture
2.4. Digestive Enzymes Inhibition and Kinetics
2.5. Anti-Hyperglycemic and Antioxidant Effect in Diabetic Model
2.5.1. Animals
2.5.2. Acute Toxicity
2.5.3. Experimental Diabetes
2.5.4. Experimental Design
2.5.5. Oral Glucose Tolerance Test
2.5.6. Behavioral Assays
2.5.7. Hematological and Biochemical Analysis
2.5.8. Key Enzymes and Markers of Oxidative Stress
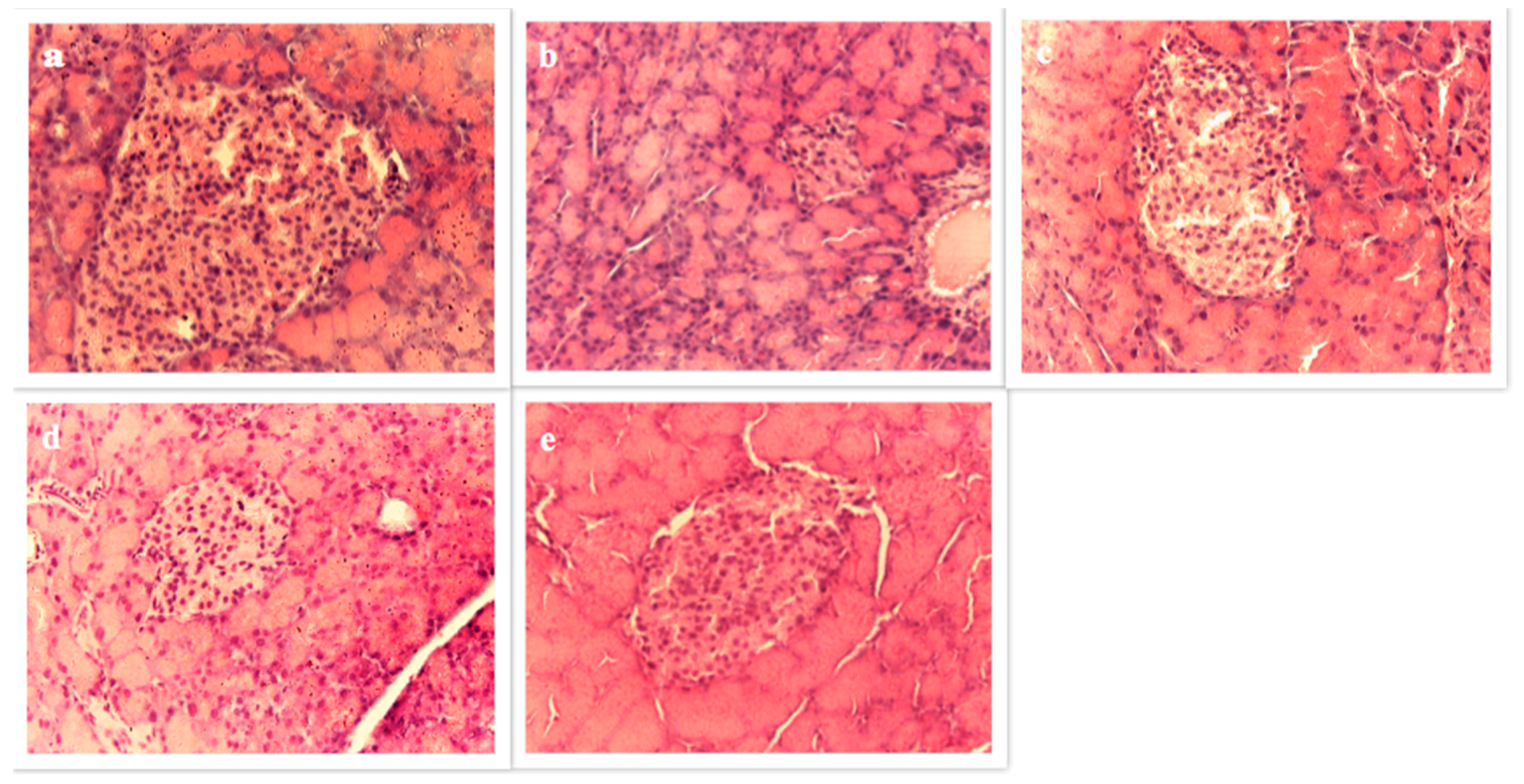
2.5.9. Histological Study of Pancreas
2.6. Statistical Analysis
3. Results
3.1. Chemical Analysis
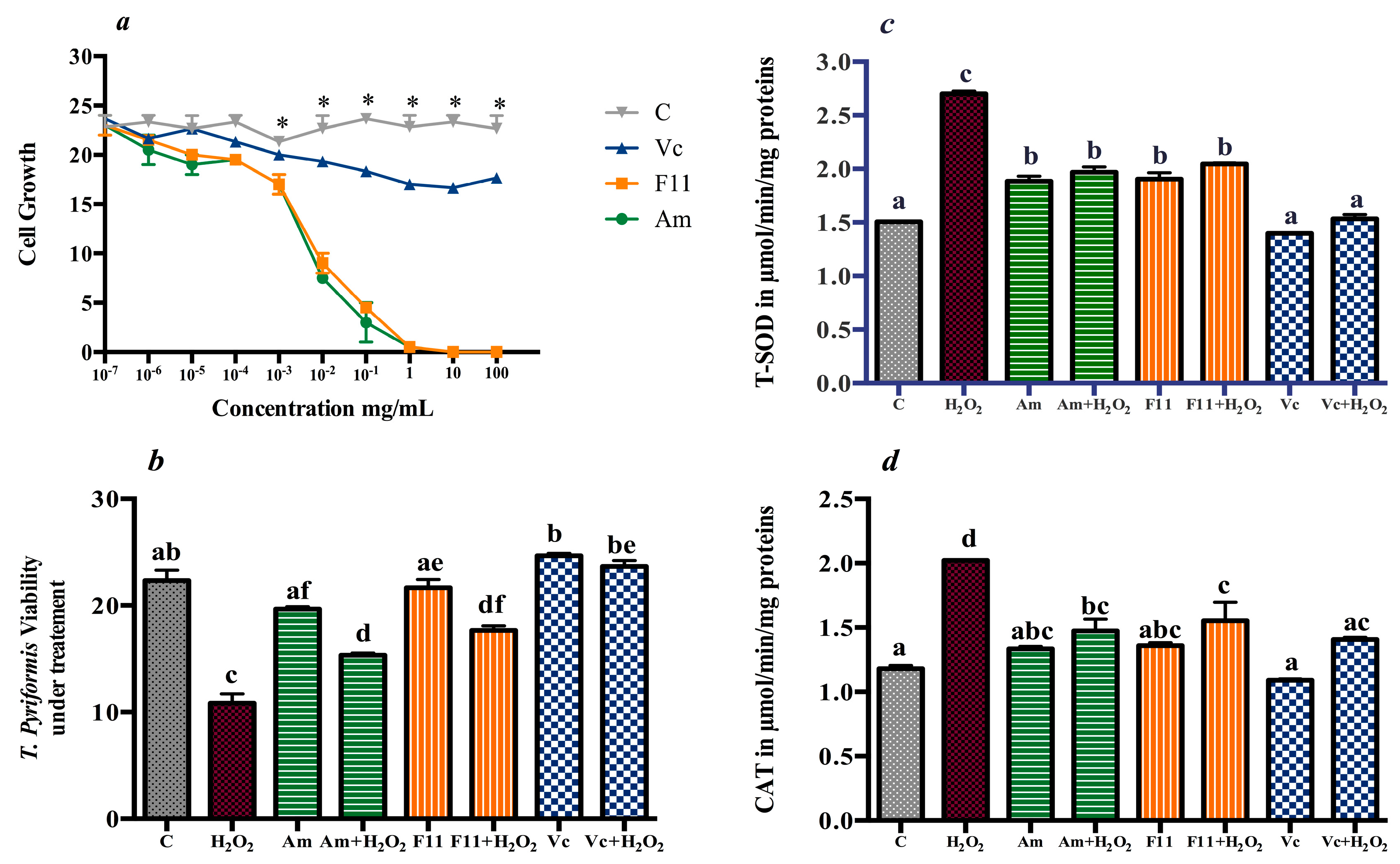
3.2. Antioxidant Effect in T. pyriformis
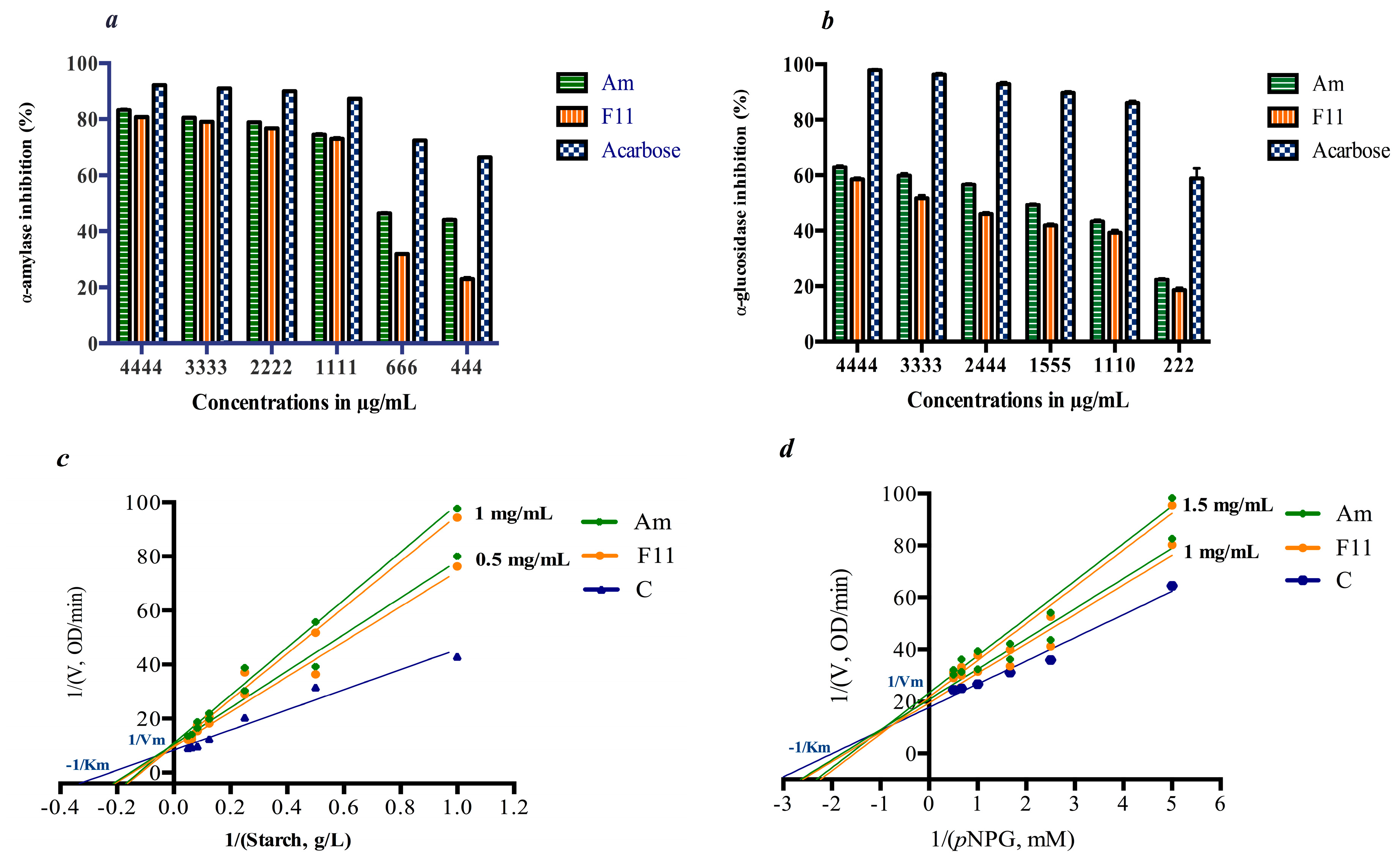
3.3. In Vitro Inhibition of Digestive Enzymes
3.4. Anti-Hyperglycemic and Antioxidant Effect in Diabetic Model
3.4.1. Acute Oral Toxicity and Metabolic Parameters
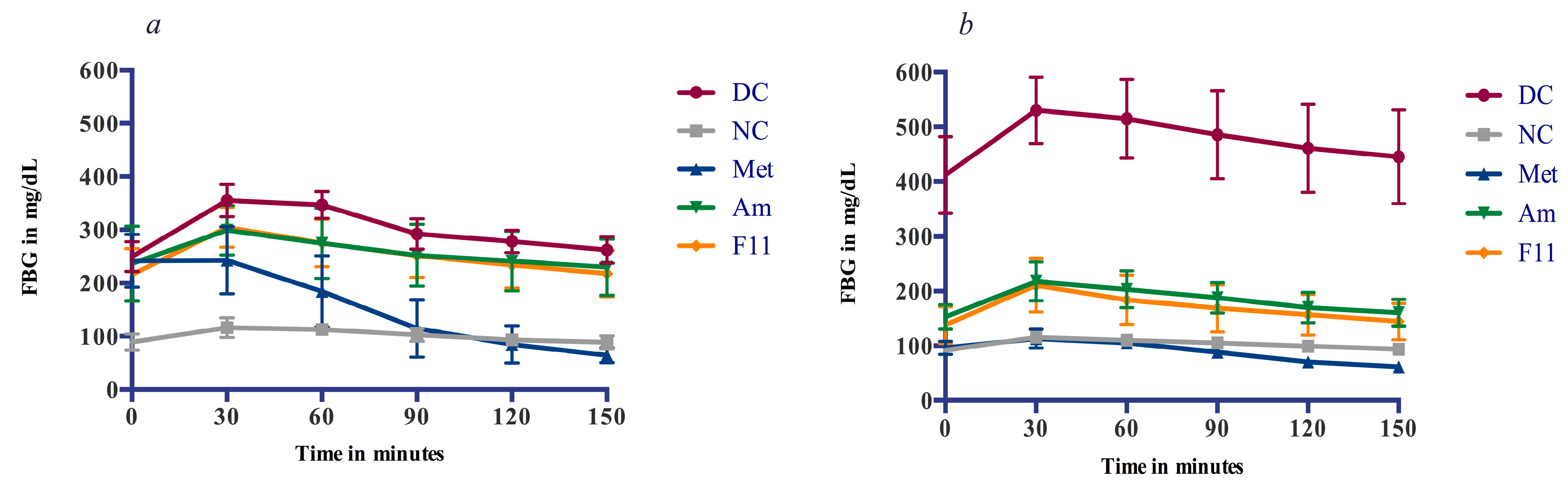
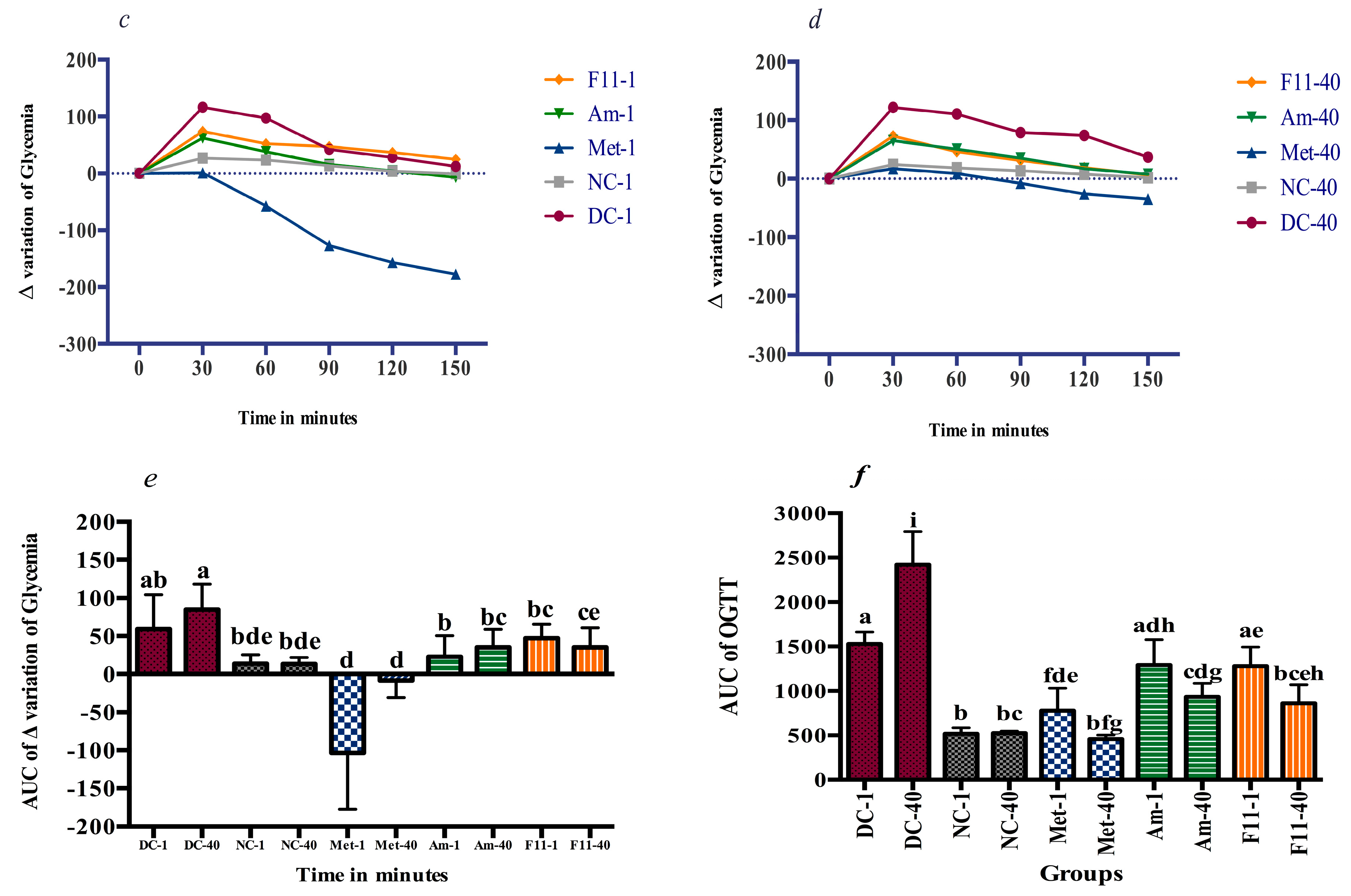
3.4.2. Glucose Tolerance and Anti-Hyperglycemic Effect
3.4.3. Hematological and Biochemical Indices
3.4.4. Oxidative Stress Markers and Antioxidants Enzymes
3.4.5. Behavioral Analysis
3.4.6. Pancreas Histopathology
4. Discussion
4.1. In Vitro Antidiabetic Activity
4.2. Oxidative Damage Prevention and Anti-Hyperglycemic Effect
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AD | Alzheimer disease |
| AI | Atherosclerosis index |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| FBG | Fasting blood glucose |
| AUC | Areas under curve |
| CAT | Catalase |
| DNS | Dinitrosalicylic acid |
| EPM | Elevated plus maze |
| GPx | Glutathione Peroxidase |
| GSH | Glutathione |
| HDL | High density lipoprotein |
| HGB | Hemoglobin |
| LDH | Lactate dehydrogenase |
| LDL | Low density lipoprotein |
| MDA | Malondialdehyde |
| p-NPG | p-Nitrophenyl-α-D-glucopyranoside |
| OFR | Overnight fasted rats |
| OFT | Open field test |
| OGTT | Oral glucose tolerance test |
| OS | Oxidative stress |
| PBS | Phosphate buffer solution |
| PPYG | Proteose-peptone yeast Glucose defined medium |
| RBC | Red blood corpuscles count |
| ROS | Radical oxygenated species |
| SOD | Superoxide dismutase |
| STZ-NA | Streptozotocine nicotinamide |
| TG | Triacylglycerols |
| HDL | High density lipoprotein |
| T2DM | Type 2 diabetes mellitus |
| WBC | White blood corpuscles count |
References
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.P. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J. Biol. Chem. 2004, 279, 42351–42354. [Google Scholar] [CrossRef] [PubMed]
- Wold, L.E.; Asli, F.C.; Jun, R. Oxidative stress and stress signaling: Menace of diabetic cardiomyopathy. Acta Pharmacol. Sin. 2005, 26, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Are Oxidative Stress—Activated Signaling Pathways Mediators of Insulin Resistance and β-Cell Dysfunction? Diabetes 2003, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, J.E.; Diamond-Stanic, M.K.; Marchionne, E.M. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic. Biol. Med. 2011, 51, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A. New Insights on Oxidative Stress and Diabetic Complications May Lead to a “Causal” Antioxidant Therapy. Diabetes Care 2003, 26, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Truswell, A.S. Cereal grains and coronary heart disease. Eur. J. Clin. Nutr. 2002, 56, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Vetrani, C.; Vitale, M.; Godos, J.; Riccardi, G.; Grosso, G. Whole Grain Intake and Glycaemic Control in Healthy Subjects: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2017, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- Marmouzi, I.; Saidi, N.; Meddah, B.; Bouksaim, M.; Gharby, S.; El Karbane, M.; Serragui, S.; Cherrah, Y.; Faouzi, M.E.A. Nutritional characteristics, biochemical composition and antioxidant activities of Moroccan Oat varieties. Food Meas. 2016, 10, 156–165. [Google Scholar] [CrossRef]
- Rahimi, R.; Nikfar, S.; Larijani, B.; Abdollahi, M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed. Pharmacother. 2005, 59, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.L.; Cai, F.L.; Dong, J.L.; Hu, X.Z. Hypoglycemic Effects and Biochemical Mechanisms of Oat Products on Streptozotocin-Induced Diabetic Mice. J. Agric. Food Chem. 2011, 59, 8895–8900. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Hu, X.; Guo, Q.; Cui, S.W.; Xian, Y.; You, S.; Chen, X.; Xu, C.; Gao, X. Physicochemical properties and regulatory effects on db/db diabetic mice of β-glucans extracted from oat, wheat and barley. Food Hydrocoll. 2014, 37, 60–68. [Google Scholar] [CrossRef]
- Detka, J.; Kurek, A.; Basta-Kaim, A.; Kubera, M.; Lasoń, W.; Budziszewska, B. Neuroendocrine link between stress, depression and diabetes. Pharmacol. Rep. 2013, 65, 1591–1600. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Domenico, F.D.; Barone, E. Elevated risk of type 2 diabetes for development of Alzheimer disease: A key role for oxidative stress in brain. Biochim. Biophys. Acta 2014, 1842, 1693–1706. [Google Scholar] [CrossRef] [PubMed]
- Rocher, A.; Colilla, F.; Ortiz, M.L.; Mendez, E. Identification of the three major coeliac immunoreactive proteins and one α-amylase inhibitor from oat endosperm. FEBS Lett. 1992, 310, 37–40. [Google Scholar] [CrossRef]
- Bischoff, B.A.G.H. Pharmacology of α-glucosidase inhibition. Eur. J. Clin. Investig. 1994, 24 (Suppl. 3), 3–10. [Google Scholar]
- Dong, J.; Cai, F.; Shen, R.; Liu, Y. Hypoglycaemic effects and inhibitory effect on intestinal disaccharidases of oat beta-glucan in streptozotocin-induced diabetic mice. Food Chem. 2011, 129, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Marmouzi, I.; El Madani, N.; Charrouf, Z.; Cherrah, Y.; Faouzi, M.E.A. Proximate analysis, fatty acids and mineral composition of processed Moroccan Chenopodium quinoa Willd and antioxidant properties according to the polarity. Phytothérapie 2015, 13, 110–117. [Google Scholar] [CrossRef]
- Saidi, N.; Saidi, S.; Hilali, A.; Benchekroun, M.; Al Faiz, C.; Bouksaim, M.; Shaimi, N.; Souihka, A.; Idrissi, S.A.; Gaboune, F.; et al. Improvement of oat hexaploid lines’s groat nutritive value via hybridisation with tetraploid oat A. magna. Am. J. Res. Commun. 2013, 1, 126–135. [Google Scholar]
- Mori, K.; Kashiwagi, A.; Yomo, T. Single-Cell Isolation and Cloning of Tetrahymena thermophila Cells with a Fluorescence-Activated Cell Sorter. J. Eukaryot. Microbiol. 2011, 58, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Lizard, G.; Gueldry, S.; Deckert, V.; Gambert, P.; Lagrost, L. Evaluation of the cytotoxic effects of some oxysterols and of cholesterol on endothelial cell growth: Methodological aspects. Pathol. Biol. 1997, 45, 281–290. [Google Scholar] [PubMed]
- Lowry, O.H.; Rosebrough, H.N.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Mannervik, B. Glutathione peroxidase. Methods Enzymol. 1985, 113, 490–495. [Google Scholar] [PubMed]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2015, 70, 1–20. [Google Scholar]
- Kee, K.T.; Koh, M.; Oong, L.X.; Ng, K. Screening culinary herbs for antioxidant and α-glucosidase inhibitory activities. Int. J. Food Sci. Technol. 2013, 48, 1884–1891. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay of lipid peroxides in animal tissue by thio barbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Efferth, T.; Egon, K. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Sun, Z. Current views on type 2 diabetes. J. Endocrinol. 2010, 204, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E. Alpha-Glucosidase Inhibitors. Endocrinol. Metab. Clin. N. Am. 1997, 26, 539–551. [Google Scholar] [CrossRef]
- Van de Laar, F.A.; Lucassen, P.L.B.J.; Akkermans, R.P.; Van de Lisdonk, E.H.; Rutten, G.E.H.M.; Van Weel, C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2005, 18, CD003639. [Google Scholar]
- Krause, H.P.; Ahr, H.J. Pharmacokinetics and Metabolism of Glucosidase Inhibitors. In Oral Antidiabetics; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 1996; Volume 119, pp. 541–555. [Google Scholar]
- Balfour, J.A.; McTavish, D. Acarbose: An Update of its Pharmacology and Therapeutic Use in Diabetes Mellitus. Drugs 1993, 46, 1025–1054. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.Y.; Kaneko, T.; Wang, Y.; Sato, A. Acarbose alone or in combination with ethanol potentiates the hepatotoxicity of carbon tetrachloride and acetaminophen in rats. J. Hepatol. 1999, 29, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Evert, A.B.; Boucher, J.L.; Cypress, M.; Dunbar, S.A.; Franz, M.J.; Mayer-Davis, E.J.; Neumiller, J.J.; Nwankwo, R.; Verdi, C.L.; Urbanski, P.; et al. Nutrition Therapy Recommendations for the Management of Adults With Diabetes. Diabetes Care 2014, 37, S120–S143. [Google Scholar] [CrossRef] [PubMed]
- Thu Phan, M.A.; Wang, J.; Tang, J.; Lee, Y.Z.; Ng, K. Evaluation of α-glucosidase inhibition potential of some flavonoids from Epimedium brevicornum. LWT Food Sci. Technol. 2013, 53, 492–498. [Google Scholar] [CrossRef]
- Ramakrishna, R.; Sarkar, D.; Schwarz, P.; Shetty, K. Phenolic linked anti-hyperglycemic bioactives of barley (Hordeum vulgare L.) cultivars as nutraceuticals targeting type 2 diabetes. Ind. Crops Prod. 2017, 107, 509–517. [Google Scholar] [CrossRef]
- Malunga, L.N.; Eck, P. Inhibition of intestinal α-glucosidase and glucose absorption by feruloylated arabinoxylan mono-and oligosaccharides from corn bran and wheat aleurone. J. Nutr. Metab. 2016, 2016, 1932532. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, P.; Bomzan, D.P.; Rao, B.S.; Sreerama, Y.N. Distribution of phenolic antioxidants in whole and milled fractions of quinoa and their inhibitory effects on α-amylase and α-glucosidase activities. Food Chem. 2016, 199, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Daou, C.; Zhang, H. Oat Beta-Glucan: Its Role in Health Promotion and Prevention of Diseases. Compr. Rev. Food Sci. Food Saf. 2012, 11, 355–365. [Google Scholar] [CrossRef]
- Shen, X.L.; Zhao, T.; Zhou, Y.; Shi, X.; Zou, Y.; Zhao, G. Effect of oat β-glucan intake on glycaemic control and insulin sensitivity of diabetic patients: A meta-analysis of randomized controlled trials. Nutrients 2016, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Wanga, Q.; Ellisa, P.R. Oat β-glucan: Physico-chemical characteristics in relation to its blood-glucose and cholesterol-lowering properties. Br. J. Nutr. 2014, 112, S4–S13. [Google Scholar] [CrossRef] [PubMed]
- Radenković, M.; Stojanović, M. Milica Prostran Experimental diabetes induced by alloxan and streptozotocin: The current state of the art. J. Pharmacol. Toxicol. Methods 2016, 78, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Masiello, P.; Broca, C.; Gross, R.; Roye, M.; Manteghetti, M.; Hillaire-Buys, D.; Novelli, M.; Ribes, G. Experimental NIDDM: Development of a New Model in Adult Rats Administered Streptozotocin and Nicotinamide. Diabetes 1998, 47, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Swanston-Flat, S.K.; Day, C.; Bailey, C.J.; Flatt, P.R. Traditional plant treatment for diabetes: Studies in normal and streptozotocin diabetic mice. Diabetologia 1990, 33, 462–464. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2015, 38, S8–S16. [Google Scholar]
- Taylor, F.; Huffman, M.D.; Macedo, A.F.; Moore, T.H.M.; Burke, M.; Davey Smith, G.; Ward, K.; Ebrahim, S. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2013, CD004816. [Google Scholar] [CrossRef]
- Ohaeri, O.C. Effect of garlic oil on the levels of various enzymes in the serum and tissue of streptozotocin diabetic rats. Biosci. Rep. 2001, 21, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Palsamy, P.; Subramanian, S. Resveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocin-nicotinamide induced experimental diabetic rats. Biomed. Pharmacother. 2008, 62, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Natali, A.; Ferrannini, E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: A systematic review. Diabetologia 2006, 49, 434–441. [Google Scholar] [CrossRef] [PubMed]
- King, G.L.; Loeken, M.R. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem. Cell. Biol. 2004, 122, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Mahadev, K.; Zilbering, A.; Zhu, L.; Goldstein, B.J. Insulin-stimulated Hydrogen Peroxide Reversibly Inhibits Protein-tyrosine Phosphatase 1B in Vivo and Enhances the Early Insulin Action Cascade. J. Biol. Chem. 2001, 276, 21938–21942. [Google Scholar] [CrossRef] [PubMed]
- Porokhovnik, L.N.; Passekov, V.P.; Gorbachevskaya, N.L.; Sorokin, A.B.; Veiko, N.N.; Lyapunova, N.A. Active ribosomal genes, translational homeostasis and oxidative stress in the pathogenesis of schizophrenia and autism. Psychiatr. Genet. 2015, 25, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Genet, S.; Kale, R.K.; Baquer, N.Z. Alterations in antioxidant enzymes and oxidative damage in experimental diabetic rat tissues: Effect of vanadate and fenugreek (Trigonella foenum graecum). Mol. Cell. Biochem. 2002, 236, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Nandakumar, N.; Rengarajan, T.; Palaniswami, R.; Gnanadhas, E.N.; Lakshminarasaiah, U.; Gopas, J.; Nishigaki, I. Antioxidants and human diseases. Clin. Chim. Acta 2014, 436, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Godin, D.V.; Wohaieb, S.A.; Garnett, M.E.; Goumeniouk, A.D. Antioxidant enzyme alterations in experimental and clinical diabetes. Mol. Cell. Biochem. 1988, 84, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.M. Oat Antioxidants. J. Cereal Sci. 2001, 33, 115–129. [Google Scholar] [CrossRef]
- Chen, C.Y.; Milbury, P.E.; Kwak, H.K.; Collins, F.W.; Samuel, P.; Blumberg, J.B. Avenanthramides and Phenolic Acids from Oats Are Bioavailable and Act Synergistically with Vitamin C to Enhance Hamster and Human LDL Resistance to Oxidation. J. Nutr. 2004, 134, 1459–1466. [Google Scholar] [PubMed]
- Wang, P.; Chen, H.; Zhu, Y.; McBride, J.; Fu, J.; Sang, S. Oat Avenanthramide-C (2c) Is Biotransformed by Mice and the Human Microbiota into Bioactive Metabolites. J. Nutr. 2015, 145, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Salkovic-Petrisic, M.; Knezovic, A.; Hoyer, S.; Riederer, P. What have we learned from the streptozotocin-induced animal model of sporadic Alzheimer’s disease, about the therapeutic strategies in Alzheimer’s research. J. Neural Transm. 2013, 120, 233–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samy, D.M.; Ismail, C.A.; Nassr, R.A.; Zeitoun, T.M.; Nomair, A.M. Down stream modulation of extrinsic apoptotic pathway in streptozotocin-induced Alzheimer's dementia in rats: Erythropoietin versus curcumin. Eur. J. Pharmacol. 2016, 770, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo-Solleiro, D.; Crespo-Ramírez, M.; Roldán-Roldán, G.; Hiriart, M.; Pérez de la Mora, M. Role of thirst and visual barriers in the differential behavior displayed by streptozotocin-treated rats in the elevated plus-maze and the open field test. Physiol. Behav. 2013, 120, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Garabadu, D.; Krishnamurthy, S. Diazepam Potentiates the Antidiabetic, Antistress and Anxiolytic Activities of Metformin in Type-2 Diabetes Mellitus with Cooccurring Stress in Experimental Animals. BioMed Res. Int. 2014, 2014, 693074. [Google Scholar] [CrossRef] [PubMed]
| Compounds | Amlal | F11-5 |
|---|---|---|
| Gallic acid | 41.08 ± 2.32 b | 13.34 ± 2.29 a |
| Chlorogenic acid | 2.56 ± 0.45 a | 1.78 ± 0.12 a |
| p-Hydroxybenzoic acid | 1840.34 ± 30.45 b | 1270.02 ± 38.34 a |
| Caffeic acid | 250.67 ± 32.11 a | 421.54 ± 12.32 b |
| Syringic acid | 1830.66 ± 90.21 b | 310.41 ± 33.09 a |
| p-Coumaric acid | 26.67 ± 3.22 b | 16.43 ± 1.90 a |
| Ferulic acid | 70.45 ± 1.87 b | 1.98 ± 0.49 a |
| Sinapic acid | 17.10 ± 2.09 b | 11.62 ± 2.78 a |
| Salicilyc acid | 4.67 ± 0.07 b | 1.78 ± 0.16 a |
| α-Tocopherol | 1.65 ± 0.22 a | 1.82 ± 0.12 b |
| Measure | NC | DC | Met | Am | F11 | |
|---|---|---|---|---|---|---|
| FBG (mg/dL) | D1 | 89.15 ± 16.48 a | 249.85 ± 28.05 a | 246.16 ± 52.60 a | 236.71 ± 70.16 a | 215.80 ± 48.83 a |
| D14 | 86.83 ± 9.64 a | 295.50 ± 28.98 b | 107.66 ± 7.14 b | 216.42 ± 25.26 a | 193.14 ± 34.78 a | |
| D28 | 91.83 ± 10.04 a | 381.80 ± 38.10 c | 100.50 ± 3.50 b | 189.85 ± 33.30 a | 176.28 ± 34.51 a | |
| D42 | 92.16 ±7.19 a | 412.20 ± 49.87 c | 92.83 ± 9.78 b | 152.50 ± 22.38 b | 137.57 ± 33.57 b | |
| Food intake (g) | D1 | 16.85 ± 1.57 a | 19.14 ± 3.02 a | 18.85 ± 2.60 a | 19.28 ± 2.13 a | 18.28 ± 2.87 a |
| D41 | 19.42 ± 3.10 a | 27.14 ± 2.19 b | 21.42 ± 1.98 a | 23.00 ± 1.41 a | 23.14 ± 2.91 a | |
| Water intake (mL) | D1 | 24.71 ± 4.46 a | 71 ± 11.71 a | 57.28 ± 7.69 a | 67.14 ± 3.71 a | 63.00 ± 7.16 a |
| D41 | 25.14 ± 5.61 a | 170 ± 14.54 b | 68.14 ± 10.17 a | 98.00 ± 7.50 b | 102.42 ± 15.05 b | |
| Urinary volume (mL) | D1 | 10.57 ± 1.98 a | 45.57 ± 6.87 a | 43.57 ± 2.69 a | 37.42 ± 5.42 a | 39.71 ± 5.46 a |
| D41 | 11.14 ± 1.77 a | 71.14 ± 2.60 b | 51.71 ± 5.61 a | 52.14 ± 2.41 b | 55.28 ± 5.58 b | |
| Body Weight (g) | D1 | 208.57 ± 25.33 a | 241.85 ± 10.52 a | 221.71 ± 56.29 a | 221.42 ± 26.45 a | 214.85 ± 57.01 a |
| D41 | 236.16 ± 25.89 a | 118.71 ± 21.62 b | 198.57 ± 13.50 a | 175.28 ± 14.39 a | 189.28 ± 58.01 a | |
| Parameters | Unit | NC | DC | Met | Am | F11 |
|---|---|---|---|---|---|---|
| Hematology | ||||||
| HGB | g/dL | 11.25 ± 0.49 a | 14.32 ± 1.27 a | 12.93 ± 0.98 a | 11.1 ± 3.39 a | 12.02 ±2.04 a |
| RBC | 10−6/uL | 6.87 ± 0.02 a | 8.36 ± 0.59 b | 7.84 ± 0.21 ab | 12.76 ± 0.50 c | 6.76 ± 1.05 a |
| WBC | 10−3/uL | 4.23 ± 0.89 a | 21.31 ± 3.69 c | 10.43 ± 5.43 ab | 18.05 ± 1.18 b | 12.83 ± 3.90 ab |
| Neutrophils | 10−3/uL | 15.50 ± 4.65 a | 21.63 ± 6.29 ab | 16.43 ± 3.47 b | 15.83 ± 3.44 b | 17.01 ± 0.10 b |
| Lymphocytes | 10−3/uL | 65.25 ± 15.34 abc | 54.05 ± 1.62 c | 45.65 ± 9.89 b | 73.93 ± 4.20 b | 55.33 ± 4.90 c |
| Monocytes | 10−3/uL | 4.95 ± 0.91 a | 9.86 ± 1.77 d | 5.24 ± 1.69 c | 6.71 ± 1.65 b | 5.73 ± 0.49 bcd |
| Eosinophils | 10−3/uL | 2.80 ± 0.97 ab | 2.77 ± 0.73 a | 1.98 ± 0.59 c | 3.91 ± 1.60 abc | 2.10 ± 0.81 bc |
| Basophils | 10−3/uL | n.d | n.d | n.d | n.d | n.d |
| Platelet count | 10−3/uL | 508 ± 89.78 ab | 516.75 ± 93.11 ab | 534.33 ± 51.18 a | 548.86 ± 41.71 ab | 584.50 ± 61.51 b |
| Liver function | ||||||
| ALT | IU/L | 66.66 ± 12.01 ab | 130.05 ± 4.56 c | 84.66 ± 17.62 b | 68.28 ± 37.66 ab | 72.40 ± 18.14 a |
| AST | IU/L | 104.5 ± 14.86 ac | 225.66 ± 53.65 d | 118.33 ± 33.26 c | 139.14 ± 29.53 a | 141.33 ± 19.75 bc |
| Total proteins | g/L | 62.00 ± 5.32 a | 63.00 ± 1.87 abcd | 68.28 ± 3.98 def | 67.11 ± 4.82 ce | 64.76 ± 5.28 bf |
| Renal function | ||||||
| LDH | U/L | 327.33 ± 26.00 ac | 693.00 ± 78.23 b | 351.88 ± 91.34 ac | 569.00 ± 89.50 a | 426.26 ± 49.25 c |
| Urea | g/L | 0.22 ± 0.04 ac | 0.62 ± 0.12 b | 0.43 ± 0.09 c | 0.49 ± 0.19 ab | 0.28 ± 0.05 a |
| Creatinine | mg/L | 4.61 ± 0.28 abcd | 7.70 ± 1.49 a | 4.18 ± 0.22 b | 4.95 ± 0.35 c | 4.68 ± 0.32 d |
| Lipid profile | ||||||
| Cholesterol | g/L | 0.44 ± 0.16 ac | 1.09 ± 0.20 b | 0.46 ± 0.17 ad | 0.66 ± 0.19 c | 0.63 ± 0.12 cd |
| TG | g/L | 0.58 ± 0.09 acde | 4.51 ± 0.26 b | 0.41 ± 0.16 c | 0.72 ± 0.21 d | 1.01 ± 0.22 e |
| HDL | g/L | 0.22 ± 0.04 a | 0.14 ± 0.04 b | 0.21 ± 0.05 a | 0.17 ± 0.05 a | 0.17 ± 0.01 a |
| LDL | g/L | 0.20 ± 0.03 a | 0.26 ± 0.05 ab | 0.21 ± 0.09 ab | 0.21 ± 0.08 b | 0.21 ± 0.12 a |
| AI | ratio | 0.91 ± 0.05 a | 2.36 ± 0.03 d | 0.99 ± 0.05 b | 1.23 ± 0.04 c | 1.23 ± 0.10 bc |
| Minerals | ||||||
| Sodium | mmol/L | 141.28 ± 1.60 a | 134.66 ± 9.71 ab | 141.14 ± 2.11 ab | 140.14 ± 1.57 b | 139.80 ± 2.48 ab |
| Potassium | mmol/L | 5.42 ± 0.54 ac | 4.41 ± 0.60 bc | 5.4 ± 1.15 ab | 5.39 ± 0.35 abc | 6.31 ± 1.33 c |
| Chlore | mmol/L | 106.71 ± 2.62 a | 97.00 ± 7.93 ab | 103.85 ± 1.95 ac | 101.42 ± 1.98 b | 98.60 ± 3.78 bc |
| MDA | T-SOD | Mn-SOD | CuZn-SOD | CAT | GSH | GPx | |
|---|---|---|---|---|---|---|---|
| Liver | |||||||
| NC | 0.42 ± 0.05 a | 7.22 ± 1.57 a | 1.66 ± 0.44 a | 3.58 ± 0.24 ab | 5.78 ± 1.44 a | 2.36 ± 0.25 a | 3.22 ± 0.27 ac |
| DC | 6.37 ± 0.60 d | 16.21 ± 2.86 c | 3.64 ± 0.63 d | 11.80 ± 2.06 c | 18.42 ± 1.81 c | 4.38 ± 0.99 c | 7.20 ± 0.42 d |
| Met | 0.55 ± 0.06 b | 7.25 ± 2.46 a | 1.96 ± 0.46 c | 4.33 ± 1.30 b | 7.17 ± 0.77 a | 3.50 ± 0.97 b | 2.05 ± 0.71 a |
| Am | 1.07 ± 0.17 c | 8.30 ± 0.48 abc | 2.72 ± 0.54 b | 3.60 ± 1.34 a | 8.57 ± 1.71 b | 3.08 ± 0.35 abc | 2.75 ± 0.23 b |
| F11 | 2.13 ± 0.98 abc | 14.52 ± 3.00 b | 2.93 ± 0.75 abc | 9.25 ± 0.50 c | 14.74 ± 3.88 abc | 3.38 ± 1.60 abc | 5.91 ± 0.94 cd |
| Kidney | |||||||
| NC | 0.182 ± 0.02 ab | 8.30 ± 0.65 ab | 2.64 ± 0.38 a | 4.32 ± 0.98 a | 8.57 ± 0.96 a | 1.46 ± 0.18 a | 3.94 ±0.68 ae |
| DC | 0.92 ± 0.05 e | 12.63 ± 1.02 c | 4.01 ± 0.12 b | 9.99 ± 1.07 b | 12.86 ± 0.81 c | 2.13 ± 0.31 d | 7.53 ± 1.41 e |
| Met | 0.42 ± 0.03 cd | 10.04 ± 0.63 a | 2.79 ± 0.36 a | 5.39 ± 0.87 a | 10.32 ± 0.72 b | 1.48 ± 0.20 ab | 5.34 ± 1.26 bde |
| Am | 0.232 ± 0.09 ac | 7.93 ± 0.95 ab | 2.83 ± 0.59 a | 5.61 ± 0.26 a | 7.99 ± 1.69 ab | 1.14 ± 0.11 bc | 6.52 ± 1.18 cde |
| F11 | 0.453 ± 0.09 bd | 7.16 ± 0.85 b | 3.44 ± 0.72 a | 4.75 ± 0.71 a | 7.82 ± 1.24 a | 1.22 ± 0.18 c | 5.45 ± 0.57 d |
| NC | DC | Met | Am | F11 | |
|---|---|---|---|---|---|
| Open Field test | |||||
| Total squares entries | 42.32 ± 5.32 c | 12.21 ± 2.11 a | 29.21 ± 3.21 b | 25.43 ± 2.42 b | 27.43 ± 5.32 b |
| Central squares entries | 19.45 ± 3.23 d | 2.12 ± 0.98 a | 9.43 ± 1.21 b | 5.32 ± 1.05 ab | 4.98 ± 1.43 ab |
| Time spent in central squares (%) | 45.95 ± 4.23 c | 17.36 ± 3.32 a | 32.28 ± 2.79 b | 20.92 ± 2.56 a | 18.15 ± 3.26 a |
| Elevated Plus Maze test | |||||
| Arm entries | 35.60 ± 4.56 d | 3.21 ± 0.45 a | 29.32 ± 2.22 d | 7.75 ± 1.45 b | 12.25 ± 1.67 c |
| Open arms entries | 23.12 ± 3.21 d | 2.50 ± 0.32 a | 21.32 ± 3.17 c | 4.75 ± 0.89 a | 7.75 ± 1.22 b |
| Time spent in open arms (%) | 26.29 ± 5.43 d | 3.34 ± 1.12 a | 16.78 ± 5.69 c | 5.88 ± 3.45 a | 9.43 ± 4.01 b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marmouzi, I.; Karym, E.M.; Saidi, N.; Meddah, B.; Kharbach, M.; Masrar, A.; Bouabdellah, M.; Chabraoui, L.; El Allali, K.; Cherrah, Y.; et al. In Vitro and In Vivo Antioxidant and Anti-Hyperglycemic Activities of Moroccan Oat Cultivars. Antioxidants 2017, 6, 102. https://doi.org/10.3390/antiox6040102
Marmouzi I, Karym EM, Saidi N, Meddah B, Kharbach M, Masrar A, Bouabdellah M, Chabraoui L, El Allali K, Cherrah Y, et al. In Vitro and In Vivo Antioxidant and Anti-Hyperglycemic Activities of Moroccan Oat Cultivars. Antioxidants. 2017; 6(4):102. https://doi.org/10.3390/antiox6040102
Chicago/Turabian StyleMarmouzi, Ilias, El Mostafa Karym, Nezha Saidi, Bouchra Meddah, Mourad Kharbach, Azlarab Masrar, Mounya Bouabdellah, Layachi Chabraoui, Khalid El Allali, Yahia Cherrah, and et al. 2017. "In Vitro and In Vivo Antioxidant and Anti-Hyperglycemic Activities of Moroccan Oat Cultivars" Antioxidants 6, no. 4: 102. https://doi.org/10.3390/antiox6040102





