Vitamin E as an Antioxidant in Female Reproductive Health
Abstract
:1. Vitamin E
1.1. Sources of Vitamin E
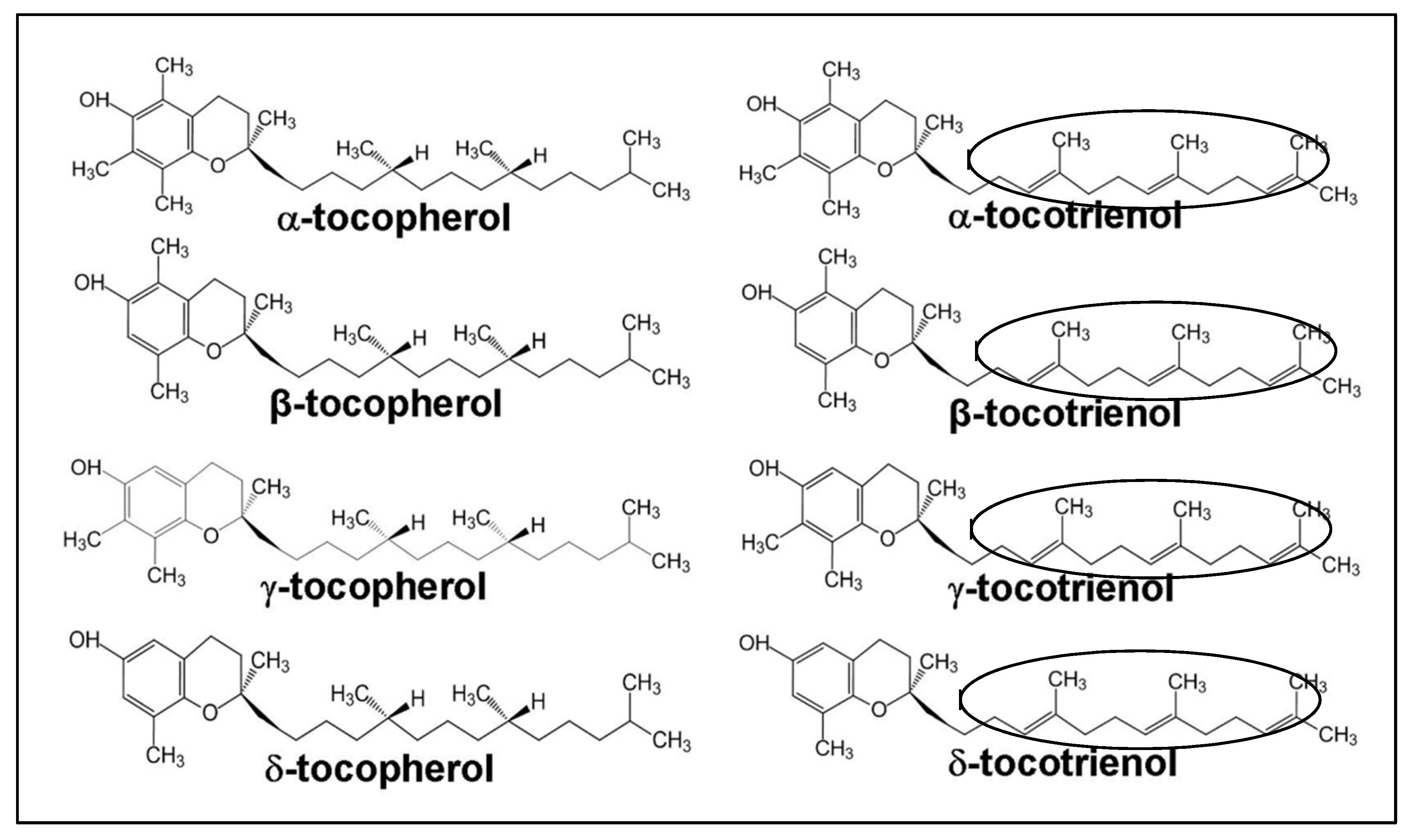
1.2. Structure of Vitamin E
2. Reproductive Disorders: The Risk Factors
Oxidative Stress (OS) as One of the Risk Factors in Reproductive Disorders
3. Antioxidants and Their Roles in Reproductive Disorders
Vitamin E as an Antioxidant in Female Reproduction: The Reported Studies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Evans, H.M.; Bishop, K.S. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 1922, 56, 650–651. [Google Scholar] [CrossRef] [PubMed]
- Tappel, A.L. Vitamin E as the biological lipid antioxidant. Vitam. Horm. 1962, 20, 493–510. [Google Scholar]
- Burton, G.W.; Ingold, K.U. Vitamin E application of the principles of physical organic chemistry to the exploration of its structure and function. Acc. Chem. Res. 1986, 19, 194–201. [Google Scholar] [CrossRef]
- Esterbauer, H.; Dieber-Rotheneder, M.; Striegl, G.; Waeg, G. Role of vitamin E in preventing the oxidation of low density lipoprotein. Am. J. Clin. Nutr. 1991, 53, 314S–321S. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, A.J.; Pennington, J.A.T.; Weihrauch, J.L. Analysis and distribution of vitamin E in vegetable oils and foods. In Vitamin E in Health and Disease; Packer, L., Fuchs, J., Eds.; Marcel Dekker: New York, NY, USA, 1993; pp. 9–31. ISBN 0-8247-8692-0. [Google Scholar]
- Ramaswamy, K.; Subash, C.G.; Ji, H.K.; Bharat, B.A. Tocotrienols fight cancer by targeting multiple cell signaling pathways. Genes Nutr. 2012, 7, 43–52. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kanno, C.; Yamauchi, K.; Tsugo, T. Identification of alpha-, beta-, gamma-, and delta-tocopherols and their contents in human milk. Biochim. Biophys. Acta 1975, 380, 282–290. [Google Scholar] [PubMed]
- Nehdi, I.; Omri, S.; Khalil, M.I.; Al-Resayes, S.I. Characteristics and chemical composition of date palm (Phoenix canariensis) seeds and seed oil. Ind. Crops Prod. 2010, 32, 360–365. [Google Scholar] [CrossRef]
- Tan, B. Vitamin E: Tocotrienols—The Science behind Tocotrienols. Available online: https://assets.kyani.net/documents/us/Tocotrienols_Science_White_Paper-1.12-EN-ALL.pdf (accessed on 14 August 2017).
- Rimbach, G.; Jennifer, M.; Patricia, H.; John, K.L. Gene-Regulatory Activity of α-Tocopherol. Molecules 2010, 15, 1746–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IUPAC-IUB Joint Commission on Biochemical Nomenclature. Nomenclature of tocopherols and related compounds. (Recommendations 1981). Eur. J. Biochem. 1982, 123, 473–475. [Google Scholar]
- Rimbach, G.; Minihane, A.M.; Majewicz, J.; Fischer, A.; Pallauf, J.; Virgli, F.; Weinberg, P.D. Regulation of cell signalling by vitamin E. Proc. Nutr. Soc. 2002, 61, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Pignitter, M.; Stolze, K.; Gartner, S.; Dumhart, B.; Stoll, C.; Steiger, G.; Kraemer, K.; Somoza, V. Cold fluorescent light as major inducer of lipid oxidation in soybean oil stored at household conditions for eight weeks. J. Agric. Food Chem. 2014, 62, 2297–2305. [Google Scholar] [CrossRef] [PubMed]
- The Structure of Vitamin, E. Available online: https://www.omicsonline.org/articles-images/2155-9899-4-137-g001.html (accessed on 23 January 2018).
- Wigle, D.T.; Arbuckle, T.E.; Turner, M.C.; Bérubé, A.; Yang, Q.; Liu, S.; Krewski, D. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J. Toxicol. Environ. Health B Crit. Rev. 2008, 11, 373–517. [Google Scholar] [CrossRef] [PubMed]
- Rider, C.V.; Furr, J.R.; Wilson, V.S.; Gray, L.E., Jr. Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity. J. Androl. 2010, 33, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Al-Gubory, K.H. Environmental pollutants and lifestyle factors induce oxidative stress and poor prenatal development. Reprod. Biomed. Online 2014, 29, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Kortenkamp, A. Ten years of mixing cocktails: A review of combination effects of endocrine-disrupting chemicals. Environ. Health Perspect. 2007, 115 (Suppl. 1), 98–105. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.C.; Liu, J.M.; Fraser, W.D. Large prospective birth cohort studies on environmental contaminants and child health—Goals, challenges, limitations and needs. Med. Hypotheses 2010, 74, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Ruder, E.H.; Hartman, T.J.; Blumberg, J.; Goldman, M.B. Oxidative stress and antioxidants: Exposure and impact on female fertility. Hum. Reprod. Update 2008, 14, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.G.; McCallum, G.P.; Chen, C.S.; Henderson, J.T.; Lee, C.J.; Perstin, J.; Preston, T.J.; Wiley, M.J.; Wong, A.W. Oxidative stress in developmental origins of disease: Teratogenesis, neurodevelopmental deficits, and cancer. Toxicol. Sci. 2009, 108, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol. 2010, 42, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Nisenblat, V.; Norman, R. Lifestyle factors in people seeking infertility treatment—A review. Aust. N. Z. J. Obstet. Gynaecol. 2010, 50, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Biedenharn, K.R.; Fedor, J.M.; Agarwal, A. Lifestyle factors and reproductive health: Taking control of your fertility. Reprod. Biol. Endocrinol. 2013, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, P.; Somanathan, R. Mechanism of teratogenesis: Electron transfer, reactive oxygen species, and antioxidants. Birth Defects Res. C Embryo Today 2006, 78, 308–325. [Google Scholar] [CrossRef] [PubMed]
- Rasch, V. Cigarette, alcohol, and caffeine consumption: Risk factors for spontaneous abortion. Acta Obstet. Gynecol. Scand. 2003, 82, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Odouli, R.; Li, D.K. Maternal caffeine consumption during pregnancy and the risk of miscarriage: A prospective cohort study. Am. J. Obstet. Gynecol. 2008, 198, 279.e1–279.e8. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Junaid, M.A. Lifestyle, pregnancy and epigenetic effects. Epigenomics 2015, 7, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Dechanet, C.; Anahory, T.; Mathieu Daude, J.C.; Quantin, X.; Reyftmann, L.; Hamamah, S.; Hedon, B.; Dechaud, H. Effects of cigarette smoking on reproduction. Hum. Reprod. Update 2011, 17, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Dembele, K.; Yao, X.H.; Chen, L.; Nyomba, B.L. Intrauterine ethanol exposure results in hypothalamic oxidative stress and neuroendocrine alterations in adult rat offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R796–R802. [Google Scholar] [CrossRef] [PubMed]
- Wentzel, P.; Eriksson, U.J. Ethanol-induced fetal dysmorphogenesis in the mouse is diminished by high antioxidative capacity of the mother. Toxicol. Sci. 2006, 92, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Wentzel, P.; Rydberg, U.; Eriksson, U.J. Antioxidative treatment diminishes ethanol-induced congenital malformations in the rat. Alcohol. Clin. Exp. Res. 2006, 30, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, M.M.; van Rooij, I.A.; Miller, R.K.; Zielhuis, G.A.; de Jong-van den Berg, L.T.; Roeleveld, N. Teratogenic mechanisms of medical drugs. Hum. Reprod. Update 2010, 16, 378–394. [Google Scholar] [CrossRef] [PubMed]
- Deavall, D.G.; Martin, E.A.; Horner, J.M.; Roberts, R. Drug-induced oxidative stress and toxicity. J. Toxicol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wells, P.G. In vivo phenytoin-initiated oxidative damage to proteins and lipids in murine maternal hepatic and embryonic tissue organelles: Potential molecular targets of chemical teratogenesis. Toxicol. Appl. Pharmacol. 1994, 125, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.M.; Harris, C. A novel hypothesis for thalidomide-induced limb teratogenesis: Redox misregulation of the NF-kappaB pathway. Antioxid. Redox Signal. 2004, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Defoort, E.N.; Kim, P.M.; Winn, L.M. Valproic acid increases conservative homologous recombination frequency and reactive oxygen species formation: A potential mechanism for valproic acid-induced neural tube defects. Mol. Pharmacol. 2006, 69, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, B.R.; Danielsson, C.; Nilsson, M.F. Embryonic cardiac arrhythmia and generation of reactive oxygen species: Common teratogenic mechanism for IKr blocking drugs. Reprod. Toxicol. 2007, 24, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Borgerding, M.; Klus, H. Analysis of complex mixtures—Cigarette smoke. Exp. Toxicol. Pathol. 2005, 57, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Feltes, B.C.; de Faria Poloni, J.; Notari, D.L.; Bonatto, D. Toxicological effects of the different substances in tobacco smoke on human embryonic development by a systems chemo-biology approach. PLoS ONE 2013, 8, e61743. [Google Scholar] [CrossRef] [PubMed]
- George, L.; Granath, F.; Johansson, A.L.; Anneren, G.; Cnattingius, S. Environmental tobacco smoke and risk of spontaneous abortion. Epidemiology 2006, 17, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Smulian, J.C.; Vintzileos, A.M. Incidence of placental abruption in relation to cigarette smoking and hypertensive disorders during pregnancy: A meta-analysis of observational studies. Obstet. Gynecol. 1999, 93, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Faiz, A.S.; Ananth, C.V. Etiology and risk factors for placenta previa: An overview and meta-analysis of observational studies. J. Matern. Fetal Neonatal Med. 2003, 13, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.H.; Hsieh, C.C.; Hsu, J.J.; Chiu, T.H.; Lo, L.M.; Hsieh, T.T. Risk factors for placenta previa in an Asian population. Int. J. Gynaecol. Obstet. 2007, 97, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Kolas, T.; Nakling, J.; Salvesen, K.A. Smoking during pregnancy increases the risk of preterm births among parous women. Acta Obstet. Gynecol. Scand. 2000, 79, 644–648. [Google Scholar] [PubMed]
- Fantuzzi, G.; Aggazzotti, G.; Righi, E.; Facchinetti, F.; Bertucci, E.; Kanitz, S.; Barbone, F.; Sansebastiano, G.; Battaglia, M.A.; Leoni, V.; et al. Preterm delivery and exposure to active and passive smoking during pregnancy: A case-control study from Italy. Paediatr. Perinat. Epidemiol. 2007, 21, 194–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaddoe, V.W.; Troe, E.J.; Hofman, A.; Mackenbach, J.P.; Moll, H.A.; Steegers, E.A.; Witteman, J.C. Active and passive maternal smoking during pregnancy and the risks of low birthweight and preterm birth: The Generation R Study. Paediatr. Perinat. Epidemiol. 2008, 22, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Wisborg, K.; Kesmodel, U.; Henriksen, T.B.; Olsen, S.F.; Secher, N.J. Exposure to tobacco smoke in utero and the risk of stillbirth and death in the first year of life. Am. J. Epidemiol. 2001, 154, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Hogberg, L.; Cnattingius, S. The influence of maternal smoking habits on the risk of subsequent stillbirth: Is there a causal relation? BJOG 2007, 114, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.A.; Milerad, J. Smoking and the sudden infant death syndrome. Rev. Environ. Health 2006, 21, 81–103. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.M.; Franks, S. Environment, lifestyle and infertility—An inter-generational issue. Nat. Cell Biol. 2002, 4, s33–s40. [Google Scholar] [CrossRef] [PubMed]
- Zenzes, M.T.; Krishnan, S.; Krishnan, B.; Zhang, H.; Casper, R.F. Cadmium accumulation in follicular fluid of women in in vitro fertilization-embryo transfer is higher in smokers. Fertil. Steril. 1995, 64, 599–603. [Google Scholar] [CrossRef]
- Younglai, E.V.; Foster, W.G.; Hughes, E.G.; Trim, K.; Jarrell, J.F. Levels of environmental contaminants in human follicular fluid, serum, and seminal plasma of couples undergoing in vitro fertilization. Arch. Environ. Contam. Toxicol. 2002, 43, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Neal, M.S.; Zhu, J.; Foster, W.G. Quantification of benzo[a]pyrene and other PAHs in the serum and follicular fluid of smokers versus non-smokers. Reprod. Toxicol. 2008, 25, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Van Voorhis, B.J.; Dawson, J.D.; Stovall, D.W.; Sparks, A.E.; Syrop, C.H. The effects of smoking on ovarian function and fertility during assisted reproduction cycles. Obstet. Gynecol. 1996, 88, 785–791. [Google Scholar] [CrossRef]
- Ness, R.B.; Grisso, J.A.; Hirschinger, N.; Markovic, N.; Shaw, L.M.; Day, N.L.; Kline, J. Cocaine and tobacco use and the risk of spontaneous abortion. N. Engl. J. Med. 1999, 340, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Okuka, M.; McLean, M.; Keefe, D.L.; Liu, L. Effects of cigarette smoke on fertilization and embryo development in vivo. Fertil. Steril. 2009, 92, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Reece, E.A. Nicotine-induced embryonic malformations mediated by apoptosis from increasing intracellular calcium and oxidative stress. Birth Defects Res. B Dev. Reprod. Toxicol. 2005, 74, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Holloway, A.C.; Kellenberger, L.D.; Petrik, J.J. Fetal and neonatal exposure to nicotine disrupts ovarian function and fertility in adult female rats. Endocrine 2006, 30, 213–216. [Google Scholar] [CrossRef]
- Mokhtar, N.; Rajikin, M.H.; Zakaria, Z. Role of tocotrienol-rich palm vitamin E on pregnancy and preimplantation embryos in nicotine treated rats. Biomed. Res. 2008, 19, 181–184. [Google Scholar]
- Rajikin, M.H.; Latif, E.S.; Mar, M.R.; Mat Top, A.G.; Mokhtar, N.M. Deleterious effects of nicotine on the ultrastructure of oocytes: Role of gamma-tocotrienol. Med. Sci. Monit. 2009, 15, BR378–BR383. [Google Scholar] [PubMed]
- Asadi, E.; Mehrdad, J.; Mohammad, J.G. Effect of vitamin E on oocytes apoptosis in nicotine-treated mice. Iran. J. Basic Med. Sci. 2012, 15, 880–884. [Google Scholar] [PubMed]
- Kamsani, Y.S.; Rajikin, M.H.; Nor-Ashikin, M.N.K.; Nuraliza, A.S.; Chatterjee, A. Nicotine-induced cessation of embryonic development is reversed by γ-tocotrienol in mice. Med. Sci. Monit. Basic Res. 2013, 19, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Phoebe, C.J.; Julie, A.M.; Emma, L.B.; Philip, M.H.; Keith, T.J. Increased zona pellucida thickness and meiotic spindle disruption in oocytes from cigarette smoking mice. Hum. Reprod. 2011, 26, 878–884. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sekhon, L.; Shah, R. Redox considerations in female reproductive function and assisted reproduction: From molecular mechanisms to health implications. Antioxid. Redox Signal. 2008, 10, 1375–1403. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.D.; Powell, S.R. Inhibition of cellular antioxidants: A possible mechanism of toxic cell injury. Environ. Health Perspect. 1984, 57, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA damage and disease: Induction, repair and significance. Mutat. Res. 2004, 567, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Dennery, P.A. Role of redox in fetal development and neonatal diseases. Antioxid. Redox Signal. 2004, 6, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Wu, M.Y.; Chen, M.J.; Chao, K.H.; Ho, H.N.; Yang, Y.S. Nitric oxide is associated with poor embryo quality and pregnancy outcome in in vitro fertilization cycles. Fertil. Steril. 2004, 82, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Agarwal, A. Role of reactive oxygen species in gynecologic diseases. Reprod. Med. Biol. 2004, 3, 177–199. [Google Scholar] [CrossRef]
- Guerin, P.; El Mouatassim, S.; Menezo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef]
- Jurisicova, A.; Varmuza, S.; Casper, R.F. Programmed cell death and human embryo fragmentation. Mol. Hum. Reprod. 1996, 2, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.W.; Wang, Y. Secretion of lipid peroxides by the human placenta. Am. J. Obstet. Gynecol. 1993, 169, 1462–1466. [Google Scholar] [CrossRef]
- Myatt, L.; Cui, X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Poston, L.; Raijmakers, M.T. Trophoblast oxidative stress, antioxidants and pregnancy outcome—A review. Placenta 2004, 25 (Suppl. A), S72–S78. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Walsh, S.W. Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta 1998, 19, 581–586. [Google Scholar] [CrossRef]
- Jauniaux, E.; Watson, A.L.; Hempstock, J.; Bao, Y.; Skepper, J.N.; Burton, G.J. Onset of maternal arterial blood flow and placental oxidative stress: A possible factor in human early pregnancy failure. Am. J. Pathol. 2000, 157, 2111–2122. [Google Scholar] [CrossRef]
- Parman, T.; Wiley, M.J.; Wells, P.G. Free radical-mediated oxidative DNA damage in the mechanism of thalidomide teratogenicity. Nat. Med. 1999, 5, 582–585. [Google Scholar] [PubMed]
- Burton, G.J.; Hempstock, J.; Jauniaux, E. Oxygen, early embryonic metabolism and free radical-mediated embryopathies. Reprod. Biomed. Online 2003, 6, 84–96. [Google Scholar] [CrossRef]
- Nicol, C.J.; Zielenski, J.; Tsui, L.C.; Wells, P.G. An embryoprotective role for glucose-6-phosphate dehydrogenase in developmental oxidative stress and chemical teratogenesis. FASEB J. 2000, 14, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.A.; Santanam, N.; Parthasarathy, S. Endometriosis: A disease of oxidative stress? Semin. Reprod. Endocrinol. 1998, 16, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Rong, R.; Ramachandran, S.; Santanam, N.; Murphy, A.A.; Parthasarathy, S. Induction of monocyte chemotactic protein-1 in peritoneal mesothelial and endometrial cells by oxidized low-density lipoprotein and peritoneal fluid from women with endometriosis. Fertil. Steril. 2002, 78, 843–848. [Google Scholar] [CrossRef]
- Szczepanska, M.; Kozlik, J.; Skrzypczak, J.; Mikolajczyk, M. Oxidative stress may be a piece in the endometriosis puzzle. Fertil. Steril. 2003, 79, 1288–1293. [Google Scholar] [CrossRef]
- Bedaiwy, M.A.; Falcone, T. Peritoneal fluid environment in endometriosis. Clinicopathological implications. Minerva Ginecol. 2003, 55, 333–345. [Google Scholar] [PubMed]
- Jackson, L.W.; Schisterman, E.F.; Dey-Rao, R.; Browne, R.; Armstrong, D. Oxidative stress and endometriosis. Hum. Reprod. 2005, 20, 2014–2020. [Google Scholar] [CrossRef] [PubMed]
- Mier-Cabrera, M.; Jimenez-Zamudio, L.; Garcia-Latorre, E.; Cruz-Orozco, O.; Hernandez-Guerrero, C. Quantitative and qualitative peritoneal immune profiles, T-cell apoptosis and oxidative stress-associated characteristics in women with minimal and mild endometriosis. BJOG 2011, 118, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Dhaliwal, L.K.; Saha, S.C.; Sangwan, S.; Dhawan, V. Role of 8-iso-prostaglandin F 2alpha and 25-hydroxycholesterol in the pathophysiology of endometriosis. Fertil. Steril. 2010, 94, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Agarwal, A.; Banerjee, J.; Alvarez, J.G. The role of oxidative stress in spontaneous abortion and recurrent pregnancy loss: A systematic review. Obstet. Gynecol. Surv. 2007, 62, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Polak, G.; Rola, R.; Gogacz, M.; Koziol-Montewka, M.; Kotarski, J. Malonyldialdehyde and total antioxidant status in the peritoneal fluid of infertile women. Ginekol. Pol. 1999, 70, 135–140. [Google Scholar] [PubMed]
- Polak, G.; Koziol-Montewka, M.; Tarkowski, R.; Kotarski, J. Peritoneal fluid and plasma 4-hydroxynonenal and malonyldialdehyde concentrations in infertile women. Ginekol. Pol. 2011, 72, 1316–1320. [Google Scholar]
- Agarwal, A.; Gupta, S.; Sikka, S. The role of free radicals and antioxidants in reproduction. Curr. Opin. Obstet. Gynecol. 2006, 18, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. Placental oxidative stress: From miscarriage to preeclampsia. J. Soc. Gynecol. Investig. 2004, 11, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.; Sargent, I.L. Placental stress and pre-eclampsia: A revised view. Placenta 2009, 30 (Suppl. A), S38–S42. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Yung, H.W.; Cindrova-Davies, T.; Charnock-Jones, D.S. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta 2009, 30 (Suppl. A), S43–S48. [Google Scholar] [CrossRef] [PubMed]
- Karowicz-Bilinska, A. Lipid peroxides concentration in women with intrauterine growth restriction. Ginekol. Pol. 2004, 75, 6–9. [Google Scholar] [PubMed]
- Biri, A.; Bozkurt, N.; Turp, A.; Kavutcu, M.; Himmetoglu, O.; Durak, I. Role of oxidative stress in intrauterine growth restriction. Gynecol. Obstet. Investig. 2007, 64, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.C.; Lee, K.H.; Yi, C.H.; Ha, E.H.; Christiani, D.C. Genetic susceptibility of term pregnant women to oxidative damage. Toxicol. Lett. 2002, 129, 255–262. [Google Scholar] [CrossRef]
- Frosali, S.; DiSimplicio, P.; Perrone, S.; DiGiuseppe, D.; Longini, M.; Tanganelli, D.; Buonocore, G. Glutathione recycling and antioxidant enzyme activities in erythrocytes of term and preterm newborns at birth. Neonatology 2004, 85, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.D.; Pathak, R.; Ahmed, T.; Ahmed, R.S.; Tripathi, A.K.; Guleria, K.; Banerjee, B.D. Association of glutathione S-transferase M1 and T1 gene polymorphisms and oxidative stress markers in preterm labor. Clin. Biochem. 2010, 43, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Suke, S.G.; Ahmed, T.; Ahmed, R.S.; Tripathi, A.K.; Guleria, K.; Sharma, C.S.; Makhijani, S.D.; Banerjee, B.D. Organochlorine pesticide residue levels and oxidative stress in preterm delivery cases. Hum. Exp. Toxicol. 2010, 29, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.V. Anti-oxidants in pregnancy Endogenous anti-oxidants in pregnancy and preeclampsia. Aust. N. Z. J. Obstet. Gynaecol. 2006, 46, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Mustacich, D.; Powis, G. Thioredoxin reductase. Biochem. J. 2000, 346, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Chae, H.Z.; Kim, K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 2005, 38, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Gasdaska, J.R.; Berggren, M.; Powis, G. Cell growth stimulation by the redox protein thioredoxin occurs by a novel helper mechanism. Cell Growth Differ. 1995, 6, 1643–1650. [Google Scholar] [PubMed]
- Saitoh, M.; Nishitoh, H.; Fujii, M.; Takeda, K.; Tobiume, K.; Sawada, Y.; Kawabata, M.; Miyazono, K.; Ichijo, H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998, 17, 2596–2606. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, J.; Arner, E.S.J. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Aerts, L.; Van, A.F.A. Taurine and taurine-deficiency in the perinatal period. J. Perinat. Med. 2002, 30, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Uriu-Adams, J.Y.; Keen, C.L. Zinc and reproduction: Effects of zinc deficiency on prenatal and early postnatal development. Birth Defects Res. B. Dev. Reprod. Toxicol. 2010, 89, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Silvia, I.A.; Castañón, S.G.; Ruata, M.L.; Aragüés, E.F.; Terraz, P.B.; Irazabal, Y.G.; González, E.G.; Rodríguez, B.G. Updating of normal levels of copper, zinc and selenium in serum of pregnant women. J. Trace Elem. Med. Biol. 2007, 21 (Suppl. 1), 49–52. [Google Scholar] [CrossRef]
- Hess, S.Y.; King, J.C. Effects of maternal zinc supplementation on pregnancy and lactation outcomes. Food Nutr. Bull. 2009, 30 (Suppl. 1), S60–S78. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Katz, A.; Padayatty, S.J.; Vitamin, C. Modern Nutrition in Health and Disease; Shils, M.E., Shike, M., Ross, A.C., Caballero, B., Cousins, R.J., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; pp. 507–524. [Google Scholar]
- Zhang, C.; Williams, M.A.; King, I.B.; Dashow, E.E.; Sorensen, T.K.; Frederick, I.O.; Thompson, M.L.; Luthy, D.A. Vitamin C and the risk of preeclampsia—Results from dietary questionnaire and plasma assay. Epidemiology 2002, 13, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Henmi, H.; Endo, T.; Kitajima, Y.; Manase, K.; Hata, H.; Kudo, R. Effects of ascorbic acid supplementation on serum progesterone levels in patients with a luteal phase defect. Fertil. Steril. 2003, 80, 459–461. [Google Scholar] [CrossRef]
- Westphal, L.M.; Polan, M.L.; Trant, A.S.; Mooney, S.B. A nutritional supplement for improving fertility in women: A pilot study. J. Reprod. Med. 2004, 49, 289–293. [Google Scholar] [PubMed]
- Rumiris, D.; Purwosunu, Y.; Wibowo, N.; Farina, A.; Sekizawa, A. Lower rate of preeclampsia after antioxidant supplementation in pregnant women with low antioxidant status. Hypertens. Pregnancy 2006, 25, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Jeffrey, A. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Fraser, W.D.; Audibert, F.; Bujold, E.; Leduc, L.; Xu, H.; Boulvain, M.; Julien, P. The Vitamin E debate: Implications for ongoing trials of pre-eclampsia prevention. BJOG 2005, 112, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Parvin, B.; Kobra, H.; Fatemeh, A.; Nazli, N. Effects of vitamin E supplementation on some pregnancy health indices: A randomized clinical trial. Int. J. Gen. Med. 2011, 4, 461–464. [Google Scholar] [CrossRef]
- De Vriese, S.R.; Dhont, M.; Christophe, A.B. Oxidative stability of low density lipoproteins and vitamin E levels increase in maternal blood during normal pregnancy. Lipids 2001, 36, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Chelchowska, M.; Laskowska-Klita, T.; Leibschang, J. The effect of tobacco smoking during pregnancy on concentration of vitamin E in blood of mothers and their newborns in umbilical cord blood. Ginekol. Pol. 2006, 77, 263–268. [Google Scholar] [PubMed]
- Von Mandach, U.; Huch, R.; Huch, A. Maternal and cord serum vitamin E levels in normal and abnormal pregnancy. Int. J. Vitam. Nutr. Res. 1994, 64, 26–32. [Google Scholar] [PubMed]
- Tamura, T.; Goldenberg, R.L.; Johnston, K.E.; Cliver, S.P.; Hoffman, H.J. Serum concentrations of zinc, folate, vitamins A and E, and proteins, and their relationships to pregnancy outcome. Acta Obstet. Gynecol. Scand. Suppl. 1997, 165, 63–70. [Google Scholar] [PubMed]
- Cicek, N.; Eryilmaz, O.G.; Sarikaya, E.; Gulerman, C.; Genc, Y. Vitamin E effect on controlled ovarian stimulation of unexplained infertile women. J. Assist. Reprod. Genet. 2012, 29, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Traber, G.M. Vitamin E Inadequacy in Humans: Causes and Consequences. Adv. Nutr. 2014, 5, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Barrie, M.M. Vitamin E deficiency in rats: Fertility in the female. Biochem. J. 1938, 32, 2134–2137. [Google Scholar] [CrossRef] [PubMed]
- Simsek, M.; Naziroglu, M.; Simsek, H.; Cay, M.; Aksakal, M.; Kumru, S. Blood plasma levels of lipoperoxides, glutathione peroxidase, beta carotene, vitamin A and E in women with habitual abortion. Cell Biochem. Funct. 1998, 16, 227–231. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Abd el-Maksoud, A.; Nassar, M.F. Nutritional stunting in Egypt: Which nutrient is responsible? East. Mediterr. Health J. 2002, 8, 272–280. [Google Scholar] [PubMed]
- Fares, S.; Sethom, M.M.; Khouaja-Mokrani, C.; Jabnoun, S.; Feki, M.; Kaabachi, N. Vitamin A, E, and D deficiencies in Tunisian very low birth weight neonates: Prevalence and risk factors. Pediatr. Neonatol. 2014, 55, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.; Omar, E.; Froemming, G.; Said, R. Tocotrienol rich fraction supplementation confers protection on the ovary from cyclophasphamide induced apoptosis. Asian Pac. J. Trop. Dis. 2014, 4, 234. [Google Scholar] [CrossRef]
- Saleh, H.; Omar, E.; Froemming, G.; Said, R. Tocotrienol preserves ovarian function in cyclophosphamide therapy. Hum. Exp. Toxicol. 2015, 34, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Nasibah, A.; Rajikin, M.H.; Nor-Ashikin, M.N.K.; Nuraliza, A.S. Tocotrienol improves the quality of impaired mouse embryos induced by corticosterone. In Proceedings of the Symposium on Humanities, Science and Engineering Research (SHUSER2012), Kuala Lumpur, Malaysia, 24–27 June 2012; pp. 135–138. [Google Scholar]
- Lee, E.; Min, S.-H.; Song, B.-S.; Yeon, J.-Y.; Kim, J.-W.; Bae, J.-H.; Park, S.-Y.; Lee, Y.-H.; Kim, S.-U.; Lee, D.-S.; et al. Exogenous γ-tocotrienol promotes preimplantation development and improves the quality of porcine embryos. Reprod. Fertil. Dev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Paumgartten, F.J.R.; De-Carvalho, R.R.; Araujo, I.B.; Pinto, F.M.; Borges, O.O.; Souza, C.A.M.; Kuriyama, S.M. Evaluation of the developmental toxicity of annatto in the rat. Food Chem. Toxicol. 2002, 40, 1595–1601. [Google Scholar] [CrossRef]
- Syairah, S.M.M.; Rajikin, M.H.; Sharaniza, A.-R.; Nor-Ashikin, M.N.K.; Anne, T.; Barrie, T. Annatto (Bixa orellana) derived δ-tocotrienol supplementation suppresses PIK3CA oncogene expression in 2- and 4-cell embryos of nicotine-induced mice. Anticancer Res. 2014, 34, 6064. [Google Scholar]
- Syairah, S.M.M.; Rajikin, M.H.; Sharaniza, A.-R. Supplementation of annatto (Bixa orellana)-derived δ-tocotrienol produced high number of morula through increased expression of 3-phosphoinositide dependent protein kinase-1 (PDK1) in mice. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2015, 9, 741–745. [Google Scholar]
- Syairah, S.M.M.; Rajikin, M.H.; Sharaniza, A.R.; Nor-Ashikin, N.K.; Kamsani, Y.S. Chromosomal status in murine preimplantation 2-cell embryos following annatto (Bixa orellana)-derived pure delta-tocotrienol supplementation in normal and nicotine-treated mice. WASJ 2016, 34, 1855–1859. [Google Scholar] [CrossRef]
- Syairah, S.M.M.; Rajikin, M.H.; Sharaniza, A.R.; Nor-Ashikin, M.N.K. Annatto (Bixa orellana) δ-TCT supplementation protected against embryonic DNA damages through alterations in PI3K/Akt-Cyclin D1 pathway. Int. J. Vitam. Nutr. Res. 2017. accepted. [Google Scholar]
- Sugahara, R.; Sato, A.; Uchida, A.; Shiozawa, S.; Sato, C.; Virgona, N.; Yano, T. Annatto tocotrienol induces a cytotoxic effect on human prostate cancer PC3 cells via the simultaneous inhibition of Src and Stat3. J. Nutr. Sci. Vitaminol. 2015, 61, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.E.; Seidel, G.E., Jr. Culture of in vitro-produced bovine embryos with vitamin E improves development in vitro and after transfer to recipients. Biol. Reprod. 2000, 62, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Suzuki, K.; Yoneda, A.; Watanabe, T. Effects of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology 2004, 62, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarajan, B.; Valivittan, K. Ameliorating effect of vitamin E on in vitro development of preimplantation buffalo embryos. J. Assist. Reprod. Genet. 2009, 26, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Shankar, M.B.; Munuswamy, D. Effect of α-tocopherol supplementation on in vitro maturation of sheep oocytes and in vitro development of preimplantation sheep embryos to the blastocyst stage. J. Assist. Reprod. Genet. 2010, 27, 483–490. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Mutalip, S.S.; Ab-Rahim, S.; Rajikin, M.H. Vitamin E as an Antioxidant in Female Reproductive Health. Antioxidants 2018, 7, 22. https://doi.org/10.3390/antiox7020022
Mohd Mutalip SS, Ab-Rahim S, Rajikin MH. Vitamin E as an Antioxidant in Female Reproductive Health. Antioxidants. 2018; 7(2):22. https://doi.org/10.3390/antiox7020022
Chicago/Turabian StyleMohd Mutalip, Siti Syairah, Sharaniza Ab-Rahim, and Mohd Hamim Rajikin. 2018. "Vitamin E as an Antioxidant in Female Reproductive Health" Antioxidants 7, no. 2: 22. https://doi.org/10.3390/antiox7020022




