Disease Prevention: An Opportunity to Expand Edible Plant-Based Vaccines?
Abstract
:1. Introduction: The Current State of Vaccination
2. The Problem of Infectious Diseases and their Outbreaks
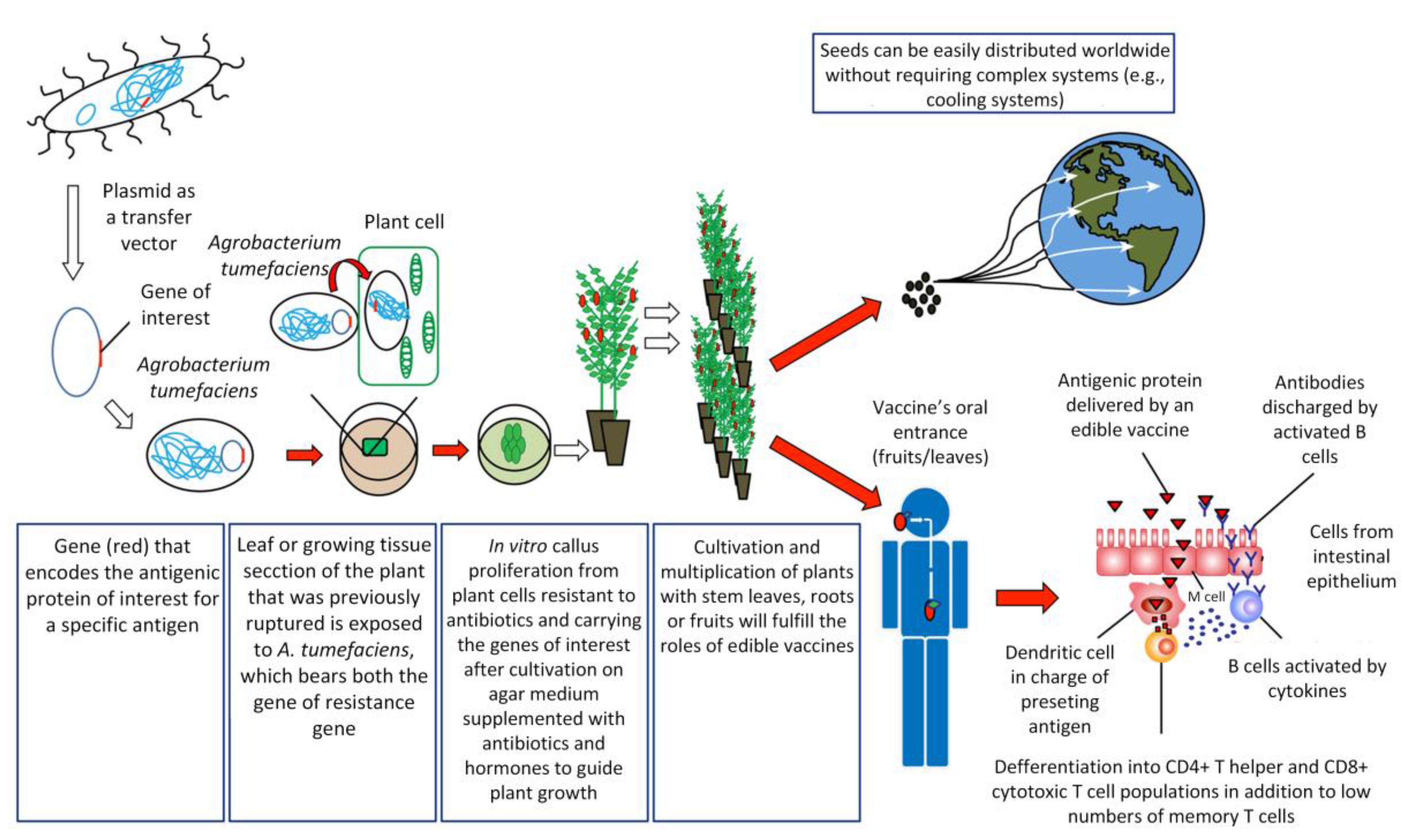
3. Edible Vaccines: What Are They and How Do They Work?
How Are Edible Vaccines Developed?
4. Edible Vaccine Advantages and Disadvantages
5. Plants Already Transformed for Use as Edible Vaccines
5.1. Potatoes
5.2. Tobacco
5.3. Tomatoes
5.4. Lettuce
5.5. Rice
5.6. Carrots
5.7. Soybeans
5.8. Alfalfa
5.9. Corn
5.10. Papaya
5.11. Quinoa
5.12. Bananas
5.13. Peas
5.14. Apples
5.15. Cherry Tomatillos
5.16. Algae
6. Current and Future Challenges
6.1. Current and Future Regulation of Edible Vaccine Production, Commercialization, and Copyright
6.2. Ethical Aspects
6.3. Bioterrorism
7. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arntzen, C.; Plotkin, S.; Dodet, B. Plant-derived vaccines and antibodies: Potential and limitations. Vaccine 2005, 23, 1753–1756. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Verma, P.; Singh, P.; Tuli, R. Plants as bioreactors for the production of vaccine antigens. Biotechnol. Adv. 2009, 27, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Hansson, M.; Nygren, P.Å.; Ståhl, S. Design and production of recombinant subunit vaccines. Biotechnol. Appl. Biochem. 2000, 32, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Langridge, W.H.R. Edible Vaccines. Sci. Am. 2000, 283, 66–71. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Mallorquín, P.; Pardo, R.; Vega, M. Vacunas de Nueva Generación; Genoma España Salud humana: Madrid, España, 2004; p. 113. [Google Scholar]
- Madigan, M.; Martinko, J.; Parker, J. Brock Biología de los Microorganismos, 12th ed.; Pearson Addison Wesley: Madrid, Spain, 2009; p. 1259. [Google Scholar]
- Glick, B.R.; Pasternak, J.J.; Patten, Ch.L. Molecular Biotechnology. Principles and Applications of Recombinant DNA, 4th ed.; ASM Press: Herndon, VA, USA, 2010; p. 999. [Google Scholar]
- Organización Mundial de la Salud (OMS). Plan de Acción Mundial sobre Vacunas; Biblioteca OMS: Ginebra, Suiza, 2013; p. 148. ISBN 9789243504988. [Google Scholar]
- Organización Mundial de la Salud (OMS); United Nations Children’s Fund (UNICEF); Banco Mundial. Vacunas e Inmunización: Situación Mundial, 3rd ed.; Organización Mundial de la Salud: Ginebra, Suiza, 2010; p. 185. [Google Scholar]
- Kumru, O.; Joshi, S.; Smith, D.; Russell, C.; Prusik, T.; Volkin, D. Vaccine instability in the cold chain: Mechanisms, analysis and formulation strategies. Biologicals 2014, 42, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.; Streatfield, S.J.; Wyckoff, K. Medical molecular farming: Production of antibiotics, biopharmaceuticals and edible vaccines in plants. Trends Plant Sci. 2001, 6, 219–226. [Google Scholar] [CrossRef]
- Mason, H.S.; Lam, D.M.K.; Arntzen, C.J. Expression of hepatitis B surface antigen in transgenic plants. Proc. Natl. Acad. Sci. USA 1992, 89, 11745–11749. [Google Scholar] [CrossRef] [PubMed]
- Aswathi, P.B.; Bhanja, S.K.; Yadav, A.S.; Rekha, V.; John, J.K.; Gopinath, D.; Sadanandan, G.V.; Shinde, A.; Jacob, A. Plant Based Edible Vaccines against Poultry Diseases: A Review. Adv. Anim. Vet. Sci. 2014, 2, 305–311. [Google Scholar] [CrossRef]
- Knipe, D.M.; Howley, P.M. Fields Virology, 6th ed.; Williams & Wilkins: Philadelphia, PA, USA, 2013; p. 2456. [Google Scholar]
- OMS. Enfermedad por el Virus del Ebola. 2014. Available online: http://www.who.int/mediacentre/factsheets/fs103/es/ (accessed on 25 March 2015).
- OMS. Diphtheria Reported Cases. 2015. Available online: http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tsincidencediphtheria.htlm (accessed on 10 July 2015).
- OMS. Measles–WHO European Region. 2015. Available online: http://www.who.int/csr/don/6-march-2015-measles/en/ (accessed on 10 July 2015).
- Bertin, X. Sarampión en Chile: Las razones del resurgimiento de las enfermedades que creíamos erradicadas. 2015. Available online: http://www.latercera.com/noticia/nacional/2015/06/680-633372-9-sarampion-en-chile-las-razones-del-resurgimiento-de-las-enfermedades-que.shtml (accessed on 10 June 2015).
- Minsal. Información Sobre Sarampión. 2015. Available online: http://web.minsal.cl/sites/default/files/7REPORTECASOSjunio2015_1.pdf (accessed on 25 June 2015).
- Polanda, G.; Jacobson, R.; Ovsyannikova, I. Trends affecting the future of vaccine development and delivery: The role of demographics, regulatory science, the anti-vaccine movement, and vaccinomics. Vaccine 2009, 27, 3240–3244. [Google Scholar] [CrossRef] [PubMed]
- Gestal, J.J.; Rodríguez, L.; Montes, A.; Takkouche, B. Emergencia en europa de la difteria y la poliomelitis. Rev. Esp. Salud Pública 1996, 70, 5–14. [Google Scholar]
- Los padres del niño con difteria se sienten “engañados” por los antivacunas. Available online: http://www.abc.es/sociedad/20150605/abci-padres-nino-difteria-destrozados-201506051436.html (accessed on 12 June 2015).
- Pogrebnyak, N.; Golovkin, M.; Andrianov, V.; Spitsin, S.; Smirnov, Y.; Egolf, R.; Koprowski, H. Severe acute respiratory syndrome (SARS) S protein production in plants: Development of recombinant vaccine. Proc. Natl. Acad. Sci. USA 2005, 102, 9062–9067. [Google Scholar] [CrossRef] [PubMed]
- Lamphear, B.J.; Streatfield, S.J.; Jilka, J.M.; Brooks, C.A.; Barker, D.K.; Turner, D.D.; Delaney, D.E.; Garcia, M.; Wiggins, B.; Woodard, S.L.; et al. Delivery of subunit vaccines in maize seed. J. Control Release 2002, 85, 169–180. [Google Scholar] [CrossRef]
- Lamphear, B.J.; Jilka, J.M.; Kesl, L.; Welter, M.; Howard, J.A.; Streatfield, S.J. A corn-based delivery system for animal vaccines: An oral transmissible gastroenteritis virus vaccine boosts lactogenic immunity in swine. Vaccine 2004, 22, 2420–2424. [Google Scholar] [CrossRef] [PubMed]
- Liew, P.S.; Hair-Bejo, M. Farming of plant-based veterinary vaccines and their applications for disease prevention in animals. Adv. Virol. 2015, 2015, 936940. [Google Scholar] [CrossRef] [PubMed]
- Tacket, C.O.; Mason, H.S.; Losonsky, G.; Clements, J.D.; Levine, M.M.; Arntzen, C.J. Immunogenicity in humans of a recombinant bacterial antigen delivered in a transgenic potato. Nat. Med. 1998, 4, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.L.; He, Z.M.; Peng, Z.Q.; Qi, Y.; Chen, Q.; Yu, S.Y. Cholera toxin B protein in transgenic tomato fruit induces systemic immune response in mice. Transgenic Res. 2007, 16, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Qian, K.X.; Su, N.; Chang, H.Y.; Liu, J.X.; Chenrlekar, G.F. Foot-and-mouth disease virus VP1 protein fused with cholera toxin B subunit expressed in Chlamydomonas reinhardtii chloroplast. Biotechnol. Lett. 2003, 25, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S. Vaccine Fact Book; Pharmaceutical Research and Manufacturers of America: Washington, DC, USA, 2013; p. 97. [Google Scholar]
- Pelosi, A.; Shepherd, R.; Guzman, G.D.; Hamill, J.D.; Meeusen, E.; Sanson, G.; Walmsley, M. The release and induced immune responses of a plant-made and delivered antigen in the mouse gut. Curr. Drug Deliv. 2011, 8, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Mabbott, N.A.; Donaldson, D.S.; Ohno, H.; Williams, I.R.; Mahajan, A. Microfold (M) cells: Important immunosurveillance posts in the intestinal epithelium. Mucosa Immunol. 2013, 6, 666–667. [Google Scholar] [CrossRef] [PubMed]
- Mildner, A.; Jung, S. Development and Function of Dendritic cells Subsets. Inmmunity 2014, 40, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Dalod, M.; Chelbi, R.; Malissen, B.; Lawrence, T. Dendritic cell maturation: Functional specialization through signaling specificity and transcriptional programming. EMBO J. 2014, 33, 1104–1116. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.; Han, J.-A.; Koh, H.; Choi, B.; Cho, Y.; Jeong, H.; Ra, J.-S.; Sung, P.S.; Shin, E.-C.; Ryu, S.; et al. CD8α− Dendritic Cells Induce Antigen-Specific T Follicular Helper Cells Generating Efficient Humoral Immune Responses. Cell Rep. 2015, 11, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Milpied, P.J.; McHeyzer-Williams, M.G. High-affinity IgA needs TH17 cell functional plasticity. Nat. Immunol. 2013, 14, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Gupta, P.; Khatri, K.; Goyal, A.; Vyas, S. Edible vaccines: A new approach to oral immunization. Indian J. Biotechnol. 2008, 7, 283–294. [Google Scholar]
- Reboldi, A.; Arnon, T.I.; Rodda, L.B.; Atakilit, A.; Sheppard, D.; Cyster, J.G. IgA production requires B cell interaction with subepithelial dendritic cells in Peyer’s patches. Science 2016, 352. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, M.; Urbano, M.; Valzasina, B.; Francolini, M.; Rotta, G.; Bonasio, R.; Granucci, F.; Kraehenbuhl, J.P.; Ricciardi-Castagnoli, P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001, 2, 361–367. [Google Scholar] [CrossRef] [PubMed]
- McDole, J.R.; Wheeler, L.W.; McDonald, K.G.; Wang, W.; Konjufca, V.; Knoop, K.A.; Newberry, R.D.; Miller, M.J. Goblet cells deliver luminal antigen to CD103+ DCs in the small intestine. Nature 2012, 483, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.; Rosas, G.; Cervantes, J.; Fragoso, G.; Rosales-Mendoza, S.; Sciutto, E. Transgenic plants: A 5-year update on oral antipathogen vaccine development. Expert Rev. Vaccines 2014, 13, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.T.; Daniell, H. Plant-made oral vaccines against human infectious diseases—Are we there yet? Plant Biotechnol. J. 2015, 13, 1056–1070. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, A.; Azegamia, T.; Kiyonoa, H. The mucosal immune system for vaccine development. Vaccine 2014, 32, 6711–6723. [Google Scholar] [CrossRef] [PubMed]
- Richman, L.K.; Chiller, J.M.; Brown, W.R.; Hanson, D.G.; Vaz, N.M. Enterically induced immunologic tolerance. I. Induction of suppressor T lymphoyctes by intragastric administration of soluble proteins. J. Immunol. 1978, 121, 2429–2434. [Google Scholar] [PubMed]
- Kesik-Brodacka, M.; Lipiec, A.; Kozak Ljunggren, M.; Jedlina, L.; Miedzinska, K.; Mikolajczak, M.; Plucienniczak, A.; Legocki, A.B.; Wedrychowicz, H. Immune response of rats vaccinated orally with various plant-expressed recombinant cysteine proteinase constructs when challenged with Fasciola hepatica metacercariae. PLoS Negl. Trop. Dis. 2017, 11, e00045451. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.L.; Paruch, L.; Dobrica, M.-O.; Caras, J.; Tucureanu, C.; Onu, A.; Ciulean, S.; Stavaru, C.; Eerde, A.; Wang, Y.; et al. Lettuce-produced hepatitis C virus E1E2 heterodimer triggers immune responses in mice and antibody production after oral vaccination. Plant Biotechnol. J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kilany, W.H.; Arafa, A.; Erfan, A.M.; Ahmed, M.S.; Nawar, A.A.; Selim, A.A.; Khoulosy, S.G.; Hassan, M.K.; Aly, M.M.; Hafez, H.M.; et al. Isolation of highly pathogenic avian influenza H5N1 from table eggs after vaccinal break in commercial layer flock. Avian Dis. 2010, 54, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Strugnell, R.; Zepp, F.; Cunningham, A.; Tantawichien, T. Vaccines Antigens. Chapter 3. In Understanding Modern Vaccines: Perspectives in Vaccinology; Garçon, N., Stern, P.L., Cunningham, A.L., Stanberry, L.R., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2011; Volume 1, pp. 61–88. [Google Scholar]
- Cebadera, M. Plantas Modificadas Genéticamente Como Vacunas Comestibles: Aspectos Científicos y Socioeconómicos. Ph.D. Thesis, Universidad Complutense Madrid, Madrid, España, 2012. [Google Scholar]
- Cañizares, M.C.; Lomonossoff, G.P.; Nicholson, L. Development of cowpea mosaic virus-based vectors for the production of vaccines in plants. Expert Rev. Vaccines. 2005, 4, 687–697. [Google Scholar]
- Kumar, B.V.; Raja, T.K.; Wani, M.R.; Sheikh, S.A.; Lone, M.A.; Nabi, G.; Azooz, M.M.; Younis, M.; Sarwat, M.; Ahmad, P. Transgenic plants as green factories for vaccine production. Afr. J. Biotechnol. 2013, 12, 6147–6158. [Google Scholar]
- Ma, J.K.; Drake, P.M.; Christou, P. The production of recombinant pharmaceultical proteins in plants. Nature 2003, 4, 794–805. [Google Scholar]
- Mason, H.S.; Warzecha, H.; Mor, T.; Arntzen, C. ; Edible plant vaccines: Applications for prophylactic and therapeutic molecular medicine. Trends Mol. Med. 2002, 8, 324–329. [Google Scholar] [CrossRef]
- Rybicki, E.P. Plant-produced vaccines: Promise and reality. Drug Discov. Today 2009, 14, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Tacket, C.O.; Mason, H.S.; Losonsky, G.; Estes, M.K.; Levine, M.M.; Arntzen, C.J. Human immune responses to a novel Norwalk virus vaccine delivered in transgenic potatoes. J. Infect. Dis. 2000, 182, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Komarova, T.V.; Baschieri, S.; Donini, M.; Marusic, C.; Benvenuto, E.; Dorokhov, Y.L. Transient expression systems for plant-derived biopharmaceuticals. Expert Rev. Vaccines 2010, 9, 859–876. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Schillberg, S.; Hellwig, S.; Twyman, R.M.; Drossard, J. GMP issues for recombinant plant-derived pharmaceutical proteins. Biotechnol. Adv. 2012, 30, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Vamvaka, E.; Twyman, R.M.; Christou, P.; Capell, T. Can plant biotechnology help break the HIV-malaria link? Biotechnol. Adv. 2014, 32, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.; Khan, M.S.; Allison, L. Milestones in chloroplast genetic engineering: An environmentally friendly era in biotechnology. Trends Plant Sci. 2002, 7, 84–91. [Google Scholar] [CrossRef]
- Guan, Z.-J.; Guo, B.; Huo, Y.; Guan, Z.-P.; Dai, J.; Wei, Y. Recent advances and safety issues of transgenic plant-derived vaccines. Appl. Microbiol. Biotechnol. 2013, 97, 2817–2840. [Google Scholar] [CrossRef] [PubMed]
- Waheed, M.T.; Sameeullah, M.; Khan, F.A.; Syed, T.; Ilahi, M.; Gottschamenl, J.; Lössi, A.G. Need of cost-effective vaccines in developing countries: Whay plant biotechnology can offer? SpringerPlus 2016, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Rybicki, E. Plant-made vaccines for humans and animals. Plant Biotechnol. J. 2010, 8, 620–637. [Google Scholar] [CrossRef] [PubMed]
- Shirbaghaee, Z.; Bolhassani, A. Different applications of virus-like particles in biology and medicine: Vaccination and delivery systems. Biopolymers 2016, 105, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Turpen, T.H.; Reinl, S.J.; Charoenvit, Y.; Hoffman, S.L.; Fallarme, V.; Grill, L.K. Malarial epitopes expressed on the surface of recombinant tobacco mosaic virus. Biotechnology 1995, 13, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Brennan, F.R.; Jones, T.D.; Hamilton, W.D. Cowpea mosaic virus as a vaccine carrier of heterologous antigens. Mol. Biotechnol. 2001, 17, 15–26. [Google Scholar] [CrossRef]
- Moxon, E.R.; Siegrist, C.A. The next decade of vaccines: Societal and scientific challenges. New decades of vaccines Series. Lancet 2011, 378, 347–359. [Google Scholar] [CrossRef]
- Huang, Z.; LePore, K.; Elkin, G.; Thanavala, Y.; Mason, H.S. High-yield rapid production of hepatitis B surface antigen in plant leaf by a viral expression system. Plant Biotechnol. J. 2007, 6, 202–209. [Google Scholar] [CrossRef]
- Musiymuck, K.; Stephenson, N.; Bi, H.; Farrance, C.E.; Orozovic, G.; Brodelius, M.; Brodelius, P.; Horsey, A.; Ugulava, N.; Shamloul, A.M.; et al. A launch vector for the production of vaccines in plants. Influenza Other Respir. Viruses 2006, 1, 19–25. [Google Scholar]
- Alvarez, M.A.; Cardineau, G.A. Prevention of bubonic and pneumonic plague using plant-derived vaccines. Biotechnol. Adv. 2010, 28, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Vaquero-Martin, C.; Sack, M.; Drossard, J.; Emans, N.; Commandeur, U. Towards molecular farming in the future: Transient protein expression in plants. Biotechnol. Appl. Biochem. 1999, 30, 113–116. [Google Scholar] [PubMed]
- Legocki, A.B.; Miedzinska, K.; Czaplin, S.M.; Płucieniczak, A.; Wedrychowicz, H. Immunoprotective properties of transgenic plants expressing E2 glycoprotein from CSFV and cysteine protease from Fasciola hepatica. Vaccine 2005, 23, 1844–1846. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, N.; Kiyono, H.; Yuki, Y. Plant-based vaccines for animals and humans: Recent advances in technology and clinical trials. Ther. Adv. Vaccines 2015, 3, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Peyret, H.; Lomonossoff, G. The pEAQ vector series: The easy and quick way to produce recombinant proteins in plants. Plant Mol. Biol. 2013, 83, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Salazar-González, J.; Bañuelos-Hernández, B.; Rosales-Mendoza, S. Current status of viral expression systems in plants and perspectives for oral vaccines development. Plant Mol. Biol. 2015, 87, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Leuzinger, K.; Dent, M.; Hurtado, J.; Stahnke, J.; Lai, H.; Zhou, X.; Chen, Q. Efficient agroinfiltration of plants for high-level transient expression of recombinant proteins. J. Vis. Exp. 2013, 77, e50521. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Lai, H.; Engle, M.; Gorlatov, S.; Gruber, C.; Steinkellner, H.; Diamond, M.S.; Chen, Q. Generation and analysis of novel plant-derived antibody-based therapeutic molecules against west nile virus. PLoS ONE 2014, 9, e93541. [Google Scholar] [CrossRef] [PubMed]
- Fulton, A.; Lai, H.; Chen, Q.; Zhang, C. Purification of monoclonal antibody against Ebola GP1 protein expressed in Nicotiana benthamiana. J. Chromatogr. A 2015, 1389, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.L.; Pinyerd, H.L.; Crisantes, J.D.; Rigano, M.M.; Pinkhasov, J.; Walmsley, A.M.; Mason, H.S.; Cardineau, G.A. Plant-made subunit vaccine against pneumonic and bubonic plague is orally immunogenic in mice. Vaccine 2006, 24, 2477–2490. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Cabrera-Ponce, J.L.; Fragoso, G.; López-Casillas, F.; Guevara-García, A.; Rosas, G. A new highly effective anticysticercosis vaccine expressed in transgenic papaya. Vaccine 2007, 25, 4252–4260. [Google Scholar] [CrossRef] [PubMed]
- Lindh, I.; Brave, A.; Hallengard, D.; Hadad, R.; Kalbina, I.; Strid, A.; Andersson, S. Oral delivery of plant-derived HIV-1 p24 antigen in low doses shows a superior priming effect in mice compared to high doses Ingrid. Vaccine 2014, 32, 2288–2293. [Google Scholar] [CrossRef] [PubMed]
- Moravec, T.; Schmidt, M.A.; Herman, E.M.; Woodford-Thomas, T. Production of Escherichia coli heat labile toxin (LT) B subunit in soybean seed and analysis of its immunogenicity as an oral vaccine. Vaccine 2007, 25, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Loza-Rubio, E.; Rojas-Anaya, E.; Lopez, J.; Olivera-Florez, M.T.; Gómez-Lim, M.; Tapia-Pérez, G. Induction of a protective immune response to rabies virus in sheep after oral immunization with transgenic maize, expressing the rabies virus glycoprotein. Vaccine 2012, 30, 5551–5556. [Google Scholar] [CrossRef] [PubMed]
- Sathish, K.; Sriraman, R.; Subramanian, B.M.; Rao, N.H.; Kasa, B.; Donikeni, J. Plant expressed coccidial antigens as potential vaccine candidates in protecting chicken against coccidiosis. Vaccine 2012, 30, 4460–4464. [Google Scholar] [CrossRef] [PubMed]
- Thanavala, Y.; Lugade, A. Oral transgenic plant-based vaccine for hepatitis B. Immunol. Res. 2010, 46, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yu, L.; Li, L.; Hu, J.; Zhou, J.; Zhou, X. Oral immunization with transgenic rice seeds expressing VP2 protein of infectious bursal disease virus induces protective immune responses in chickens. Plant Biotechnol. J. 2007, 5, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Scissum-Gunn, K.; Singh, N.K.; Giambrone, J.J. Towards development of an edible vaccine for avian reovirus. Avian Dis. 2009, 53, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Kaminuma, O.; Yang, L.; Takai, T.; Mori, A.; Umezu-Goto, M.; Ohtomo, T.; Ohmachi, Y.; Noda, Y.; Hirose, S.; et al. Prevention of allergic asthma by vaccination with transgenic rice seed expressing mite allergen: Induction of allergen-specific oral tolerance without bystander suppression. Plant Biotechnol. J. 2011, 9, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Ashraf, M.; Younis, M.; Hu, X.; Kumar, A.; Akram, N.; Al-Qurainy, F. Role of transgenic plants in agriculture and biopharming. Biotechnol. Adv. 2012, 30, 524–540. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Daniell, H. The engineered chloroplast genome just got smarter. Trends Plant Sci. 2015, 20, 622–640. [Google Scholar] [CrossRef] [PubMed]
- Sack, M.; Hofbauer, A.; Fischer, R.; Stoger, E. The increasing value of plant-made proteins. Curr. Opin. Biotechnol. 2015, 32, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.P.; Trivedi, M.N.; Vachhani, U.D.; Joshi, V. Edible vaccine: A better way for immunization. Int. J. Curr. Pharm. Res. 2011, 3, 53–56. [Google Scholar]
- Bora, A.; Kumar Gogoi, H.; Veer, V. Molecular farming for production of biopharmaceutical and edible vaccines in plants. In Herbal Insecticides, Repellents and Biomedicines: Effectiveness and Commercialization, 1st ed.; Veer, V., Gopalakrishnan, R., Eds.; Springer: New Delhi, India, 2016; p. 264. [Google Scholar]
- Merlin, M.; Pezzotti, M.; Avesani, L. Edible plants for oral delivery of biopharmaceuticals. Br. J. Clin. Pharmacol. 2017, 83, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Juarez, P.; Virdi, V.; Depicker, A.; Orzaez, D. Biomanufacturing of protective antibodies and other therapeutics in edible plant tissues for oral applications. Plant. Biotechnol. J. 2016, 14, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Cherian, S.; Sumithra, T.G.; Raina, O.K.; Sankar, M. Edible vaccines against veterinary parasitic diseases-Current status and future prospects. Vaccine 2013, 31, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, V.; Kumar, J. Edible vaccines. Sri Ramachandra J. Med. 2006, 1, 33–34. [Google Scholar]
- Aryamvally, A.; Gunasekaran, V.; Narenthiran, K.R.; Pasupathi, R. New strategies toward edible vaccines: An overview. J. Diet. Suppl. 2016. [Google Scholar] [CrossRef] [PubMed]
- Qui, X.; Wong, G.; Audet, J.; Bello, A.; Fernando, L.; Alimonti, J.B.; Fausther-Bovendo, H.; Wei, H.; Aviles, J.; Hiatt, E.; et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014, 514, 47–53. [Google Scholar]
- Organización Mundial de la Salud (OMS). Cómo enfrentar los eventos supuestamente atribuidos a la vacunación o inmunización, Vacunación segura; Organización Mundial de la Salud: Washington, DC, USA, 2002. Available online: http://www.who.int/immunization_safety/publications/aefi/en/vacunacion_segura_S.pdf (accessed on 18 June 2015).
- Fernández, A.; Ortigosa, S.; Hervás, S.; Corral, P.; Seguí, J.; Gaétan, J.; Coursaget, P.; Veramendi, J. Human papillomavirus L1 protein expressed in tobacco chloroplasts self-assembles into virus-like particles that are highly immunogenic. Plant Biotechnol. J. 2008, 6, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Specht, E.A.; Mayfield, S.P. Algae-based oral recombinant vaccines. Front. Microbiol. 2014, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Mason, H.S.; Haq, T.A.; Clements, J.D. Edible vaccine protects mice against Escherichia coli heat-labile enterotoxin (LT): Potatoes expressing a synthetic LT-B gene. Vaccine 1998, 16, 1336–1343. [Google Scholar] [CrossRef]
- Arakawa, T.; Chong, D.K.X.; Langridge, W.H.R. Efficacy of a food plant-based oral cholera toxin B subunit vaccine. Nat. Biotechnol. 1998, 16, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Dalsgaard, K.; Uttenthal, A.; Jones, T.D.; Xu, F.; Merryweather, A.; Hamilton, W.D.; Langeveld, J.P.; Boshuizen, R.S.; Kamstrup, S.; Lomonossoff, G.P.; et al. Plant-derived vaccine protects target animals against a viral disease. Nat. Biotechnol. 1997, 15, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Castañon, S.; Marín, M.S.; Martín-Alonso, J.M.; Boga, J.A.; Casais, R.; Humara, J.M.; Ordás, R.J.; Parra, F. Immunization with potato plants expressing VP60 protein protects against rabbit hemorrhagic disease virus. J. Virol. 1999, 73, 4452–4455. [Google Scholar] [PubMed]
- Hahn, B.S.; Jeon, I.S.; Jung, Y.J.; Kim, J.B.; Park, J.S.; Ha, S.H.; Kim, K.H.; Kim, H.M.; Yang, J.S.; Kim, Y.H. Expression of hemagglutinin-neuraminidase protein of Newcastle disease virus in transgenic tobacco. Plant Biotechnol. Rep. 2007, 1, 85–92. [Google Scholar] [CrossRef]
- Mason, H.S.; Ball, J.M.; Shi, J.J.; Jiang, X.; Estes, M.K.; Arntzen, C.J. Expression of Norwlak virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc. Natl. Acad. Sci. USA 1996, 93, 5335–5340. [Google Scholar] [CrossRef] [PubMed]
- Lacorte, C.; Lohuis, H.; Goldbach, R.; Prins, M. Assessing the expression of chicken anemia virus proteins in plants. Virus Res. 2007, 129, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Kostrzak, A.; Cervantes, M.; Guetard, D.; Nagaraju, D.B.; Wain-Hobson, S.; Tepfer, D.; Pniewski, T.; Sala, M. Oral administration of low doses of plant-based HBsAg induced antigen-specific IgAs and IgGs in mice, without increasing levels of regulatory T cells. Vaccine 2009, 27, 4798–4807. [Google Scholar] [CrossRef] [PubMed]
- Gómez, E.; Zoth, S.C.; Asurmendi, S.; Rovere, C.V.; Berinstein, A. Expression of Hemagglutinin-Neuraminidase glycoprotein of Newcastle Disease Virus in agroinfiltrated Nicotiana benthamiana. Plants Biotechnol. J. 2009, 144, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Kanagarajan, S.; Tolf, C.; Lundgren, A.; Waldenstrom, J.; Brodelius, P.E. Transient Expression of Hemagglutinin Antigen from Low Pathogenic Avian Influenza A (H7N7) in Nicotiana benthamiana. PLoS ONE 2012, 7, e33010. [Google Scholar] [CrossRef] [PubMed]
- Shoji, Y.; Farrance, C.E.; Bautista, J.; Bi, H.; Musiychuk, K.; Horsey, A.; Park, H.; Jaje, J.; Green, B.J.; Shamloul, M.; et al. A plant-based system for rapid production of influenza vaccine antigens. Influ. Other Respir Viruses 2012, 6, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Gorantala, J.; Grover, G.; Rahi, A.; Chaudhary, P.; Rajwanshi, R.; Sarin, L.B.; Bhatnagar, R. Generation of protective immune response against anthrax by oralimmunization with protective antigen plant-based vaccine. J. Biotechnol. 2014, 176, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Buehner, N.; Hutson, A.; Estes, M.; Manson, H. Tomato is a highly effective vehicle for expresión and oral immunization with Norwalk virus capsid protein. Plant Biotechnol. J. 2006, 4, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.M.; Yao, Q.H.; Zhang, Z.; Peng, R.H.; Xiong, A.S.; Wang, H.K. Expression of the human hepatitis B virus large surface antigen gene in transgenic tomato plants. Clin. Vaccine Immunol. 2007, 14, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, L.; Kumar, G.; Ganapathi, T.R.; Revathi, C.J.; Bapat, V.A. Transient and stable expression of hepatitis b surface antigen in tomato (Lycopersicon esculentum). Plant Biotechnol. Rep. 2008, 2, 1–6. [Google Scholar] [CrossRef]
- Youm, J.W.; Jeon, J.H.; Kim, H.; Kim, Y.H.; Ko, K.; Joung, H.; Kim, H. Transgenic tomatos expressinghuman beta-amyloid for use as a vaccine against Alzheimer’s disease. Biotechnol. Lett. 2008, 30, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Kim, M.Y.; Kim, B.G.; Kang, T.J.; Kim, Y.S.; Jang, Y.S.; Arntzen, C.J.; Yang, M.S. Syntesis and assembly of Escherichia coli heat-labile enterotoxin B subunit in transgenic lettuce (Lactuca sativa). Protein Expr. Purif. 2007, 51, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Spök, A. Molecular farming on the rise-GMO regulators still walking a tightrope. Trends Biotechnol. 2007, 25, 74–82. [Google Scholar]
- Oszvald, M.; Kang, T.J.; Tomoskozi, S.; Tamas, C.; Tamas, L.; Kim, T.G.; Yang, M.S. Expression of a synthetic neutralizing epitope of porcine epidemic diarrhea virus fused with synthetic b subunit of Escherichia coli heat labile enterotoxin in rice endosperm. Mol. Biotechnol. 2007, 35, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.J.; Shen, H.F.; Liang, W.Q.; Guo, X.M.; Zhang, C.; Wang, Y.; Li, G.; Wu, A.; Cao, K.; Zhang, D. Immunogenicity of recombinant hepatitis b virus surface antigen fused with pres1 epitopes expressed in rice seeds. Transgenic Res. 2008, 17, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Oszvald, M.; Kang, T.J.; Tomoskozi, S.; Jenes, B.; Kim, T.G.; Cha, Y.S.; Tamas, L.; Yang, M.S. Expression of cholera toxin B subunit in transgenic rice endosperm. Mol. Biotechnol. 2008, 40, 261–268. [Google Scholar] [CrossRef] [PubMed]
- USDA. Rice World Markets and Trade. Foreign Agricultural Service/USDA. Office of Global Analysis, January 2017. Available online: https://apps.fas.usda.gov/psdonline/circulars/grain-rice.pdf (accessed on 5 May 2017).
- Rosales-Mendoza, S.; Alpuche-Solís, A.; Soria-Guerra, R.; Moreno-Fierros, L.; Martínez-González, L.; Herrera-Díaz, A.; Korban, S.S. Expression of an Escherichia coli antigenic fusion protein comprising the heat labile toxin B subunit and the heat stable toxin and its assembly as a functional oligomer in transplastomic tobacco plants. Plant J. 2008, 57, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, M.; Li, Y.; Zhao, Y.; He, H.; Yang, G.; Zheng, C. Oral immunogenicity and protective efficacy in mice of a carrot-derived vaccine candidate expressing UreB subunit against Helicobacter pylori. Protein Expr. Purif. 2010, 69, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Ekam, V.S.; Udosen, E.O.; Chighu, A.E. Comparative Effect of Carotenoid Complex from Goldenneo-Life Dynamite and Carrot Extracted Carotenoids on Immune Parameters in Albino Wistar Rats. Niger. J. Physiol. Sci. 2006, 21, 1–4. [Google Scholar] [PubMed]
- Wigdorovitz, A.; Pérez Filgueira, D.M.; Robertson, N.; Carrillo, C.; Sadir, A.M.; Morris, T.J.; Borca, M.V. Protection of mice against challenge with foot and mouth disease virus (FMDV) by immunization with foliar extracts from plants infected with recombinant tobacco mosaic virus expressing the FMDV structural protein VP1. Virology 1999, 264, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Wigdorovitz, A.; Mozovoj, M.; Santos, M.; Parreno, V.; Gomez, C.; Perez-Filgueira, D.M.; Trono, K.G.; Ríos, R.D.; Franzone, P.M.; Fernández, F.; et al. Protective lactogenic immunity conferred by an edible peptide vaccine to bovine rotavirus produced in transgenic plants. J. Gen. Virol. 2004, 85, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.K.; Liao, S.C.; Chang, C.C.; Liu, H.J. Expression of avian reovirus σC protein in transgenic plants. J. Virol. Methods 2006, 134, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Yan-Ju, Y.E.; Wen-Gui, L.I. Immunoprotection of transgenic alfalfa (Medicago sativa) containing Eg95-EgA31 fusion gene of Echinococcus granulosus against Eg protoscoleces. J. Trop. Med. 2010, 3, 10–13. [Google Scholar]
- Guerrero-Andrade, O.; Loza-Rubio, E.; Olivera-Flores, T.; Fehérvári-Bone, T.; Gómez-Lim, M.A. Expression of the Newcastle disease virus fusion protein in transgenic maize and immunological studies. Transgenic Res. 2006, 15, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Chen, T.H.; Hu, C.C.; Liao, J.T.; Lee, C.W.; Liao, J.W.; Lin, M.Y.; Liu, H.J.; Wang, M.Y.; Lin, N.S.; et al. Induction of protective immunity in chickens immunized with plant–made chimeric Bamboo mosaic virus particles expressing very virulent Infectious bursal disease virus antigen. Virus Res. 2012, 166, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.B.S.; Ganapathi, T.R.; Revathi, C.J.; Srinivas, L.; Bapat, V.A. Expression of hepatitis B surface antigen in transgenic banana plants. Planta 2005, 222, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.-J.; Guo, B.; Huo, Y.-L.; Guan, Z.-P.; Wei, Y.-H. Overview of expression of hepatitis B surface antigen in transgenic plants. Vaccine 2010, 28, 7351–7362. [Google Scholar] [CrossRef] [PubMed]
- Satyavathi, V.V.; Prasad, V.; Khandelwal, A.; Shaila, M.S.; Sita, G.L. Expression of hemagglutinin protein of Rinderpest virus in transgenic pigeon pea [Cajanus cajan (L.) Millsp.] plants. Plant Cell Rep. 2003, 21, 651–658. [Google Scholar] [PubMed]
- Lau, J.M.; Korban, S.S. Transgenic apple expressing an antigenic protein of the human respiratory synsytial virus. J. Plant Physiol. 2010, 167, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ma, Y.; Li, M.; Cheng, T.; Li, S.-W.; Zhang, J.; Xia, N.-S. Oral immunization of animals with transgenic cherry tomatillo expressing HBsAg. World J. Gastroenterol. 2003, 9, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.; Zhou, B.; Pettersson, P.L.; Gonzalez, M.J.; Mayfield, S.P. Synthesis and assembly of a full-length human monoclonal antibody in algal chloroplasts. Biotechnol. Bioeng. 2009, 104, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Fan, C.; Chen, Y.; Hu, Z. The Potential for Microalgae as Bioreactors to Produce Pharmaceuticals. Int. J. Mol. Sci. 2016, 17, e962. [Google Scholar] [CrossRef] [PubMed]
- Franconi, R.; Demurtas, O.C.; Massa, S. Plant-derived vaccines and other therapeutics produced in contained systems. Expert Rev. Vaccines 2010, 9, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Dreesen, I.A.; Charpin-El, H.G.; Fussenegger, M. Heat-stable oral alga-based vaccine protects mice from Staphylococcus aureus infection. J. Biotechnol. 2010, 145, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.A.; Topol, A.B.; Doerner, D.Z.; Mayfield, S. Alga-produced cholera toxin-pfs25 fusion proteins as oral vaccines. Appl. Environ. Microbiol. 2013, 79, 3917–3925. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.E.; Mayfield, S.P. Recent developments in the production of human therapeutic proteins in eukaryotic algae. Expert Opin. Biol. Ther. 2005, 5, 225–235. [Google Scholar] [CrossRef] [PubMed]
- He, D.M.; Qian, K.X.; Shen, G.F.; Zhang, Z.F.; Li, Y.N.; Su, Z.L.; Shao, H.B. Recombination and expression of classical swine fever virus (CSFV) structural protein E2 gene in Chlamydomonas reinhardtii chroloplasts. Colloids Surf. B Biointerphases 2007, 55, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Van der Laan, J.W.; Minor, P.; Mahoney, R.; Arntzen, C.; Shin, J.; Wood, D. WHO informal consultation on scientific basis for regulatory evaluation of candidate human vaccines from plants, Geneva, Switzerland, 24–25 January 2005. Vaccine 2006, 24, 4271–4278. [Google Scholar] [CrossRef] [PubMed]
- OMS. Immunization, Vaccines and Biologicals. 2014. Available online: http://www.who.int/immunization_standards/vaccine_regulation/en/# (accessed on 25 March 2015).
- Maxwell, S. Analysis of laws governing combination products, transgenic food, pharmaceutical products and their applicability to edible vaccines. BYU Law Rev. 2014, 28, 65–82. [Google Scholar]
- Lal, P.; Ramachandran, V.G.; Goyal, R.; Sharma, R. Edible vaccines: Current status and future. Indian J. Med. Microbiol. 2007, 25, 93–102. [Google Scholar] [PubMed]
- Colson, P.; Richet, H.; Desnues, D.; Balique, B.; Moal, V.; Grob, J.-J.; Bernis, P.; Lecoq, H.; Harlé, J-R.; Berland, Y.; et al. Pepper Mild Mottle Virus, a Plant Virus Associated with Specific Immune Responses, Fever, Abdominal Pains, and Pruritus in Humans. PLoS ONE 2010, 5, e1004. [Google Scholar] [CrossRef] [PubMed]
- Hirlekar, R.; Bhairy, S. Edible vaccines: An advancement in oral immunization. Asian J. Pharm. Clin. Res. 2017, 10, 82–88. [Google Scholar]
- Amin, L.; Ayuni, N.; Azlan, A.; Ahmad, J. Ethical perception of human gene in transgenic banana. Afr. J. Biotechnol. 2011, 10, 12486–12496. [Google Scholar]
- Zapanta, P.E.; Ghorab, S. Age of bioterrorism: Are you prepared? Review of bioweapons and their clinical presentation for otolaryngologist. Otolaryngol. Head Neck 2014, 151, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Arntzen, C. Plant-made pharmaceuticals: From “edible vaccines” to ebola therapeutics. Plant Biotechnol. J. 2015, 13, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Mbongue, J.C.; Nicholas, D.A.; Zhang, K.; Kim, N.S.; Hamilton, B.N.; Larios, M.; Zhang, G.; Umezawa, K.; Firek, A.F.; Langridge, W.H. Induction of indoleamine 2, 3-dioxygenase in human dendritic cells by a cholera toxin B subunit-proinsulin vaccine. PLoS ONE 2015, 10, e0118562. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.S.; Mbongue, J.C.; Nicholas, D.A.; Esebanmen, G.E.; Unternaehrer, J.J.; Firek, A.F.; Langridge, W.H. Chimeric Vaccine Stimulation of Human Dendritic Cell Indoleamine 2, 3-Dioxygenase Occurs via the Non-Canonical NF-kB Pathway. PLoS ONE 2016, 11, e0147509. [Google Scholar]
| Infectious Diseases | Number of Countries Affected | Year(s) of Outbreak Occurrence (Since 2010) | Edible Vaccines Already Tested in Animals (Not Humans) |
|---|---|---|---|
| Zika | 29 | 2015, 2016 | |
| Poliomyelitis | 19 | 2010, 2011, 2013 to 2016 | |
| Measles | 17 | 2010, 2011, 2013 to 2015 | |
| Coronavirus (MERS-CoV) | 15 | 2012 to 2016 | Tomato [23], Corn [24,25,26] |
| Ebola | 12 | 2011, 2012, 2014, 2015 | |
| Yellow fever | 12 | 2010 to 2013, 2016 | |
| Cholera | 8 | 2010 to 2013, 2015 | Potato [27], Tomato [28], Algae [29] |
| Lassa fever | 7 | 2012, 2015, 2016 | |
| Chikungunya | 6 | 2014, 2015, 2016 | |
| Dengue | 5 | 2010, 2012, 2015, 2016 | |
| Avian influenza, H5N1 virus | 5 | 2010 to 2014 | |
| Rift Valley fever | 4 | 2010, 2012, 2016 | |
| West Nile virus | 3 | 2011, 2014, 2015 | |
| Microcephaly | 3 | 2015, 2016 | |
| Meningococcal disease | 2 | 2010, 2015 | |
| Plagues (bubonic, pneumonic) | 2 | 2010, 2015 | |
| Rubella | 2 | 2014, 2015 | Tomato [23] |
| Monkeypox | 1 | 2016 | |
| Marburg hemorrhagic fever | 1 | 2012 | |
| Typhoid fever | 1 | 2015 | |
| Hantavirus | 1 | 2012 | |
| Enterovirus D68 | 1 | 2014 | |
| Elizabethkingia | 1 | 2016 | |
| Oropouche virus | 1 | 2016 | |
| Avian influenza, H7N9 virus | 1 | 2013 to 2016 | |
| Avian influenza, H5N6 virus | 1 | 2014 to 2016 | |
| Crimean-Congo hemorrhagic fever | 1 | 2010 | |
| Hemolytic uremic syndrome | 1 | 2011 | |
| Diphtheria | 1 | 2015 | |
| Enterohemorrhagic Escherichia coli | 1 | 2016 |
| Year | Plant | Disease or Infectious Agent | Antigen | References |
|---|---|---|---|---|
| 1998 | Potato | Enteritis produced by Escherichia col | - | [103] |
| 1998 | Potato | Norwalk virus capsid | - | [104] |
| 1998 | Potato | Non-toxic subunit (CT-B) of Vibrio cholerae enterotoxin | - | [27] |
| 1998 | Potato | Rabbit hemorrhagic | Protein VP60 | [106] |
| 2003 | Algae | Foot-and-mouth disease virus | Viral structural protein VP1 | [29] |
| 2003 | Cherry tomatillo | Hepatitis B | HBsAg (surface protein of Hepatitis B) | [138] |
| 2003 | Pea | Rinderpest virus | Hemagglutinin protein (H) | [98,136] |
| 2004 | Alfalfa | Hog rotavirus (BVR) | Antigen eBRV4 | [129] |
| 2005 | Banana | Hepatitis B | HBsAg (surface protein of Hepatitis B) | [134,135] |
| 2005 | Lettuce | Hog pest virus | Glycoprotein E2 | [72] |
| 2005 | Potato | Hepatitis B | - | [72] |
| 2005 | Tomato | Coronavirus | - | [23] |
| 2006 | Tomato | Norwalk virus | Surface protein | [50,115] |
| 2007 | Algae | Swine fever (CSFV) disease | Surface protein E2 | [102,145] |
| 2007 | Papaya | Cysticercosis caused by Taenia solium | Synthetic peptides | [80] |
| 2007 | Rice | Infectious bursitis | VP2 protein | [86] |
| 2007 | Tomato | Vibrio cholerae B toxin | CT-B protein | [28] |
| 2007 | Tomato | Hepatitis B | HBsAg (surface protein of Hepatitis B) | [50,116,117] |
| 2007 | Tobacco * | Chicken infectious anemia | Virus VP1 protein | [109] |
| 2008 | Rice | Hepatitis B | HBsAg (surface protein of Hepatitis B) | [122,123] |
| 2010 | Carrot | Helicobacter pylori | Subunidad UreB | [126] |
| 2010 | Corn | Rabies virus | Antigen glycoproteins | [13,85] |
| 2012 | Tobacco * | Avian flu virus | HPAIV H5N1 | [112,113] |
| 2012 | Quinoa | Infectious bursitis virus | VP2 protein | [133] |
| 2014 | Algae | Diabetes | Glutamic acid decarboxylase | [102] |
| 2014 | Algae | Human Papilloma Virus | E7 protein | [102] |
| 2014 | Algae | Hepatitis B | HBsAg (surface protein of Hepatitis B) | [102] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Concha, C.; Cañas, R.; Macuer, J.; Torres, M.J.; Herrada, A.A.; Jamett, F.; Ibáñez, C. Disease Prevention: An Opportunity to Expand Edible Plant-Based Vaccines? Vaccines 2017, 5, 14. https://doi.org/10.3390/vaccines5020014
Concha C, Cañas R, Macuer J, Torres MJ, Herrada AA, Jamett F, Ibáñez C. Disease Prevention: An Opportunity to Expand Edible Plant-Based Vaccines? Vaccines. 2017; 5(2):14. https://doi.org/10.3390/vaccines5020014
Chicago/Turabian StyleConcha, Christopher, Raúl Cañas, Johan Macuer, María José Torres, Andrés A. Herrada, Fabiola Jamett, and Cristian Ibáñez. 2017. "Disease Prevention: An Opportunity to Expand Edible Plant-Based Vaccines?" Vaccines 5, no. 2: 14. https://doi.org/10.3390/vaccines5020014







