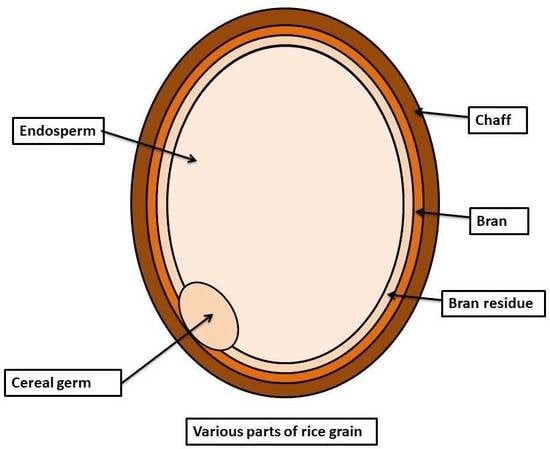
Phytochemical Profile of Brown Rice and Its Nutrigenomic Implications
Abstract
:1. Introduction
Search Strategy
2. Phytochemical Compounds in Brown Rice
Microbial Profiling in Brown Rice
3. Nutrigenomic Implications of Brown Rice
3.1. Anti-Diabetic Effect
3.2. Anti-Dyslipoproteinemia
3.3. Anti-Cancer Effect
3.4. Lowering Cholesterol
3.5. Cardio-Protective Effect
3.6. Antioxidant Effect
4. Conclusions and Future Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, Q.; Spiegelman, D.; van Dam, R.M.; Holmes, M.D.; Malik, V.S.; Willett, W.C.; Hu, F.B. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch. Intern. Med. 2010, 170, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, P.; Binodh, A.K.; Kumar, U.; Sugitha, T.; Anandan, A. Microbial association in brown rice and their influence on human health. In Brown Rice; Manickavasagan, A., Santhakumar, C., Venkatachalapathy, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 159–181. [Google Scholar]
- Fairhurst, T.; Dobermann, A. Rice in the global food supply. Better Crops Int. 2002, 16, 3–6. [Google Scholar]
- Zareiforoush, H.; Minaei, S.; Alizadeh, M.R.; Banakar, A. Qualitative classification of milled rice grains using computer vision and metaheuristic techniques. J. Food Sci. Technol. 2016, 53, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.J.; Jha, S.K.; Jha, G.K.; Sinha, J.P.; Lal, S.B. Soaking induced changes in chemical composition, glycemic index and starch characteristics of basmati rice. Rice Sci. 2015, 22, 227–236. [Google Scholar] [CrossRef]
- Chen, H.; Siebenmorgen, T.J.; Griffin, K. Quality characteristics of long-grain rice milled in two commercial systems. Cereal Chem. J. 1998, 75, 560–565. [Google Scholar] [CrossRef]
- Vetha Varshini, P.; Azhagu Sundharam, K.; Vijay Praveen, P. Brown rice—Hidden nutrients. J. Biosci. Tech. 2013, 4, 503–507. [Google Scholar]
- Liu, L.; Guo, J.; Zhang, R.; Wei, Z.; Deng, Y.; Guo, J.; Zhang, M. Effect of degree of milling on phenolic profiles and cellular antioxidant activity of whole brown rice. Food Chem. 2015, 185, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Ohtsubo, K.I.; Suzuki, K.; Yasui, Y.; Kasumi, T. Bio-functional components in the processed pre-germinated brown rice by a twin-screw extruder. J. Food Compos. Anal. 2005, 18, 303–316. [Google Scholar] [CrossRef]
- Wu, F.; Yang, N.; Touré, A.; Jin, Z.; Xu, X. Germinated brown rice and its role in human health. Crit. Rev. Food Sci. Nutr. 2013, 53, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.H.; Lim, S.T. Germinated brown rice and its bio-functional compounds. Food Chem. 2016, 196, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Foster-Powell, K.; Holt, S.H.; Brand-Miller, J.C. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 2002, 76, 5–56. [Google Scholar] [CrossRef] [PubMed]
- Umadevi, M.; Pushpa, R.; Sampathkumar, K.; Bhowmik, D. Rice—Traditional medicinal plant in India. J. Pharmacogn. Phytochem. 2012, 1, 6–12. [Google Scholar]
- Burlando, B.; Cornara, L. Therapeutic properties of rice constituents and derivatives (Oryza sativa L.): A review update. Trends Food Sci. Technol. 2014, 40, 82–98. [Google Scholar] [CrossRef]
- Hegde, S.; Yenagi, N.; Kasturiba, B. Indigenous knowledge of the traditional and qualified ayurveda practitioners on the nutritional significance and use of red rice in medications. Indian J. Tradit. Knowl. 2013, 12, 506–511. [Google Scholar]
- Isaac, R.S.R.; Nair, A.S.; Varghese, E.; Chavali, M. Phytochemical, antioxidant and nutrient analysis of medicinal rice (Oryza sativa L.) varieties found in south India. Adv. Sci. Lett. 2012, 11, 86–90. [Google Scholar] [CrossRef]
- Okarter, N.; Liu, R.H. Health benefits of whole grain phytochemicals. Crit. Rev. Food Sci. Nutr. 2010, 50, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.D.; Subhasree, R.; Bhakyaraj, R.; Vidhyalakshmi, R. Brown rice-beyond the color reviving a lost health food—A review. Am.-Eurasian J. Agron. 2009, 2, 67–72. [Google Scholar]
- Gong, E.S.; Luo, S.J.; Li, T.; Liu, C.M.; Zhang, G.W.; Chen, J.; Zeng, Z.C.; Liu, R.H. Phytochemical profiles and antioxidant activity of brown rice varieties. Food Chem. 2017, 227, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E. Scientific evidence of rice by-products for cancer prevention: Chemopreventive properties of waste products from rice milling on carcinogenesis in vitro and in vivo. BioMed Res. Int. 2017, 2017, 9017902. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Whole grain phytochemicals and health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- Adom, K.K.; Sorrells, M.E.; Liu, R.H. Phytochemicals and antioxidant activity of milled fractions of different wheat varieties. J. Agric. Food Chem. 2005, 53, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Martínez, I.; Lattimer, J.M.; Hubach, K.L.; Case, J.A.; Yang, J.; Weber, C.G.; Louk, J.A.; Rose, D.J.; Kyureghian, G.; Peterson, D.A.; et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 2013, 7, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Nakamura, K.; Kayahara, H. Analysis of phenolic compounds in white rice, brown rice, and germinated brown rice. J. Agric. Food Chem. 2004, 52, 4808–4813. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, P.J.; Peñas, E.; Martinez-Villaluenga, C.; Amigo, L.; Frias, J. Enhancement of biologically active compounds in germinated brown rice and the effect of sun-drying. J. Cereal Sci. 2017, 73, 1–9. [Google Scholar] [CrossRef]
- Adebamowo, S.N.; Eseyin, O.; Yilme, S.; Adeyemi, D.; Willett, W.C.; Hu, F.B.; Spiegelman, D.; Adebamowo, C.A. A mixed-methods study on acceptability, tolerability, and substitution of brown rice for white rice to lower blood glucose levels among Nigerian adults. Front. Nutr. 2017, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.B.; Khan, M.K. Germinated brown rice as a value added rice product: A review. J. Food Sci. Technol. 2011, 48, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T.M.; Ziegler, R.G.; Graubard, B.I.; Swanson, C.A.; Greenberg, R.S.; Schoenberg, J.B.; Swanson, G.M.; Hayes, R.B.; Mayne, S.T. Serum selenium and risk of prostate cancer in U.S. blacks and whites. Int. J. Cancer 2003, 103, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Hanna, T.J. Whole grains and protection against coronary heart disease: What are the active components and mechanisms? Am. J. Clin. Nutr. 1999, 70, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Jiamyangyuen, S.; Ooraikul, B. The physico-chemical, eating and sensorial properties of germinated brown rice. J. Food Agric. Environ. 2008, 6, 119–124. [Google Scholar]
- Aune, D.; Chan, D.S.M.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. Br. Med. J. 2011, 343, d6617. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Chan, D.S.; Greenwood, D.C.; Vieira, A.R.; Rosenblatt, D.A.; Vieira, R.; Norat, T. Dietary fiber and breast cancer risk: A systematic review and meta-analysis of prospective studies. Ann. Oncol. 2012, 23, 1394–1402. [Google Scholar] [PubMed]
- Verschoyle, R.D.; Greaves, P.; Cai, H.; Edwards, R.E.; Steward, W.P.; Gescher, A.J. Evaluation of the cancer chemopreventive efficacy of rice bran in genetic mouse models of breast, prostate and intestinal carcinogenesis. Br. J. Cancer 2007, 96, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Morino, K.; Nishio, Y.; Ishikado, A.; Arima, H.; Nakao, K.; Nakagawa, F.; Nikami, F.; Sekine, O.; Nemoto, K.I.; et al. Fiber-rich diet with brown rice improves endothelial function in type 2 diabetes mellitus: A randomized controlled trial. PLoS ONE 2017, 12, e0179869. [Google Scholar] [CrossRef] [PubMed]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, gamma-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Gaddi, A. Rice bran oil and gamma-oryzanol in the treatment of hyperlipoproteinaemias and other conditions. Phytother. Res. 2001, 15, 277–289. [Google Scholar] [PubMed]
- Sato, S.; Soga, T.; Nishioka, T.; Tomita, M. Simultaneous determination of the main metabolites in rice leaves using capillary electrophoresis mass spectrometry and capillary electrophoresis diode array detection. Plant J. 2004, 40, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Tiansawang, K.; Luangpituksa, P.; Varanyanond, W.; Hansawasdi, C. GABA production, antioxidant activity in some germinated dietary seeds and the effect of cooking on their GABA content. Food Sci. Technol. 2016, 36, 313–321. [Google Scholar] [CrossRef]
- Zhou, Z.; Blanchard, C.; Helliwell, S.; Robards, K. Fatty acid composition of three rice varieties following storage. J. Cereal Sci. 2003, 37, 327–335. [Google Scholar] [CrossRef]
- Eshun Naomi, A.; Emmanuel, A.; Barimah, J.; VM, D.; Van Twisk, C. Amino acid profiles of some varieties of rice, soybean and groundnut grown. J. Food Process. Technol. 2015, 6, 420. [Google Scholar]
- Zubair, M.; Anwar, F.; Ashraf, M.; Uddin, M.K. Characterization of high-value bioactives in some selected varieties of Pakistani Rice (Oryza sativa L.). Int. J. Mol. Sci. 2012, 13, 4608–4622. [Google Scholar] [CrossRef] [PubMed]
- Srisawat, U.; Panunto, W.; Kaendee, N.; Tanuchit, S.; Itharat, A.; Lerdvuthisopon, N.; Hansakul, P. Determination of phenolic compounds, flavonoids, and antioxidant activities in water extracts of Thai red and white rice cultivars. J. Med. Assoc. Thail. 2010, 93, S83–S91. [Google Scholar]
- Horwitz, W. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Cao, Y.; Jia, F.; Han, Y.; Liu, Y.; Zhang, Q. Study on the optimal moisture adding rate of brown rice during germination by using segmented moisture conditioning method. J. Food Sci. Technol. 2015, 52, 6599–6606. [Google Scholar] [CrossRef] [PubMed]
- Perera, I.; Seneweera, S.; Hirotsu, N. Manipulating the phytic acid content of rice grain toward improving micronutrient bioavailability. Rice 2018, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Godber, J.S. Purification and identification of components of γ-oryzanol in rice bran oil. J. Agric. Food Chem. 1999, 47, 2724–2728. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Pan, Z.; Yue, T.; Atungulu, G.G.; Berrios, J. Effect of ultrasonic treatment of brown rice at different temperatures on cooking properties and quality. Cereal Chem. J. 2010, 87, 403–408. [Google Scholar] [CrossRef]
- Lu, Z.H.; Zhang, Y.; Li, L.T.; Curtis, R.B.; Kong, X.L.; Fulcher, R.G.; Zhang, G.; Cao, W. Inhibition of microbial growth and enrichment of gamma-aminobutyric acid during germination of brown rice by electrolyzed oxidizing water. J. Food Prot. 2010, 73, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Dhillon, B.; Sodhi, N.S. Effect of degree of milling (Dom) on overall quality of rice—A review. Int. J. Adv. Biotechnol. Res. 2014, 5, 474–489. [Google Scholar]
- Cottyn, B.; Regalado, E.; Lanoot, B.; De Cleene, M.; Mew, T.W.; Swings, J. Bacterial populations associated with rice seed in the tropical environment. Phytopathology 2001, 91, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Bang, J.; Kim, H.; Kim, Y.; Kim, B.S.; Beuchat, L.R.; Ryu, J.H. Bacillus cereus and Bacillus thuringiensis spores in Korean rice: Prevalence and toxin production as affected by production area and degree of milling. Food Microbiol. 2014, 42, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Pengnoi, P.; Mahawan, R.; Khanongnuch, C.; Lumyong, S. Antioxidant properties and production of monacolin k, citrinin, and red pigments during solid state fermentation of purple rice (Oryzae sativa) varieties by Monascus purpureus. Czech J. Food Sci. 2017, 35, 32–39. [Google Scholar]
- Tanaka, K.; Sago, Y.; Zheng, Y.; Nakagawa, H.; Kushiro, M. Mycotoxins in rice. Int. J. Food Microbiol. 2007, 119, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Shortt, C. Living it up for dinner. Chem. Ind. 1998, 8, 300–303. [Google Scholar]
- Kim, K.-S.; Kim, B.-H.; Kim, M.-J.; Han, J.-K.; Kum, J.-S.; Lee, H.-Y. Quantitative microbiological profiles of brown rice and germinated brown rice. Food Sci. Biotechnol. 2012, 21, 1785–1788. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhang, H. Phytochemical constituents, health benefits, and industrial applications of grape seeds: A mini-review. Antioxidants 2017, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.F.; Lee, Y.Y. Virgin coconut oil and its cardiovascular health benefits. Nat. Prod. Commun. 2016, 11, 1151–1152. [Google Scholar]
- Zhang, H.; Ma, Z.F. Phytochemical and pharmacological poperties of Capparis spinosa as a medicinal plant. Nutrients 2018, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ma, Z.; Zhang, H.; Jin, Y.; Zhang, Y.; Hayford, F. Phytochemical properties and nutrigenomic implications of yacon as a potential source of prebiotic: Current evidence and future directions. Foods 2018, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Azmi, N.H.; Ismail, N.; Imam, M.U.; Ismail, M. Ethyl acetate extract of germinated brown rice attenuates hydrogen peroxide-induced oxidative stress in human SH-SY5Y neuroblastoma cells: Role of anti-apoptotic, pro-survival and antioxidant genes. BMC Complement. Altern. Med. 2013, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Imam, M.U.; Ismail, M. Nutrigenomic effects of germinated brown rice and its bioactives on hepatic gluconeogenic genes in type 2 diabetic rats and HEPG2 cells. Mol. Nutr. Food Res. 2013, 57, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Imam, M.U.; Musa, S.N.A.; Azmi, N.H.; Ismail, M. Effects of white rice, brown rice and germinated brown rice on antioxidant status of type 2 diabetic rats. Int. J. Mol. Sci. 2012, 13, 12952–12969. [Google Scholar] [CrossRef] [PubMed]
- Imam, M.U.; Ismail, M.; Omar, A.R. Nutrigenomic effects of germinated brown rice bioactives on antioxidant genes. Free Radic. Biol. Med. 2012, 53, S105–S106. [Google Scholar] [CrossRef]
- Imam, M.U.; Azmi, N.H.; Ismail, M. Upregulation of superoxide dismutase gene is involved in antioxidant effects of germinated rown rice. Free Radic. Biol. Med. 2012, 53, S84. [Google Scholar] [CrossRef]
- Imam, M.U.; Ismail, M.; Omar, A.R.; Ithnin, H. The hypocholesterolemic effect of germinated brown rice involves the upregulation of the apolipoprotein A1 and low-density lipoprotein receptor genes. J. Diabetes Res. 2013, 2013, 134694. [Google Scholar] [CrossRef] [PubMed]
- Imam, M.U.; Ishaka, A.; Ooi, D.-J.; Zamri, N.D.M.; Sarega, N.; Ismail, M.; Esa, N.M. Germinated brown rice regulates hepatic cholesterol metabolism and cardiovascular disease risk in hypercholesterolaemic rats. J. Funct. Foods 2014, 8, 193–203. [Google Scholar] [CrossRef]
- Terashima, Y.; Nagai, Y.; Kato, H.; Ohta, A.; Tanaka, Y. Eating glutinous brown rice for one day improves glycemic control in Japanese patients with type 2 diabetes assessed by continuous glucose monitoring. Asia Pac. J. Clin. Nutr. 2017, 26, 421–426. [Google Scholar] [PubMed]
- Nakayama, T.; Nagai, Y.; Uehara, Y.; Nakamura, Y.; Ishii, S.; Kato, H.; Tanaka, Y. Eating glutinous brown rice twice a day for 8 weeks improves glycemic control in Japanese patients with diabetes mellitus. Nutr. Diabetes 2017, 7, e273. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, S.A.; Lee, I.K.; Kim, J.G.; Park, K.G.; Jeong, J.Y.; Jeon, J.H.; Shin, J.Y.; Lee, D.H. Effect of a brown rice based vegan diet and conventional diabetic diet on glycemic control of patients with type 2 diabetes: A 12-week randomized clinical trial. PLoS ONE 2016, 11, e0155918. [Google Scholar] [CrossRef] [PubMed]
- Shobana, S.; Lakshmipriya, N.; Bai, M.R.; Gayathri, R.; Ruchi, V.; Sudha, V.; Malleshi, N.G.; Krishnaswamy, K.; Henry, C.K.; Anjana, R.M.; et al. Even minimal polishing of an Indian parboiled brown rice variety leads to increased glycemic responses. Asia Pac. J. Clin. Nutr. 2017, 26, 829–836. [Google Scholar] [PubMed]
- Ito, Y.; Mizukuchi, A.; Kise, M.; Aoto, H.; Yamamoto, S.; Yoshihara, R.; Yokoyama, J. Postprandial blood glucose and insulin responses to pre-germinated brown rice in healthy subjects. J. Med. Investig. 2005, 52, 159–164. [Google Scholar] [CrossRef]
- Mohan, V.; Spiegelman, D.; Sudha, V.; Gayathri, R.; Hong, B.; Praseena, K.; Anjana, R.M.; Wedick, N.M.; Arumugam, K.; Malik, V.; et al. Effect of brown rice, white rice, and brown rice with legumes on blood glucose and insulin responses in overweight Asian Indians: A randomized controlled trial. Diabetes Technol. Ther. 2014, 16, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Panlasigui, L.N.; Thompson, L.U. Blood glucose lowering effects of brown rice in normal and diabetic subjects. Int. J. Food Sci. Nutr. 2006, 57, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Kozuka, C.; Yabiku, K.; Takayama, C.; Matsushita, M.; Shimabukuro, M. Natural food science based novel approach toward prevention and treatment of obesity and type 2 diabetes: Recent studies on brown rice and gamma-oryzanol. Obes. Res. Clin. Pract. 2013, 7, e165–e172. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.; Kwok, K.; Li, Y.; Fu, L. In vitro study of possible role of dietary fiber in lowering postprandial serum glucose. J. Agric. Food Chem. 2001, 49, 1026–1029. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Nagase, R.; Torimitsu, M.; Yanagi, M.; Ito, Y.; Kise, M.; Mizukuchi, A.; Fujimura, N.; Hayamizu, K.; Ariga, T. Insoluble fiber is a major constituent responsible for lowering the post-prandial blood glucose concentration in the pre-germinated brown rice. Biol. Pharm. Bull. 2005, 28, 1539–1541. [Google Scholar] [CrossRef] [PubMed]
- Jaskari, J.; Kontula, P.; Siitonen, A.; Jousimies-Somer, H.; Mattila-Sandholm, T.; Poutanen, K. Oat beta-glucan and xylan hydrolysates as selective substrates for Bifidobacterium and Lactobacillus strains. Appl. Microbiol. Biotechnol. 1998, 49, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Benno, Y.; Endo, K.; Miyoshi, H.; Okuda, T.; Koishi, H.; Mitsuoka, T. Effect of rice fiber on human fecal microflora. Microbiol. Immunol. 1989, 33, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, R.; Yamsaki, T.; Uchida, A.; Ohara, K.; Ushio, H. Gamma-oryzanol recovers mouse hypoadiponectinemia induced by animal fat ingestion. Phytomedicine 2011, 18, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Ohara, K.; Kiyotani, Y.; Uchida, A.; Nagasaka, R.; Maehara, H.; Kanemoto, S.; Hori, M.; Ushio, H. Oral administration of gamma-aminobutyric acid and gamma-oryzanol prevents stress-induced hypoadiponectinemia. Phytomedicine 2011, 18, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Usuki, S.; Ito, Y.; Morikawa, K.; Kise, M.; Ariga, T.; Rivner, M.; Yu, R.K. Effect of pre-germinated brown rice intake on diabetic neuropathy in streptozotocin-induced diabetic rats. Nutr. Metab. 2007, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Usuki, S.; Tsai, Y.Y.; Morikawa, K.; Nonaka, S.; Okuhara, Y.; Kise, M.; Yu, R.K. IGF-1 induction by acylated steryl beta-glucosides found in a pre-germinated brown rice diet reduces oxidative stress in streptozotocin-induced diabetes. PLoS ONE 2011, 6, e28693. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Varbo, A. Triglycerides and cardiovascular disease. Lancet 2014, 384, 626–635. [Google Scholar] [CrossRef]
- Shen, K.-P.; Hao, C.-L.; Yen, H.-W.; Chen, C.-Y.; Chen, J.-H.; Chen, F.-C.; Lin, H.-L. Pre-germinated brown rice prevented high fat diet induced hyperlipidemia through ameliorating lipid synthesis and metabolism in C57BL/6J mice. J. Clin. Biochem. Nutr. 2016, 59, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.N.; Son, M.E.; Lim, W.C.; Lim, S.T.; Cho, H.Y. Anti-obesity effects of germinated brown rice extract through down-regulation of lipogenic genes in high fat diet-induced obese mice. Biosci. Biotechnol. Biochem. 2012, 76, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Miura, D.; Ito, Y.; Mizukuchi, A.; Kise, M.; Aoto, H.; Yagasaki, K. Hypocholesterolemic action of pre-germinated brown rice in hepatoma-bearing rats. Life Sci. 2006, 79, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Roohinejad, S.; Omidizadeh, A.; Mirhosseini, H.; Saari, N.; Mustafa, S.; Yusof, R.M.; Hussin, A.S.; Hamid, A.; Abd Manap, M.Y. Effect of pre-germination time of brown rice on serum cholesterol levels of hypercholesterolaemic rats. J. Sci. Food Agric. 2010, 90, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Albarracin, M.; Weisstaub, A.R.; Zuleta, A.; Drago, S.R. Extruded whole grain diets based on brown, soaked and germinated rice. Effects on cecum health, calcium absorption and bone parameters of growing Wistar rats. Part I. Food Funct. 2016, 7, 2722–2728. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.N.; Le, T.H.; Nguyen, D.H.; Tran, Q.B.; Nguyen, T.L.; Le, D.T.; Do, V.A.; Vu, A.L.; Aoto, H.; Okuhara, Y.; et al. Pre-germinated brown rice reduced both blood glucose concentration and body weight in Vietnamese women with impaired glucose tolerance. J. Nutr. Sci. Vitaminol. 2014, 60, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.F.; Kise, M.; Wang, M.F.; Ito, Y.; Yang, M.D.; Aoto, H.; Yoshihara, R.; Yokoyama, J.; Kunii, D.; Yamamoto, S. Effects of pre-germinated brown rice on blood glucose and lipid levels in free-living patients with impaired fasting glucose or type 2 diabetes. J. Nutr. Sci. Vitaminol. 2008, 54, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Uno, Y.; Umemoto, T.; Sugiyama, C.; Matsumoto, M.; Wada, Y.; Ishizuka, T. Effect of gamma-aminobutyric acid-rich germinated brown rice on indexes of life-style related diseases. Nihon Ronen Igakkai Zasshi 2004, 41, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ye, Z.; Li, H.; Wu, S.; Deng, D.; Zhu, Y.; Wong, M. Do radial oxygen loss and external aeration affect iron plaque formation and arsenic accumulation and speciation in rice? J. Exp. Bot. 2012, 63, 2961–2970. [Google Scholar] [CrossRef] [PubMed]
- Saki, E.; Saiful Yazan, L.; Mohd Ali, R.; Ahmad, Z. Chemopreventive effects of germinated rough rice crude extract in inhibiting azoxymethane-induced aberrant crypt foci formation in sprague-dawley rats. BioMed Res. Int. 2017, 2017, 9517287. [Google Scholar] [CrossRef] [PubMed]
- Latifah, S.Y.; Armania, N.; Tze, T.H.; Azhar, Y.; Nordiana, A.H.; Norazalina, S.; Hairuszah, I.; Saidi, M.; Maznah, I. Germinated brown rice (GBR) reduces the incidence of aberrant crypt foci with the involvement of beta-catenin and COX-2 in azoxymethane-induced colon cancer in rats. Nutr. J. 2010, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.H.; Oh, S.H. Effects of germinated brown rice extracts with enhanced levels of GABA on cancer cell proliferation and apoptosis. J. Med. Food 2004, 7, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Schuller, H.M.; Al-Wadei, H.A.; Majidi, M. Gamma-aminobutyric acid, a potential tumor suppressor for small airway-derived lung adenocarcinoma. Carcinogenesis 2008, 29, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Har, C.H.; Keong, C.K. Effects of tocotrienols on cell viability and apoptosis in normal murine liver cells (BNL CL.2) and liver cancer cells (BNL 1ME A.7R.1), in vitro. Asia Pac. J. Clin. Nutr. 2005, 14, 374–380. [Google Scholar] [PubMed]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S.; et al. Heart Disease and Stroke Statistics—2014 Update: A Report From the American Heart Association. Circulation 2014, 129, e28–e292. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh, M.; Safavi, S.M.; Nematollahi, S.; Nourieh, Z. Effect of brown rice consumption on inflammatory marker and cardiovascular risk factors among overweight and obese non-menopausal female adults. Int. J. Prev. Med. 2014, 5, 478–488. [Google Scholar] [PubMed]
- Ebizuka, H.; Sasaki, C.; Kise, M.; Arita, M. Effects of retort pouched rice containing pre-germinated brown rice on daily nutrition and physical status in healthy subjects. J. Integr. Study Diet. Habits 2007, 18, 216–222. [Google Scholar] [CrossRef]
- Ebizuka, H.; Ihara, M.; Arita, M. Antihypertensive effect of pre-germinated brown rice in spontaneously hypertensive rats. Food Sci. Technol. Res. 2009, 15, 625–630. [Google Scholar] [CrossRef]
- Choi, H.-D.; Kim, Y.-S.; Choi, I.-W.; Park, Y.-K.; Park, Y.-D. Hypotensive effect of germinated brown rice on spontaneously hypertensive rats. Korean J. Food Sci. Technol. 2006, 38, 448–451. [Google Scholar]
- Suzuki, A.; Kagawa, D.; Fujii, A.; Ochiai, R.; Tokimitsu, I.; Saito, I. Short- and long-term effects of ferulic acid on blood pressure in spontaneously hypertensive rats. Am. J. Hypertens. 2002, 15, 351–357. [Google Scholar] [CrossRef]
- Ohsaki, Y.; Shirakawa, H.; Koseki, T.; Komai, M. Novel effects of a single administration of ferulic acid on the regulation of blood pressure and the hepatic lipid metabolic profile in stroke-prone spontaneously hypertensive rats. J. Agric. Food Chem. 2008, 56, 2825–2830. [Google Scholar]
- Shao, Y.; Bao, J. Polyphenols in whole rice grain: Genetic diversity and health benefits. Food Chem. 2015, 180, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Hayashi, T.; Hayashi, K.; Murai, F.; Hori, M.; Kimoto, K.; Murakami, K. Pre-germinated brown rice could enhance maternal mental health and immunity during lactation. Eur. J. Nutr. 2007, 46, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Wunjuntuk, K.; Kettawan, A.; Rungruang, T.; Charoenkiatkul, S. Anti-fibrotic and anti-inflammatory effects of parboiled germinated brown rice (Oryza sativa ‘KDML 105′) in rats with induced liver fibrosis. J. Funct. Foods 2016, 26, 363–372. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Z.F.; Luo, X.; Li, X. Effect of mulberry fruit (Morus alba L.) consumption on health outcomes: A mini-review. Antioxidants 2018, 7, 69. [Google Scholar] [CrossRef]

| Family | Compounds |
|---|---|
| Phenolics | Gallic acid, protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, syringic acid, chlorogenic acid, caffeic acid, p-coumaric acid, sinapic acid, ferulic acid, cinnamic acid, ellagic acid |
| Flavonoids | Luteolin, apigenin, tricin, quercetin, kaempferol, isorhamnetin, myricetin |
| Anthocyanins and proanthocyanins | Peonidin-3-O-glucoside, cyanidin-3-O-glucoside, cyanidin-3-O-galactoside, cyanidin-3-O-rutinoside, catechin, epicatechin |
| Vitamins | Tocopherols, tocotrienols, B vitamins (B1, B3, B6) |
| Amino acids | Alanine, arginine, aspartic acid, cystine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, valine |
| Phytosterols | Stigmasterol, stigmastanol, β-sitosterol, campesterol, δ5-avenasterol, δ7-avenasterol |
| γ-Oryzanol | Cycloartanyl ferulate, 24-methylene cycloartanyl ferulate, campesteryl ferulate, β-sitosteryl ferulate |
| Others | Dietary fibre, phytic acid, minerals |
| Phytochemical Compounds in Rice | Analytical Methods | References |
|---|---|---|
| Phenolic acids | Microwave-assisted extraction (MAE) Ultrasound-assisted extraction (UAE) | Sato et al. (2004) [38] |
| Antioxidants | Microwave-assisted extraction (MAE) Ultrasound-assisted extraction (UAE) | Sato et al. (2004) [38] |
| Anthocyanins and proanthocyanins | UV-visible spectroscopy | Sato et al. (2004) [38] |
| Dietary fibre | Enzymatic-gravimetric method | Tiansawang et al. (2016) [39] |
| Functional lipid | Gravimetric method | Zhou et al. (2003) [40] |
| Essential amino acid | HPLC method | Naomi et al. (2014) [41] |
| Phytosterols | Gas chromatography | Zubair et al. (2012). [42] |
| Flavonoids | Fluorescent DCF | Srisawat et al. (2011) [43] |
| Tocopherols and tocotrienols | Fluorescent DCF | Srisawat et al. (2011) [43] |
| Minerals | Ashing method | Horwitz (2000) [44] |
| Gamma aminobutyric acid (GABA) | Amino acid auto analyser | Cao et al. (2015) [45] |
| γ-oryzanol | Reversed-phase HPLC method | Xu and Godber (1999) [47] |
| Phytic acid | UV-Vis spectroscopy | Perera et al. (2018) [46] |
| Group | Microbes | Microbial Association | References |
|---|---|---|---|
| Gram positive bacteria | Brevibacillus laterosporus, Brevibacillus brevis, Brevibacterium spp. | Production of amino acids | Cottyn et al. [51] |
| Cellulomonas flavigena | Degradation of cellulose | Cottyn et al. [51] | |
| Bacillus thuringiensis and Bacillus cereus | Production of enterotoxin | Kim et al. [52] | |
| Staphylococcus saprophyticus | Food-borne pathogen | Cottyn et al. [51] | |
| Fungi | Monascus purpureus | Production of red pigment | Pengnoi et al. [53] |
| Fusarium fujikuroi, Aspergillus flavus and Candida | Production of toxin | Tanaka et al. [54] | |
| Yeast | Torulopsis etchellsii, Hansenula anomala, Trichosporon pullulans, Geotrichum candidum, Saccharomyces sp. | An increase in the essential amino acids; a decrease in phytic acid and enzyme inhibitors | Panneerselvam et al. [2]; Shortt [55] |
| Property | Potential Underlying Nutrigenomic Mechanism | References |
|---|---|---|
| Antioxidative | An increase in antioxidant status and a reduction in oxidative stress via v-akt murine thyromoma viral oncogene (AKT), nuclear factor beta (NF-Kβ), mitogen activated protein kinase (p38 MAPK), c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK1/2), p53 tumour suppressor genes, catalase, insulin-like growth factor 2 (IGF2) and superoxide dismutase (SOD) | Azmi et al. (2013) [61]; Imam et al. (2013) [62]; Imam et al. (2012a) [63]; Imam et al. (2012b) [64] |
| Anti-hyperglycemia | A decrease in the level of blood glucose via the suppression of fbp and pck genes, which are gluconeogenic | Imam and Ismail [62] |
| Anti-hypocholesterolaemia | A decrease in low density lipoprotein (LDL) and total cholesterol, as well as an increase in high density lipoprotein (HDL) via the transcriptional regulation of hepatic LDL receptor, lipoprotein lipase (LPL), adiponectin, peroxisome proliferator-activator receptor (PPAR) γ, ATP binding cassette (ABCA) 1, AKT and apolipoprotein genes | Imam et al. (2013) [66]; Imam et al. [67] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravichanthiran, K.; Ma, Z.F.; Zhang, H.; Cao, Y.; Wang, C.W.; Muhammad, S.; Aglago, E.K.; Zhang, Y.; Jin, Y.; Pan, B. Phytochemical Profile of Brown Rice and Its Nutrigenomic Implications. Antioxidants 2018, 7, 71. https://doi.org/10.3390/antiox7060071
Ravichanthiran K, Ma ZF, Zhang H, Cao Y, Wang CW, Muhammad S, Aglago EK, Zhang Y, Jin Y, Pan B. Phytochemical Profile of Brown Rice and Its Nutrigenomic Implications. Antioxidants. 2018; 7(6):71. https://doi.org/10.3390/antiox7060071
Chicago/Turabian StyleRavichanthiran, Keneswary, Zheng Feei Ma, Hongxia Zhang, Yang Cao, Chee Woon Wang, Shahzad Muhammad, Elom K. Aglago, Yihe Zhang, Yifan Jin, and Binyu Pan. 2018. "Phytochemical Profile of Brown Rice and Its Nutrigenomic Implications" Antioxidants 7, no. 6: 71. https://doi.org/10.3390/antiox7060071