Antioxidant Tocols as Radiation Countermeasures (Challenges to be Addressed to Use Tocols as Radiation Countermeasures in Humans)
Abstract
:1. Introduction
2. Radiation Induced Injuries
3. Radiation Countermeasures
3.1. Tocopherols and Tocopherol Succinate
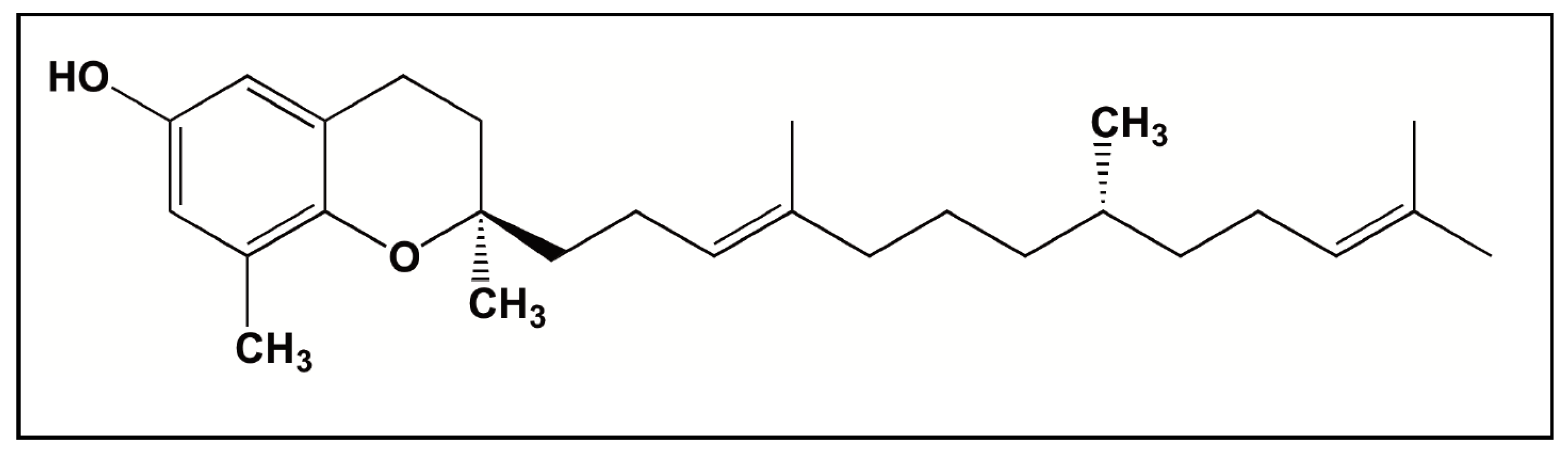
3.2. Tocotrienols
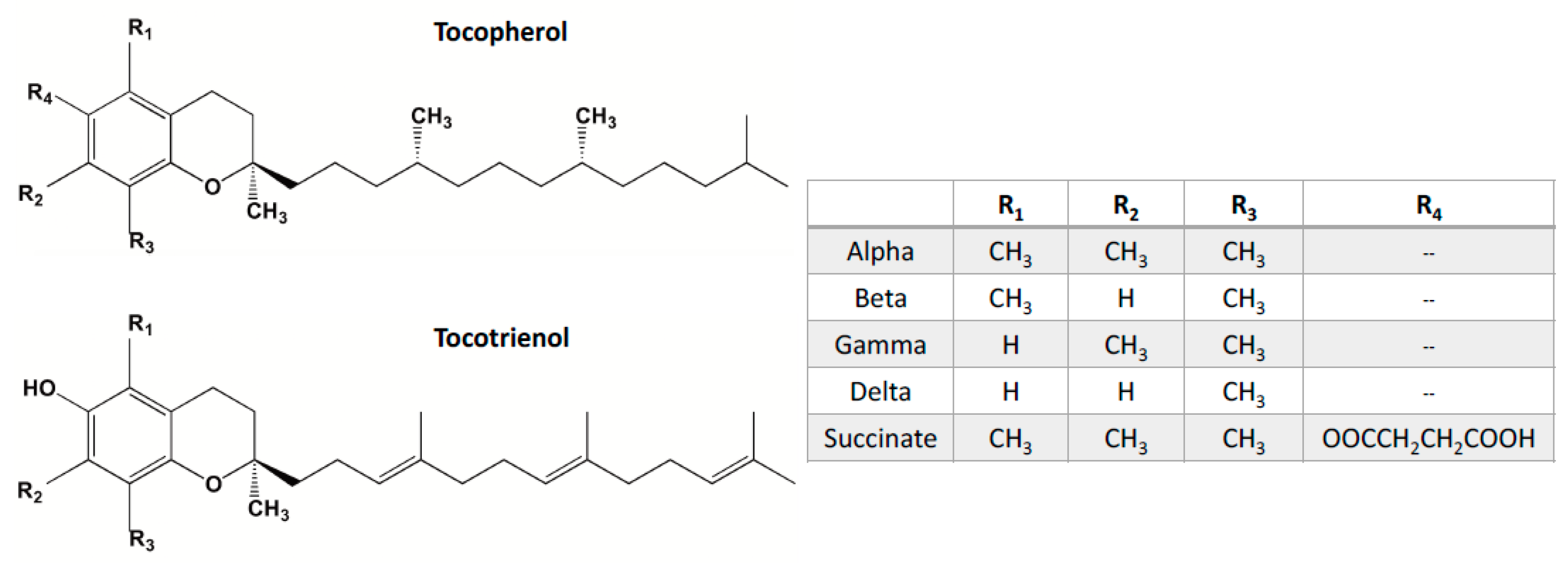
4. Vitamin E Tocols
4.1. α-Tocopherol Transfer Protein
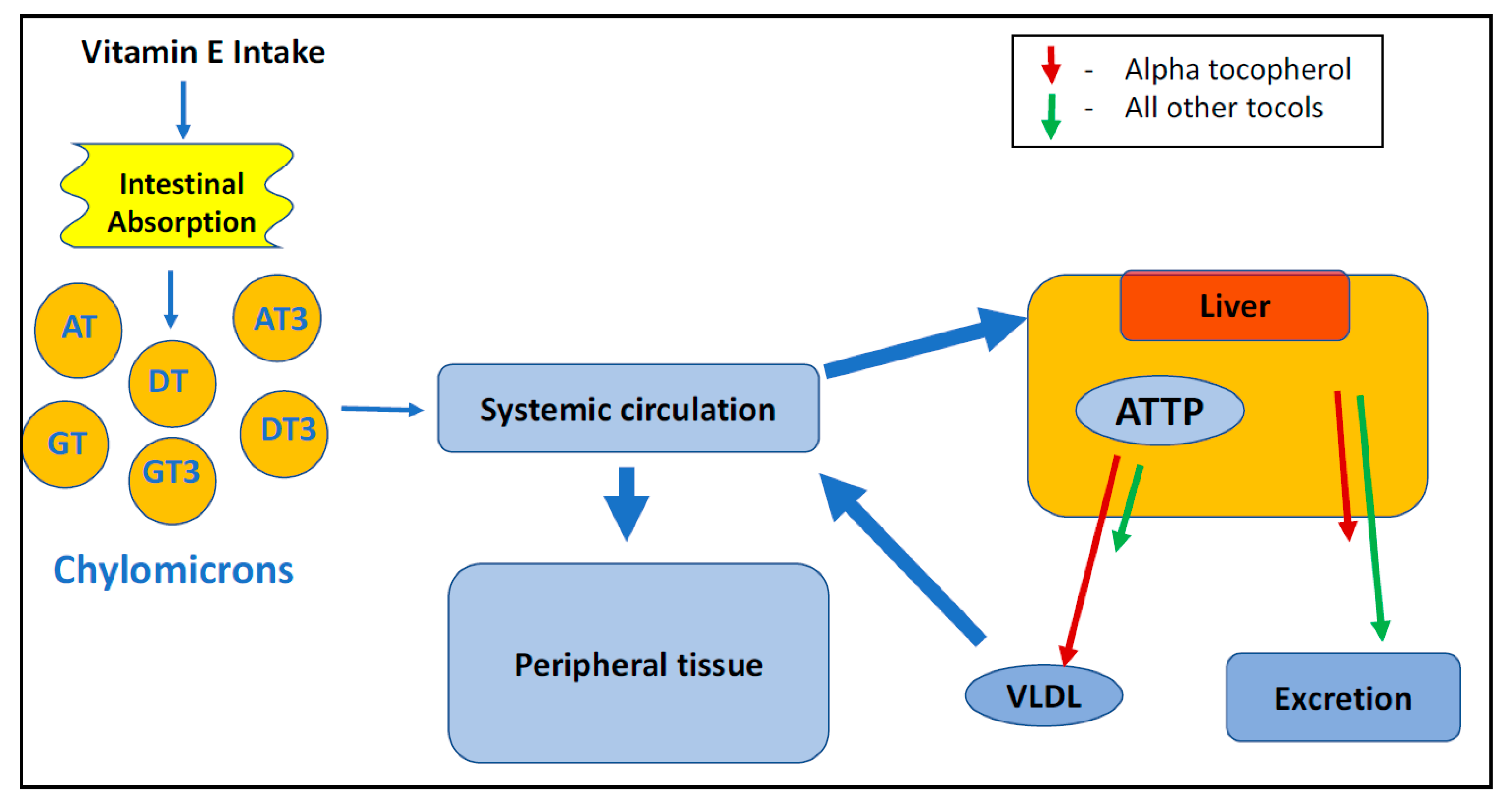
4.2. Absorption and Distribution
5. Conclusions and Future Directions
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Singh, P.K.; Krishnan, S. Vitamin E analogs as radiation response modifiers. Evid.-Based Complement. Altern. Med. 2015, 2015, 741301. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.; Ouattara, B. Combined industrial processes with irradiation to assure innocuity and preservation of food products—A review. Food Res. Int. 2000, 33, 719–724. [Google Scholar] [CrossRef]
- Pereira, E.; Antonio, A.L.; Barreira, J.C.; Barros, L.; Bento, A.; Ferreira, I.C. Gamma irradiation as a practical alternative to preserve the chemical and bioactive wholesomeness of widely used aromatic plants. Food Res. Int. 2015, 67, 338–348. [Google Scholar] [CrossRef]
- Kobashigawa, S.; Kashino, G.; Suzuki, K.; Yamashita, S.; Mori, H. Ionizing radiation-induced cell death is partly caused by increase of mitochondrial reactive oxygen species in normal human fibroblast cells. Radiat. Res. 2015, 183, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chai, Y.C.; Mazumder, S.; Jiang, C.; Macklis, R.M.; Chisolm, G.M.; Almasan, A. The late increase in intracellular free radical oxygen species during apoptosis is associated with cytochrome c release, caspase activation, and mitochondrial dysfunction. Cell Death Differ. 2003, 10, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Beattie, L.A.; Seed, T.M. Vitamin E: Tocopherols and tocotrienols as potential radiation countermeasures. J. Radiat. Res. 2013, 54, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Shafran, R.L.; Jackson, W.E.; Seed, T.M.; Kumar, K.S. Induction of cytokines by radio protective tocopherol analogs. Exp. Mol. Pathol. 2006, 81, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Wise, S.Y.; Ducey, E.J.; Brown, D.S.; Singh, V.K. Radioprotective efficacy of tocopherol succinate is mediated through granulocyte-colony stimulating factor. Cytokine 2011, 56, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Singh, P.K.; Wise, S.Y.; Posarac, A.; Fatanmi, O.O. Radioprotective properties of tocopherol succinate against ionizing radiation in mice. J. Radiat. Res. 2012, 54, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Chitra, S.; Devi, C.S. Effect of alpha-tocopherol on pro-oxidant and antioxidant enzyme status in radiation-treated oral squamous cell carcinoma. Indian J. Med. Sci. 2008, 62, 141. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.C.; Ramos-Perez, F.M.; Perez, D.E.; Novaes, P.D.; Boscolo, F.N.; Almeida, S.M. Radioprotective effect of vitamin E in parotid glands: A morphometric analysis in rats. Braz. Dent. J. 2013, 24, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Pouget, J.P.; Voisin, P. Modulation of DNA damage by pentoxifylline and α-tocopherol in skin fibroblasts exposed to gamma rays. Radiat. Res. 2005, 164, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.R.; Fleck, J.F.; Diehl, A.; Barletta, D.; Braga-Filho, A.; Barletta, A.; Ilha, L. Protective effect of alpha-tocopherol in head and neck cancer radiation-induced mucositis: A double-blind randomized trial. Head Neck 2004, 26, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, V.; Tripathi, P.; Sharma, S.; Corry, P.M.; Moros, E.G.; Singh, A.; Compadre, C.M.; Hauer-Jensen, M.; Boerma, M. Effects of late administration of pentoxifylline and tocotrienols in an image-guided rat model of localized heart irradiation. PLoS ONE 2013, 8, e68762. [Google Scholar] [CrossRef] [PubMed]
- Misirlioglu, C.H.; Demirkasimoglu, T.; Kucukplakci, B.; Sanri, E.; Altundag, K. Pentoxifylline and alpha-tocopherol in prevention of radiation-induced lung toxicity in patients with lung cancer. Med. Oncol. 2007, 24, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control. Acute Radiation Syndrome: A Brochure for Physicians. U.S. Department of Health and Human Services. Available online: https://emergency.cdc.gov/radiation/pdf/ars.pdf (accessed on 5 January 2018).
- Singh, V.K.; Ducey, E.J.; Brown, D.S.; Whitnall, M.H. A review of radiation countermeasure works ongoing at the Armed Forces Radiobiology Research Institute. Int. J. Radiat. Boil. 2012, 88, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.F.; Landauer, M.R. History and development of radiation-protective agents. Int. J. Radiat. Boil. 2009, 85, 539–573. [Google Scholar] [CrossRef] [PubMed]
- Dumont, F.; Roux, A.L.; Bischoff, P. Radiation countermeasure agents: An update. Expert Opin. Ther. Pat. 2010, 20, 73–101. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.F.; Landauer, M.R. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology 2003, 189, 1–20. [Google Scholar] [CrossRef]
- Agarwal, S.S.; Singh, V.K. Immunomodulators: A review of studies on Indian medicinal plants and synthetic peptides. Part I: Medicinal plants. Proc. Ind. Nat. Sci. Acad. B 1999, 65, 179–204. [Google Scholar]
- Satyamitra, M.M.; Kulkarni, S.; Ghosh, S.P.; Mullaney, C.P.; Condliffe, D.; Srinivasan, V. Hematopoietic recovery and amelioration of radiation-induced lethality by the vitamin E isoform δ-tocotrienol. Radiat. Res. 2011, 175, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.P.; Kulkarni, S.; Hieber, K.; Toles, R.; Romanyukha, L.; Kao, T.C.; Hauer-Jensen, M.; Kumar, K.S. Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector. Int. J. Radiat. Boil. 2009, 85, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Compadre, C.M.; Singh, A.; Thakkar, S.; Zheng, G.; Breen, P.J.; Ghosh, S.; Kiaei, M.; Boerma, M.; Varughese, K.I.; Hauer-Jensen, M. Molecular dynamics guided design of tocoflexol: A new radio protectant tocotrienol with enhanced bioavailability. Drug Dev. Res. 2014, 75, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Felemovicius, I.; Bonsack, M.E.; Baptista, M.L.; Delaney, J.P. Intestinal radioprotection by vitamin E (alpha-tocopherol). Ann. Surg. 1995, 222, 504. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, W.; Yunoki, R.; Yoshimura, H. Intestinal epithelial cells absorb γ-tocotrienol faster than α-tocopherol. Lipids 2007, 42, 163. [Google Scholar] [CrossRef] [PubMed]
- Serbinova, E.; Kagan, V.; Han, D.; Packer, L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic. Biol. Med. 1991, 10, 263–275. [Google Scholar] [CrossRef]
- Pearce, B.C.; Parker, R.A.; Deason, M.E.; Qureshi, A.A.; Wright, J.K. Hypocholesterolemic activity of synthetic and natural tocotrienols. J. Med. Chem. 1992, 35, 3595–3606. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Pearce, B.C.; Nor, R.M.; Gapor, A. Dietary alpha-tocopherol attenuates the impact of gamma-tocotrienol on hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in chickens. J. Nutr. 1996, 126, 389. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Weiss, J.F. Radioprotection by vitamin E: Injectable vitamin E administered alone or with WR-3689 enhances survival of irradiated mice. Int. J. Radiat. Oncol. Biol. Phys. 1992, 23, 841–845. [Google Scholar] [CrossRef]
- Sarma, L.; Kesavan, P.C. Protective effects of vitamins C and E against γ-ray-induced chromosomal damage in mouse. Int. J. Radiat. Boil. 1993, 63, 759–764. [Google Scholar] [CrossRef]
- Kumar, K.S. Nutritional approaches to radioprotection: Vitamin, E. Mil. Med. 2002, 167, 57. [Google Scholar] [PubMed]
- Boerma, M.; Roberto, K.A.; Hauer-Jensen, M. Prevention and treatment of functional and structural radiation injury in the rat heart by pentoxifylline and alpha-tocopherol. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Empey, L.R.; Papp, J.D.; Jewell, L.D.; Fedorak, R.N. Mucosal protective effects of vitamin E and misoprostol during acute radiation-induced enteritis in rats. Dig. Dis. Sci. 1992, 37, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.M.; Petrella, M.; Shateri, H. Effects of administering tocopherol after irradiation on survival and proliferation of murine lymphocytes. Pharmacol. Ther. 1988, 39, 393–395. [Google Scholar] [CrossRef]
- Singh, V.K.; Brown, D.S.; Kao, T.C. Tocopherol succinate: A promising radiation countermeasure. Int. Immunopharmacol. 2009, 9, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Wise, S.Y.; Ducey, E.J.; Fatanmi, O.O.; Elliott, T.B.; Singh, V.K. α-Tocopherol succinate protects mice against radiation-induced gastrointestinal injury. Radiat. Res. 2011, 177, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Parekh, V.I.; Brown, D.S.; Kao, T.C.; Mog, S.R. Tocopherol succinate: Modulation of antioxidant enzymes and oncogene expression, and hematopoietic recovery. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Newman, V.L.; Romaine, P.L.; Wise, S.Y.; Seed, T.M. Radiation countermeasure agents: An update (2011–2014). Expert Opin. Ther. Pat. 2014, 24, 1229–1255. [Google Scholar] [CrossRef] [PubMed]
- Kamal-Eldin, A.; Appelqvist, L.Å. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef] [PubMed]
- Pearce, B.C.; Parker, R.A.; Deason, M.E.; Dischino, D.D.; Gillespie, E.; Qureshi, A.A.; Wright, J.K.; Volk, K. Inhibitors of cholesterol biosynthesis. 2. hypocholesterolemic and antioxidant activities of benzopyran and tetrahydronaphthalene analogs of the tocotrienols. J. Med. Chem. 1994, 37, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Wise, S.Y.; Scott, J.R.; Romaine, P.L.; Newman, V.L.; Fatanmi, O.O. Radioprotective efficacy of delta-tocotrienol, a vitamin E isoform, is mediated through granulocyte colony-stimulating factor. Life Sci. 2014, 98, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Ghosh, S.P.; Satyamitra, M.; Mog, S.; Hieber, K.; Romanyukha, L.; Gambles, K.; Toles, R.; Kao, T.C.; Hauer-Jensen, M.; Kumar, K.S. Gamma-tocotrienol protects hematopoietic stem and progenitor cells in mice after total-body irradiation. Radiat. Res. 2010, 173, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Kulkarni, S.; Fatanmi, O.O.; Wise, S.Y.; Newman, V.L.; Romaine, P.L.; Hendrickson, H.; Gulani, J.; Ghosh, S.P.; Kumar, K.S.; et al. Radio protective efficacy of gamma-tocotrienol in nonhuman primates. Radiat. Res. 2016, 185, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Fu, D.; Latif, N.H.; Mullaney, C.P.; Ney, P.H.; Mog, S.R.; Whitnall, M.H.; Srinivasan, V.; Xiao, M. δ-tocotrienol protects mouse and human hematopoietic progenitors from γ-irradiation through extracellular signal-regulated kinase/mammalian target of rapamycin signaling. Haematologica 2010, 95, 1996–2004. [Google Scholar] [CrossRef] [PubMed]
- Loose, D.S.; Kumar, K.S.; Berbée, M.; Boerma, M.; Hauer-Jensen, M.; Fu, Q. Mechanisms underlying the radioprotective properties of γ-tocotrienol: Comparative gene expression profiling in tocol-treated endothelial cells. Genes Nutr. 2012, 7, 75. [Google Scholar]
- Kulkarni, S.; Singh, P.K.; Ghosh, S.P.; Posarac, A.; Singh, V.K. Granulocyte colony-stimulating factor antibody abrogates radioprotective efficacy of gamma-tocotrienol, a promising radiation countermeasure. Cytokine 2013, 62, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Aggarwal, A.K.; Shukla, D.K.; Thimothy, G.; Rani, S. To study and evaluation of role of gamma and delta tocotrienol in radiation induced fibrosis. Pharma Innov. 2017, 6 Pt B, 91. [Google Scholar]
- Yang, C.S.; Lee, M.J.; Zhao, Y.; Yang, Z. Metabolism of tocotrienols in animals and synergistic inhibitory actions of tocotrienols with atorvastatin in cancer cells. Genes Nutr. 2012, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Shimozawa, M.; Kuroda, M.; Nakabe, N.; Manabe, H.; Katada, K.; Kokura, S.; Ichikawa, H.; Yoshida, N.; Noguchi, N.; et al. Tocotrienols reduce 25-hydroxycholesterol-induced monocyte-endothelial cell interaction by inhibiting the surface expression of adhesion molecules. Atherosclerosis 2005, 180, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Berbee, M.; Fu, Q.; Boerma, M.; Pathak, R.; Zhou, D.; Kumar, K.S.; Hauer-Jensen, M. Reduction of radiation-induced vascular nitrosative stress by the vitamin E analog γ-tocotrienol: Evidence of a role for tetrahydrobiopterin. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Ma, X.; Li, X.; Wang, X.; Mei, Q.; Li, X.; Wu, Z.; Han, W. The accomplices of NF-κB lead to radioresistance. Curr. Protein Pept. Sci. 2015, 16, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Park, N.Y.; Jang, Y.; Ma, A.; Jiang, Q. Vitamin E γ-tocotrienol inhibits cytokine-stimulated NF-κB activation by induction of anti-inflammatory A20 via stress adaptive response due to modulation of sphingolipids. J. Immunol. 2015, 195, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Berbée, M.; Fu, Q.; Boerma, M.; Wang, J.; Kumar, K.S.; Hauer-Jensen, M. γ-Tocotrienol ameliorates intestinal radiation injury and reduces vascular oxidative stress after total-body irradiation by an HMG-CoA Reductase-dependent mechanism. Radiat. Res. 2009, 171, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Bjørneboe, A.; Bjørneboe, G.E.; Drevon, C.A. Absorption, transport and distribution of vitamin, E. J. Nutr. 1990, 120, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.P.; Yuen, K.H.; Wong, J.W. Pharmacokinetics and bioavailability of α-, γ-and δ-tocotrienols under different food status. J. Pharm. Pharmacol. 2001, 53, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.P.; Yuen, K.H.; Lim, A.B. Influence of route of administration on the absorption and disposition of α, γ-and δ-tocotrienols in rats. J. Pharm. Pharmacol. 2003, 55, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Abuasal, B.; Sylvester, P.W.; Kaddoumi, A. Intestinal absorption of γ-tocotrienol is mediated by Niemann-Pick C1-Like 1: In situ rat intestinal perfusion studies. Drug Metab. Dispos. 2010, 38, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Ouahchi, K.; Arita, M.; Kayden, H.; Hentati, F.; Hamida, M.B.; Sokol, R.; Arai, H.; Inoue, K.; Mandel, J.L.; Koenig, M. Ataxia with isolated vitamin E deficiency is caused by mutations in the α–tocopherol transfer protein. Nat. Genet. 1995, 9, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Tomizaki, T.; Schulze-Briese, C.; Baumann, U.; Stocker, A. The molecular basis of vitamin E retention: Structure of human α-tocopherol transfer protein. J. Mol. Biol. 2003, 331, 725–734. [Google Scholar] [CrossRef]
- Traber, M.G. Vitamin E bioavailability. In The Encyclopedia of Vitamin E; Preedy, V.R., Watson, R.R., Eds.; CABI: Oxon, UK, 2007; pp. 221–230. [Google Scholar]
- Liu, X.; Gujarathi, S.; Zhang, X.; Shao, L.; Boerma, M.; Compadre, C.M.; Crooks, P.A.; Hauer-Jensen, M.; Zhou, D.; Zheng, G. Synthesis of (2R,8′S,3′E)-δ-tocodienol, a tocoflexol family member designed to have a superior pharmacokinetic profile compared to δ-tocotrienol. Tetrahedron 2106, 72, 4001–4006. [Google Scholar] [CrossRef] [PubMed]
- Stocker, A.; Azzi, A. Tocopherol-binding proteins: Their function and physiological significance. Antioxid. Redox Signal. 2000, 2, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Ramakrishnan, R.; Kayden, H.J. Human plasma vitamin E kinetics demonstrate rapid recycling of plasma RRR-alpha-tocopherol. Proc. Natl. Acad. Sci. USA. 1994, 91, 10005–10008. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Breen, P.J.; Ghosh, S.; Kumar, S.K.; Varughese, K.I.; Crooks, P.A.; Hauer-Jensen, M.; Compadre, C.M. Structural modification of tocotrienols to improve bioavailability. In Tocotrienols: Vitamin E Beyond Tocopherols, 2nd ed.; Tan, B., Watson, R.R., Preedy, V.R., Eds.; American Oil Chemists Society and Taylor & Francis: Urbana, IL, USA, 2012; pp. 359–370. [Google Scholar]
- Kawakami, Y.; Tsuzuki, T.; Nakagawa, K.; Miyazawa, T. Distribution of tocotrienols in rats fed a rice bran tocotrienol concentrate. Biosci. Biotechnol. Biochem. 2007, 71, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Hayes, K.C.; Pronczuk, A.; Liang, J.S. Differences in the plasma transport and tissue concentrations of tocopherols and tocotrienols: Observations in humans and hamsters. Proc. Soc. Exp. Biol. Med. 1993, 202, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Toyoshima, K.; Yamashita, K. Dietary sesame seeds elevate α-and γ-tocotrienol concentrations in skin and adipose tissue of rats fed the tocotrienol-rich fraction extracted from palm oil. J. Nutr. 2001, 131, 2892–2897. [Google Scholar] [CrossRef] [PubMed]
- Okabe, Y.; Watanabe, A.; Shingu, H.; Kushibiki, S.; Hodate, K.; Ishida, M.; Ikeda, S.; Takeda, T. Effects of α-tocopherol level in raw venison on lipid oxidation and volatiles during storage. Meat Sci. 2002, 62, 457–462. [Google Scholar] [CrossRef]
- Min, K.C.; Kovall, R.A.; Hendrickson, W.A. Crystal structure of human α-tocopherol transfer protein bound to its ligand: Implications for ataxia with vitamin E deficiency. Proc. Natl. Acad. Sci. USA 2003, 100, 14713–14718. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E.; Klein, A.; Bietrix, F.; Gleize, B.; Malezet-Desmoulins, C.; Schneider, M.; Margotat, A.; Lagrost, L.; Collet, X.; Borel, P. Scavenger receptor class B type I (SR-BI) is involved in vitamin E transport across the enterocyte. J. Biol. Chem. 2006, 281, 4739–4745. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, N.; Wakao, Y.; Tomono, S.; Tatemichi, M.; Yano, T.; Ohshima, H. The enhancement of the oral bioavailability of γ-tocotrienol in mice by γ-cyclodextrin inclusion. J. Nutr. Biochem. 2011, 22, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.P.; Yuen, K.H. Influence of lipolysis and droplet size on tocotrienol absorption from self-emulsifying formulations. Int. J. Pharm. 2004, 281, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Fairus, S.; Nor, R.M.; Cheng, H.M.; Sundram, K. Postprandial metabolic fate of tocotrienol-rich vitamin E differs significantly from that of α-tocopherol. Am. J. Clin. Nutr. 2006, 84, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Fairus, S.; Nor, R.M.; Cheng, H.M.; Sundram, K. Alpha-tocotrienol is the most abundant tocotrienol isomer circulated in plasma and lipoproteins after postprandial tocotrienol-rich vitamin E supplementation. Nutr. J. 2012, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Khan, D.A.; Saleem, S.; Silswal, N.; Trias, A.M. Pharmacokinetics and bioavailability of annatto δ-tocotrienol in healthy fed subjects. J. Clin. Exp. Cardiol. 2015, 6. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Khan, D.A.; Silswal, N.; Saleem, S.; Qureshi, N. Evaluation of Pharmacokinetics, and Bioavailability of Higher Doses of Tocotrienols in Healthy Fed Humans. J. Clin. Exp. Cardiol. 2016, 7, 434. [Google Scholar] [CrossRef] [PubMed]
- Springett, G.M.; Husain, K.; Neuger, A.; Centeno, B.; Chen, D.T.; Hutchinson, T.Z.; Lush, R.M.; Sebti, S.; Malafa, M.P. A Phase I Safety, Pharmacokinetic, and Pharmacodynamic Presurgical Trial of Vitamin E δ-tocotrienol in Patients with Pancreatic Ductal Neoplasia. eBioMedicine 2015, 2, 1987–1995. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.Y.; Che, H.L.; Tan, D.M.; Teng, K.T. Bioavailability of tocotrienols: Evidence in human studies. Nutr. Metab. 2014, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Wahlqvist, M.L.; Krivokuca-Bogetic, Z.; Lo, C.S.; Hage, B.; Smith, R.; Lukito, W. Differential serum responses of tocopherols and tocotrienols during vitamin supplementation in hypercholesterolaemic individuals without change in coronary risk factors. Nutr. Res. 1992, 12, S181–S201. [Google Scholar] [CrossRef]
- Rasool, A.H.; Yuen, K.H.; Yusoff, K.; Wong, A.R.; Rahman, A.R. Dose dependent elevation of plasma tocotrienol levels and its effect on arterial compliance, plasma total antioxidant status, and lipid profile in healthy humans supplemented with tocotrienol rich vitamin, E. J. Nutr. Sci. Vitaminol. 2006, 52, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Rasool, A.H.; Rahman, A.R.; Yuen, K.H.; Wong, A.R. Arterial compliance and vitamin E blood levels with a self-emulsifying preparation of tocotrienol rich vitamin, E. Arch. Pharm. Res. 2008, 31, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Sami, S.A.; Salser, W.A.; Khan, F.A. Dose-dependent suppression of serum cholesterol by tocotrienol-rich fraction (TRF 25) of rice bran in hypercholesterolemic humans. Atherosclerosis 2002, 161, 199–207. [Google Scholar] [CrossRef]
- Chin, S.F.; Hamid, N.A.; Latiff, A.A.; Zakaria, Z.; Mazlan, M.; Yusof, Y.A.; Karim, A.A.; Ibahim, J.; Hamid, Z.; Ngah, W.Z. Reduction of DNA damage in older healthy adults by Tri E® Tocotrienol supplementation. Nutrition 2008, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.F.; Ibahim, J.; Makpol, S.; Hamid, N.A.; Latiff, A.A.; Zakaria, Z.; Mazlan, M.; Yusof, Y.A.; Karim, A.A.; Ngah, W.Z. Tocotrienol rich fraction supplementation improved lipid profile and oxidative status in healthy older adults: A randomized controlled study. Nutr. Metab. 2011, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Heng, E.C.; Karsani, S.A.; Rahman, M.A.; Hamid, N.A.; Hamid, Z.; Ngah, W.Z. Supplementation with tocotrienol-rich fraction alters the plasma levels of Apolipoprotein AI precursor, Apolipoprotein E precursor, and C-reactive protein precursor from young and old individuals. Eur. J. Nutr. 2013, 52, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Khan, D.A.; Mahjabeen, W.; Qureshi, N. Dose-dependent Modulation of Lipid Parameters, Cytokines and RNA by [delta]-tocotrienol in Hypercholesterolemic Subjects Restricted to AHA Step-1 Diet. Br. J. Med. Med. Res. 2015, 6, 351. [Google Scholar] [CrossRef]
- Khor, H.T.; Chieng, D.Y.; Ong, K.K. Tocotrienols inhibit liver HMG CoA reductase activity in the guinea pig. Nutr. Res. 1995, 15, 537–544. [Google Scholar] [CrossRef]
- Husain, K.; Francois, R.A.; Yamauchi, T.; Perez, M.; Sebti, S.M.; Malafa, M.P. Vitamin E δ-Tocotrienol Augments the Anti-tumor Activity of Gemcitabine and Suppresses Constitutive NF-κB Activation in Pancreatic Cancer. Mol. Cancer Ther. 2011. [Google Scholar] [CrossRef] [PubMed]
- Meganathan, P.; Jabir, R.S.; Fuang, H.G.; Bhoo-Pathy, N.; Choudhury, R.B.; Taib, N.A.; Nesaretnam, K.; Chik, Z. A new formulation of Gamma Delta Tocotrienol has superior bioavailability compared to existing Tocotrienol-Rich Fraction in healthy human subjects. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Drotleff, A.M.; Bohnsack, C.; Schneider, I.; Hahn, A.; Ternes, W. Human oral bioavailability and pharmacokinetics of tocotrienols from tocotrienol-rich (tocopherol-low) barley oil and palm oil formulations. J. Funct. Foods 2014, 7, 150–160. [Google Scholar] [CrossRef]
- Abuasal, B.S.; Qosa, H.; Sylvester, P.W.; Kaddoumi, A. Comparison of the intestinal absorption and bioavailability of γ-tocotrienol and α-tocopherol: In vitro, in situ and in vivo studies. Biopharm. Drug Dispos. 2012, 33, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Abuasal, B.S.; Lucas, C.; Peyton, B.; Alayoubi, A.; Nazzal, S.; Sylvester, P.W.; Kaddoumi, A. Enhancement of intestinal permeability utilizing solid lipid nanoparticles increases γ-tocotrienol oral bioavailability. Lipids 2012, 47, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 2006, 29, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Charman, S.A.; Charman, W.N.; Rogge, M.C.; Wilson, T.D.; Dutko, F.J.; Pouton, C.W. Self-emulsifying drug delivery systems: Formulation and biopharmaceutic evaluation of an investigational lipophilic compound. Pharm. Res. 1992, 9, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Alayoubi, A.; Nazzal, S.; Sylvester, P.W.; Kaddoumi, A. Enhanced solubility and oral bioavailability of γ-Tocotrienol using a self-emulsifying drug delivery system (SEDDS). Lipids 2014, 49, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Alayoubi, A.; Nazzal, S.; Sylvester, P.W.; Kaddoumi, A. Nonlinear absorption kinetics of self-emulsifying drug delivery systems (SEDDS) containing tocotrienols as lipophilic molecules: In vivo and in vitro studies. AAPS J. 2013, 15, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Traber, M.G. Alpha-tocopherol transfer protein (α-TTP): Insights from alpha-tocopherol transfer protein knockout mice. Nutr. Res. Pract. 2007, 1, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Gohil, K.; Schock, B.C.; Chakraborty, A.A.; Terasawa, Y.; Raber, J.; Farese, R.V.; Packer, L.; Cross, C.E.; Traber, M.G. Gene expression profile of oxidant stress and neurodegeneration in transgenic mice deficient in α-tocopherol transfer protein. Free Radic. Biol. Med. 2003, 35, 1343–1354. [Google Scholar] [CrossRef]
- Jishage, K.I.; Arita, M.; Igarashi, K.; Iwata, T.; Watanabe, M.; Ogawa, M.; Ueda, O.; Kamada, N.; Inoue, K.; Arai, H.; Suzuki, H. α-Tocopherol transfer protein is important for the normal development of placental labyrinthine trophoblasts in mice. J. Biol. Chem. 2001, 276, 1669–1672. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.W.; Terasawa, Y.; Farese, R.V.; Traber, M.G. Incorporation of deuterated RRR-or all-rac-α-tocopherol in plasma and tissues of α-tocopherol transfer protein–null mice. Am. J. Clin. Nutr. 2002, 75, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Schock, B.C.; Van der Vliet, A.; Corbacho, A.M.; Leonard, S.W.; Finkelstein, E.; Valacchi, G.; Obermueller-Jevic, U.; Cross, C.E.; Traber, M.G. Enhanced inflammatory responses in α-tocopherol transfer protein null mice. Arch. Biochem. Biophys. 2004, 423, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Terasawa, Y.; Ladha, Z.; Leonard, S.W.; Morrow, J.D.; Newland, D.; Sanan, D.; Packer, L.; Traber, M.G.; Farese, R.V. Increased atherosclerosis in hyperlipidemic mice deficient in α-tocopherol transfer protein and vitamin, E. Proc. Natl. Acad. Sci. USA 2000, 97, 13830–13834. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Igarashi, K.; Uchihara, T.; Jishage, K.I.; Tomita, H.; Inaba, A.; Li, Y.; Arita, M.; Suzuki, H.; Mizusawa, H.; et al. Delayed-onset ataxia in mice lacking α-tocopherol transfer protein: Model for neuronal degeneration caused by chronic oxidative stress. Proc. Natl. Acad. Sci. USA 2001, 98, 15185–15190. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, A.; Arita, M.; Sato, Y.; Kiyose, C.; Ueda, T.; Igarashi, O.; Arai, H.; Inoue, K. Affinity for α-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 1997, 409, 105–108. [Google Scholar] [CrossRef]
- Li, X.H.; Ghosh, S.P.; Ha, C.T.; Fu, D.; Elliott, T.B.; Bolduc, D.L.; Villa, V.; Whitnall, M.H.; Landauer, M.R.; Xiao, M. Delta-tocotrienol protects mice from radiation-induced gastrointestinal injury. Radiat. Res. 2013, 180, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Little, M.P.; Tawn, E.J.; Tzoulaki, I.; Wakeford, R.; Hildebrandt, G.; Paris, F.; Tapio, S.; Elliott, P. A systematic review of epidemiological associations between low and moderate doses of ionizing radiation and late cardiovascular effects, and their possible mechanisms. Radiat. Res. 2008, 169, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A.; Manuel, F.K.; Jones, J.; Iszard, G.; Murrey, J.; Djojonegro, B.; Wear, M. Space radiation and cataracts in astronauts. Radiat. Res. 2001, 156, 460–466. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Schimmerling, W.; Wilson, J.W.; Peterson, L.E.; Badhwar, G.D.; Saganti, P.B.; Dicello, J.F. Space radiation cancer risks and uncertainties for Mars missions. Radiat. Res. 2001, 156, 682–688. [Google Scholar] [CrossRef]
- Boerma, M.; Nelson, G.A.; Sridharan, V.; Mao, X.W.; Koturbash, I.; Hauer-Jensen, M. Space radiation and cardiovascular disease risk. World J. Cardiol. 2015, 7, 882. [Google Scholar] [CrossRef] [PubMed]
| Acute Radiation Syndrome | |||
|---|---|---|---|
| Hematopoietic Sub-Syndrome | Gastro-Intestinal Sub-Syndrome | Neuro/Cerebrovascular Sub-Syndrome | |
| Quantity of radiation | >2–3 Gy | 5–12 Gy | 10–20 Gy |
| Prodromal stage symptoms | Anorexia, nausea and vomiting | Anorexia, severe nausea, vomiting, cramps and diarrhea | Extreme nervousness and confusion; severe nausea, vomiting, and watery diarrhea; loss of consciousness; and burning sensations of the skin. |
| Latent Stage symptoms | Stem cells in bone marrow are dying, although patient may appear and feel well. | Stem cells in bone marrow and cells lining GI tract are dying, although patient may appear and feel well. | Patient may return to partial functionality. |
| Manifest Phase/Illness Phase symptoms | Anorexia, fever, and malaise. Drop in all blood cell counts occurs for several weeks. | Malaise, anorexia, severe diarrhea, fever, dehydration, and electrolyte imbalance. | Watery diarrhea, convulsions, and coma. |
| Recovery or death | Bone marrow cells will begin to repopulate the marrow. | >10 Gy radiation leads to death due to gastro-intestinal syndrome | No recovery is expected. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nukala, U.; Thakkar, S.; Krager, K.J.; Breen, P.J.; Compadre, C.M.; Aykin-Burns, N. Antioxidant Tocols as Radiation Countermeasures (Challenges to be Addressed to Use Tocols as Radiation Countermeasures in Humans). Antioxidants 2018, 7, 33. https://doi.org/10.3390/antiox7020033
Nukala U, Thakkar S, Krager KJ, Breen PJ, Compadre CM, Aykin-Burns N. Antioxidant Tocols as Radiation Countermeasures (Challenges to be Addressed to Use Tocols as Radiation Countermeasures in Humans). Antioxidants. 2018; 7(2):33. https://doi.org/10.3390/antiox7020033
Chicago/Turabian StyleNukala, Ujwani, Shraddha Thakkar, Kimberly J. Krager, Philip J. Breen, Cesar M. Compadre, and Nukhet Aykin-Burns. 2018. "Antioxidant Tocols as Radiation Countermeasures (Challenges to be Addressed to Use Tocols as Radiation Countermeasures in Humans)" Antioxidants 7, no. 2: 33. https://doi.org/10.3390/antiox7020033





