Anion- or Cation-Exchange Membranes for NaBH4/H2O2 Fuel Cells?
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
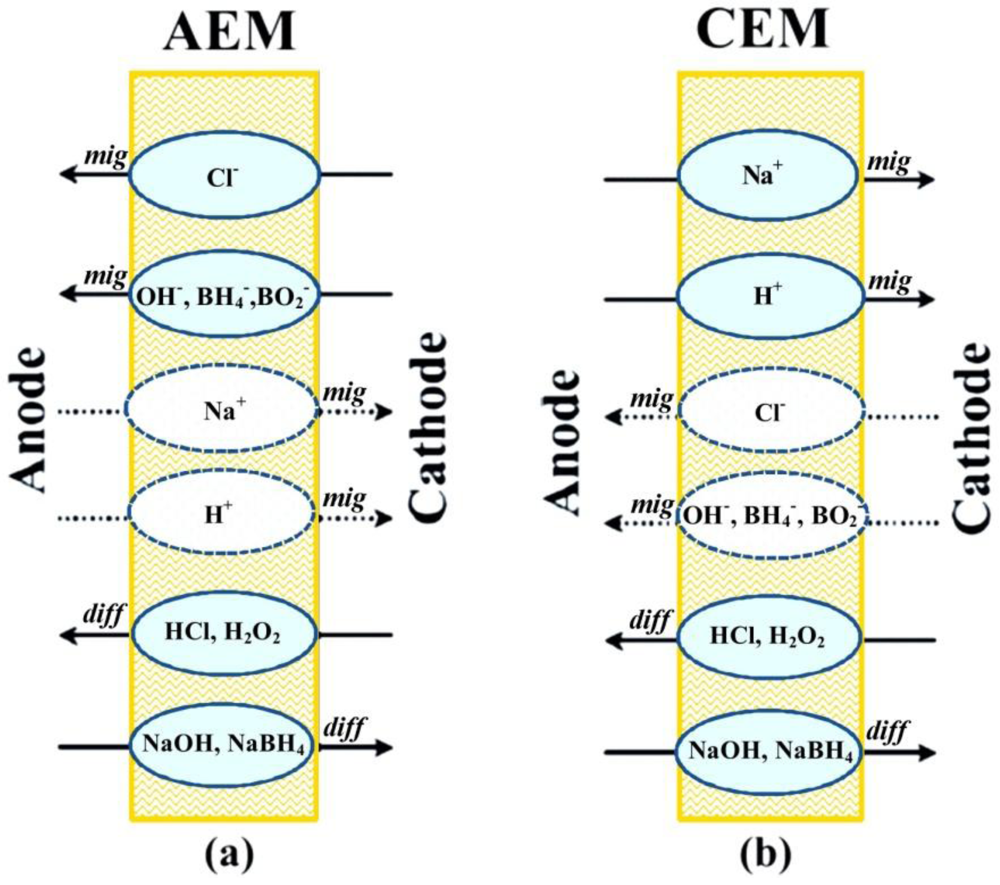
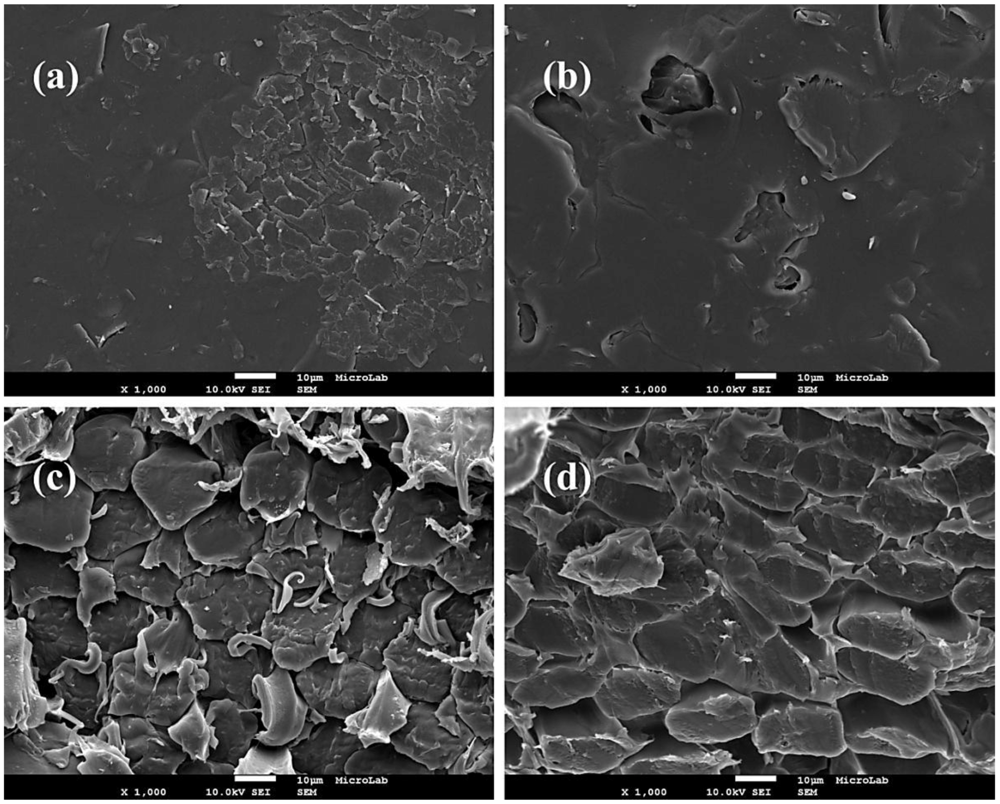
3.1. Membranes Characterization
| AMI-7001S | CMI-7000S | |
|---|---|---|
| Membrane type | heterogeneous strong base anion exchange membrane | heterogeneous strong acid cation exchange membrane |
| Polymer structure | polystyrene gel cross linked with divinylbenzene | |
| Functional group | quaternary ammonium | sulfonic acid |
| Ionic form (as shipped) | Cl− | Na+ |
| Standard thickness (mm) | 0.45 | |
| Electrical resistance (Ω cm2) 0.5 M NaCl | <40 | <30 |
| Permselectivity (0.1 mol KCl kg−1/0.5 mol KCl kg−1) | 90 | 94 |
| Total exchange capacity (meq·g−1) | 1.3 | 1.6 |
| Water permeability (cm3·h−1·m−2 @ 35 kPa) | <32 | |
| Mullen burst strength test (MPa) | >0.62 | |
| Thermal stability (°C) | 90 | |
| Membrane cost (€ per m2) | 75 | |
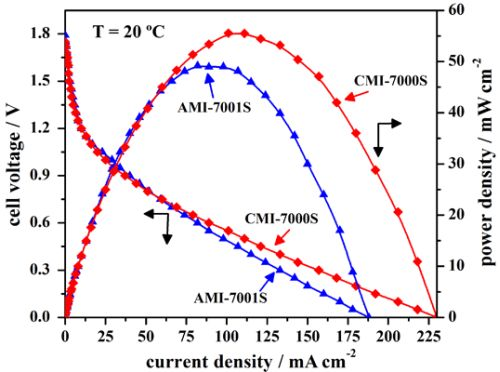
3.2. Cell Performance Study
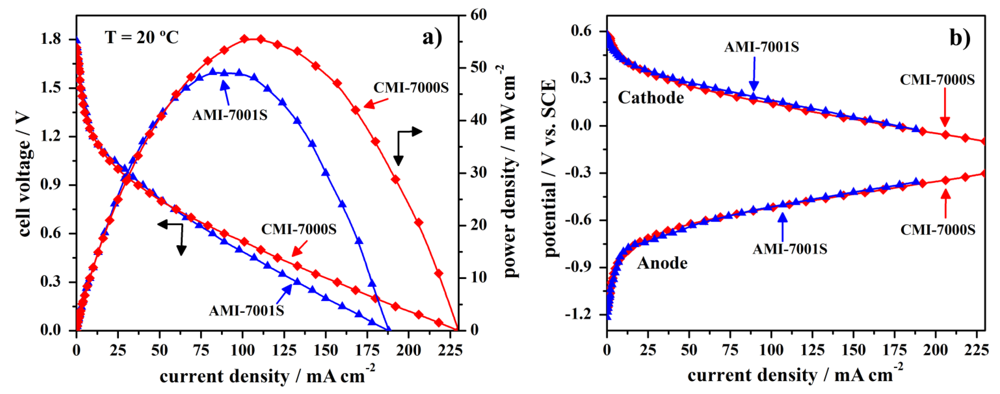
| Open-circuit voltage (V) | Short circuit current density (mA·cm−2) | Peak power density (mW·cm−2) | Cell voltage at peak power density (V) | Current density at peak power density (mA·cm−2) | |
|---|---|---|---|---|---|
| AMI-7001S | 1.79 | 188 | 49 | 0.60 | 82 |
| CMI-7000S | 1.75 | 230 | 56 | 0.55 | 101 |
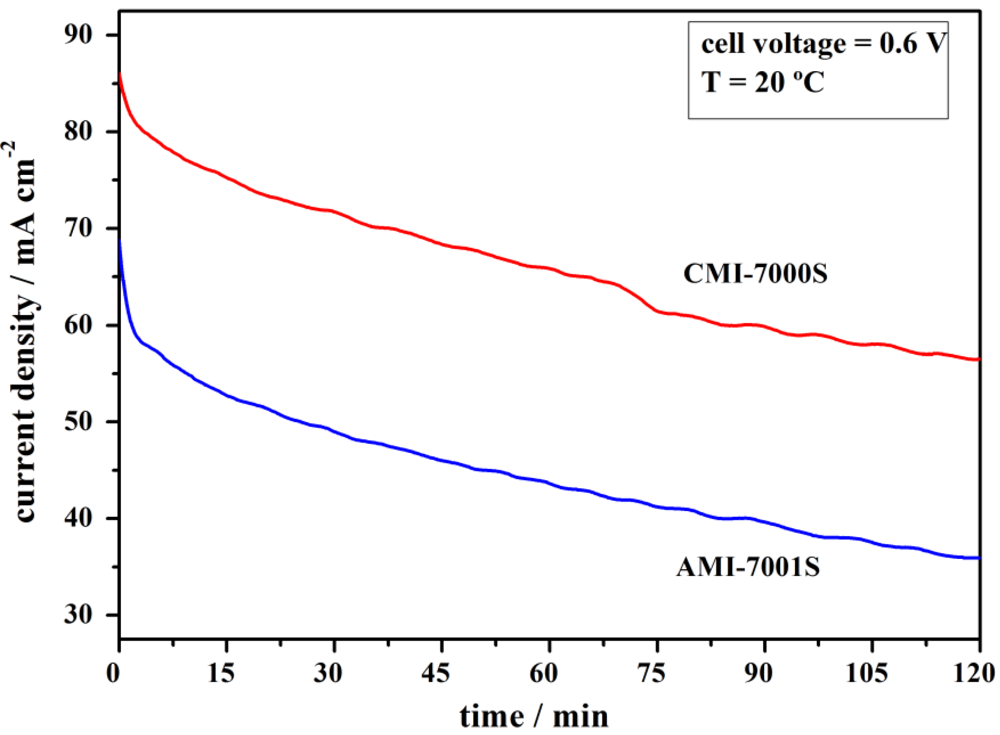
3.3. Cell Stability Study
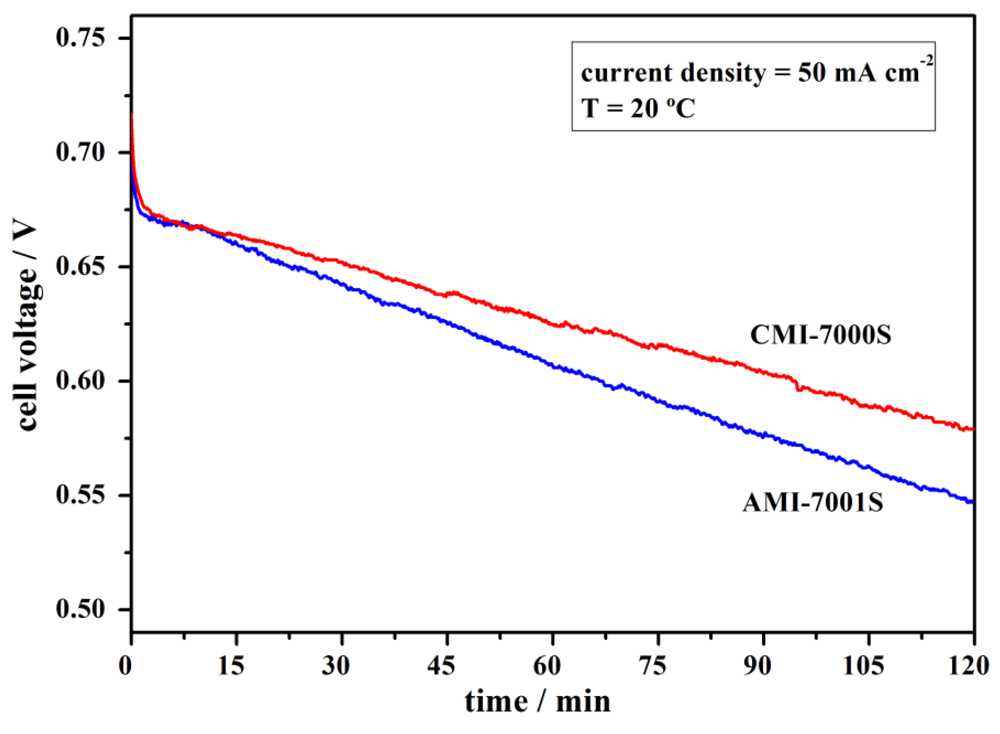
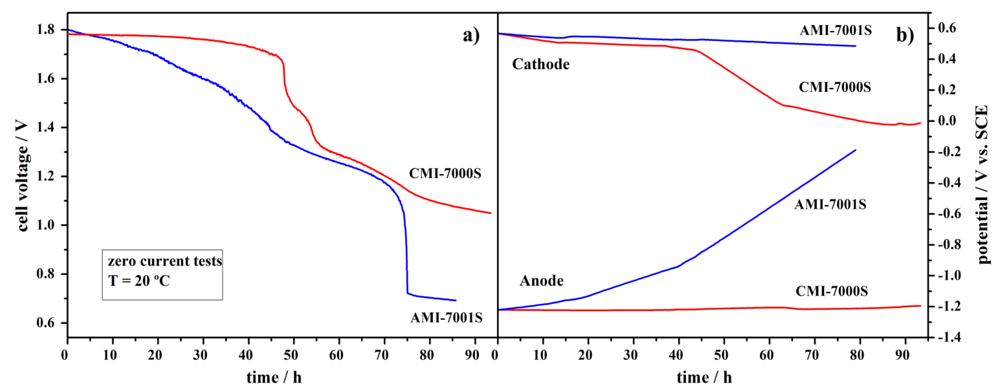
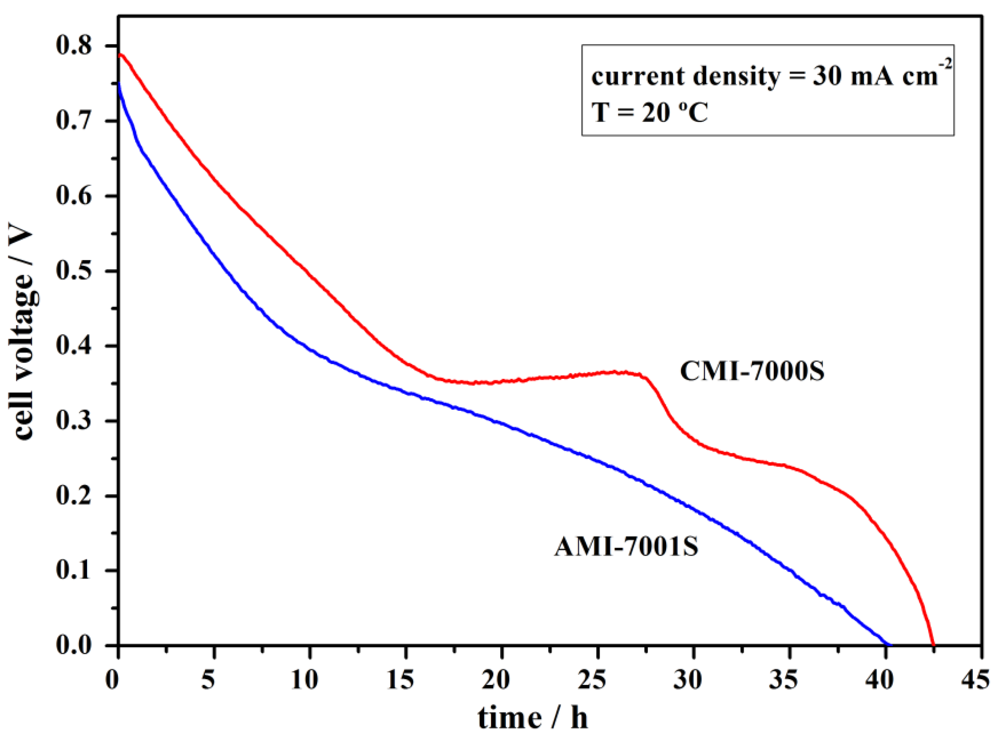
4. Conclusions
Acknowledgments
References
- Santos, D.M.F.; Sequeira, C.A.C. Sodium borohydride as a fuel for the future. Renew. Sustain. Energy Rev. 2011, 15, 3980–4001. [Google Scholar] [CrossRef]
- Ma, J.; Choudhury, N.A.; Sahai, Y. A comprehensive review of direct borohydride fuel cells. Renew. Sustain. Energy Rev. 2010, 14, 183–199. [Google Scholar] [CrossRef]
- Ponce de Leon, C.; Walsh, F.C. Fuel cells—Exploratory fuel cells: Sodium borohydride fuel cells. In Encyclopedia of Electrochemical Power Sources, 1st; Garche, J., Ed.; Elsevier: Amsterdam, the Netherlands, 2009; pp. 192–205. [Google Scholar]
- Ponce de Leon, C.; Walsh, F.C.; Pletcher, D.; Browning, D.J.; Lakeman, J.B. Direct borohydride fuel cells. J. Power Sources 2006, 155, 172–181. [Google Scholar] [CrossRef]
- Demirci, U.B. Direct liquid-feed fuel cells: Thermodynamic and environmental concerns. J. Power Sources 2007, 169, 239–246. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Sequeira, C.A.C. On the electrosynthesis of sodium borohydride. Int. J. Hydrog. Energy 2010, 35, 9851–9861. [Google Scholar] [CrossRef]
- Choudhury, N.A.; Raman, R.K.; Sampath, S.; Shukla, A.K. An alkaline direct borohydride fuel cell with hydrogen peroxide as oxidant. J. Power Sources 2005, 143, 1–8. [Google Scholar] [CrossRef]
- Raman, R.K.; Choudhury, N.A.; Shukla, A.K. A high output voltage direct borohydride fuel cell. Electrochem. Solid-State Lett. 2004, 7, A488–A491. [Google Scholar] [CrossRef]
- Demirci, U.B. Direct borohydride fuel cell: Main issues met by the membrane-electrodes-assembly and potential solutions. J. Power Sources 2007, 172, 676–687. [Google Scholar] [CrossRef]
- Yi, L.; Song, Y.; Yi, W.; Wang, X.; Wang, H.; He, P.; Hu, B. Carbon supported Pt hollow nanospheres as anode catalysts for direct borohydride-hydrogen peroxide fuel cells. Int. J. Hydrog. Energy 2011, 36, 11512–11518. [Google Scholar]
- Santos, D.M.F.; Sequeira, C.A.C. Cyclic voltammetry investigation of borohydride oxidation at a gold electrode. Electrochim. Acta 2010, 55, 6775–6781. [Google Scholar]
- Santos, D.M.F.; Sequeira, C.A.C. Chronopotentiometric investigation of borohydride oxidation at a gold electrode. J. Electrochem. Soc. 2010, 157, F16–F21. [Google Scholar] [CrossRef]
- Yi, L.; Song, Y.; Wang, X.; Yi, L.; Hu, J.; Su, G.; Yi, W.; Yan, H. Carbon supported palladium hollow nanospheres as anode catalysts for direct borohydride-hydrogen peroxide fuel cells. J. Power Sources 2012, 205, 63–70. [Google Scholar] [CrossRef]
- Sanli, E.; Uysal, B.Z.; Aksu, M.L. The oxidation of NaBH4 on electrochemically treated silver electrodes. Int. J. Hydrog. Energy 2008, 33, 2097–2104. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P.; Suda, S. Anodic oxidation of alkali borohydrides catalyzed by nickel. J. Electrochem. Soc. 2003, 150, A398–A402. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Sequeira, C.A.C. Zinc anode for direct borohydride fuel cells. J. Electrochem. Soc. 2010, 157, B13–B19. [Google Scholar] [CrossRef]
- Tegou, A.; Armyanov, S.; Valova, E.; Steenhaut, O.; Hubin, A.; Kokkinidis, G.; Sotiropoulos, S. Mixed platinum-gold electrocatalysts for borohydride oxidation prepared by the galvanic replacement of nickel deposits. J. Electroanal. Chem. 2009, 634, 104–110. [Google Scholar] [CrossRef]
- Molina Concha, B.; Chatenet, M. Direct oxidation of sodium borohydride on Pt, Ag and alloyed Pt-Ag electrodes in basic media Part I: Bulk electrodes. Electrochim. Acta 2009, 54, 6119–6129. [Google Scholar] [CrossRef]
- Cao, D.; Chen, D.; Lan, J.; Wang, G. An alkaline direct NaBH4-H2O2 fuel cell with high power density. J. Power Sources 2009, 190, 346–350. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Saturnino, P.G.; Macciò, D.; Saccone, A.; Sequeira, C.A.C. Platinum-rare earth intermetallic alloys as anode electrocatalysts for borohydride oxidation. Catal. Today 2011, 170, 134–140. [Google Scholar] [CrossRef]
- Yi, L.; Song, Y.; Liu, X.; Wang, X.; Zou, G.; He, P.; Yi, W. High activity of Au-Cu/C electrocatalyst as anodic catalyst for direct borohydride-hydrogen peroxide fuel cell. Int. J. Hydrog. Energy 2011, 36, 15775–15782. [Google Scholar]
- Atwan, M.H.; Northwood, D.O.; Gyenge, E.L. Evaluation of colloidal Os and Os-alloys (Os-Sn, Os-Mo and Os-V) for electrocatalysis of methanol and borohydride oxidation. Int. J. Hydrog. Energy 2005, 30, 1323–1331. [Google Scholar] [CrossRef]
- Cao, D.; Sun, L.; Wang, G.; Lv, Y.; Zhang, M. Kinetics of hydrogen peroxide electroreduction on Pd nanoparticles in acidic medium. J. Electroanal. Chem. 2008, 621, 31–37. [Google Scholar] [CrossRef]
- Bessette, R.R.; Cichon, J.M.; Dischert, D.W.; Dow, E.G. A study of cathode catalysis for the aluminium hydrogen peroxide semi-fuel cell. J. Power Sources 1999, 80, 248–253. [Google Scholar] [CrossRef]
- Yang, W.; Yang, S.; Sun, W.; Sun, G.; Xin, Q. Nanostructured palladium-silver coated nickel foam cathode for magnesium-hydrogen peroxide fuel cells. Electrochim. Acta 2006, 52, 9–14. [Google Scholar] [CrossRef]
- Adams, B.D.; Ostrom, C.K.; Chen, A. Highly active Pd-Pt catalysts for the electrochemical reduction of H2O2. J. Electrochem. Soc. 2011, 158, B434–B439. [Google Scholar]
- Sun, L.; Cao, D.; Wang, G. Pd-Ru/C as the electrocatalyst for hydrogen peroxide reduction. J. Appl. Electrochem. 2008, 38, 1415–1419. [Google Scholar] [CrossRef]
- Miao, X.M.; Yuan, R.; Chai, Y.Q.; Shi, Y.T.; Yuan, Y.Y. Direct electrocatalytic reduction of hydrogen peroxide based on Nafion and copper oxide nanoparticles modified Pt electrode. J. Electroanal. Chem. 2008, 612, 157–163. [Google Scholar] [CrossRef]
- Stewart, K.L.; Gewirth, A.A. Mechanism of electrochemical reduction of hydrogen peroxide on copper in acidic sulfate solutions. Langmuir 2007, 23, 9911–9918. [Google Scholar] [CrossRef]
- Gu, L.; Luo, N.; Miley, G.H. Cathode electrocatalyst selection and deposition for a direct borohydride/hydrogen peroxide fuel cell. J. Power Sources 2007, 173, 77–85. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Saturnino, P.G.; Lobo, R.F.M.; Sequeira, C.A.C. Direct borohydride/peroxide fuel cells using Prussian Blue cathodes. J. Power Sources 2012, 208, 131–137. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Sequeira, C.A.C. Effect of membrane separators on the performance of direct borohydride fuel cells. J. Electrochem. Soc. 2012, 159, B126–B132. [Google Scholar] [CrossRef]
- Couture, G.; Alaaeddine, A.; Boschet, F.; Ameduri, B. Polymeric materials as anion-exchange membranes for alkaline fuel cells. Prog. Polym. Sci. 2011, 36, 1521–1557. [Google Scholar] [CrossRef]
- Wu, H.; Wang, C.; Liu, Z.; Mao, Z. Influence of operation conditions on direct NaBH4/H2O2 fuel cell performance. Int. J. Hydrog. Energy 2010, 35, 2648–2651. [Google Scholar] [CrossRef]
- Ma, J.; Choudhury, N.A.; Sahai, Y.; Buchheit, R.G. A high performance direct borohydride fuel cell employing cross-linked chitosan membrane. J. Power Sources 2011, 196, 8257–8264. [Google Scholar]
- Aziznia, A.; Oloman, C.W.; Gyenge, E.L. A Swiss-roll liquid-gas mixed-reactant fuel cell. J. Power Sources 2012, 212, 154–160. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Sequeira, C.A.C. Polymeric membranes for direct borohydride fuel cells: A comparative study. ECS Transactions 2010, 25, 111–122. [Google Scholar]
- Smith, B.; Sridhar, S.; Khan, A.A. Solid polymer electrolyte membranes for fuel cell applications—A review. J. Membrane Sci. 2005, 259, 10–26. [Google Scholar] [CrossRef]
- Slade, S.; Campbell, S.A.; Ralph, T.R.; Walsh, F.C. Ionic conductivity of an extruded Nafion 1100 EW series of membranes. J. Electrochem. Soc. 2002, 149, A1556–A1564. [Google Scholar] [CrossRef]
- Bendert, J.C.; Papadias, D.D.; Myers, D.J. The effect of Na+ impurities on the conductivity and water uptake of Nafion 115 polymer electrolyte fuel cell membranes. J. Electrochem. Soc. 2010, 157, B1486–B1490. [Google Scholar] [CrossRef]
- Prakash, P.; Hoskins, D.; SenGupta, A.K. Application of homogeneous and heterogeneous cathion-exchange membranes in coagulant recovery from water treatment plant residuals using Donnan membrane process. J. Membrane Sci. 2004, 237, 131–144. [Google Scholar] [CrossRef]
- Sahu, A.K.; Pitchumani, S.; Sridhar, P.; Shukla, A.K. Nafion and modified-Nafion membranes for polymer electrolyte fuel cells: An overview. Bull. Mater. Sci. 2009, 32, 285–294. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, H.; Shen, P.K. Methanol-blocking Nafion composite membranes fabricated by layer-by-layer self-assembly for direct methanol fuel cells. Int. J. Hydrog. Energy 2012, 37, 6875–6879. [Google Scholar] [CrossRef]
- Yu, T.L. Nafion/PTFE composite membranes for fuel cell applications. J. Polym. Res. 2004, 11, 217–224. [Google Scholar] [CrossRef]
- Cheng, H.; Scott, K.; Lovell, K.V.; Horsfall, J.A.; Waring, S.C. Evaluation of new ion exchange membranes for direct borohydride fuel cells. J. Membrane Sci. 2007, 288, 168–174. [Google Scholar] [CrossRef]
- Li, Z.P.; Liu, B.H.; Arai, K.; Suda, S. Development of the direct borohydride fuel cell. J. Alloy. Compd. 2005, 404-406, 648–652. [Google Scholar] [CrossRef]
- Yang, C.-C.; Li, Y.J.; Chiu, S.-J.; Lee, K.-T.; Chien, W.-C.; Huang, C.-A. A direct borohydride fuel cell based on poly(vinyl alcohol)/hydroxyapatite composite polymer electrolyte membrane. J. Power Sources 2008, 184, 95–98. [Google Scholar] [CrossRef]
- Ma, J.; Sahai, Y.; Buchheit, R.G. Evaluation of multivalent phosphate cross-linked chitosan biopolymer membrane for direct borohydride fuel cells. J. Power Sources 2012, 202, 18–27. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Sequeira, C.A.C. Sodium borohydride determination by measurement of open circuit potentials. J. Electroanal. Chem. 2009, 627, 1–8. [Google Scholar] [CrossRef]
- Sequeira, C.A.C.; Hooper, A. Solid State Batteries; Martin Nijhoff Publishers: Dordrecht, the Netherlands, 1985. [Google Scholar]
- Lakeman, J.B.; Rose, A.; Pointon, K.D.; Browning, D.J.; Lovell, K.V.; Waring, S.C.; Horsfall, J.A. The direct borohydride fuel cell for UUV propulsion power. J. Power Sources 2006, 162, 765–772. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Šljukić, B.; Morais, A.L.; Santos, D.M.F.; Sequeira, C.A.C. Anion- or Cation-Exchange Membranes for NaBH4/H2O2 Fuel Cells? Membranes 2012, 2, 478-492. https://doi.org/10.3390/membranes2030478
Šljukić B, Morais AL, Santos DMF, Sequeira CAC. Anion- or Cation-Exchange Membranes for NaBH4/H2O2 Fuel Cells? Membranes. 2012; 2(3):478-492. https://doi.org/10.3390/membranes2030478
Chicago/Turabian StyleŠljukić, Biljana, Ana L. Morais, Diogo M. F. Santos, and César A. C. Sequeira. 2012. "Anion- or Cation-Exchange Membranes for NaBH4/H2O2 Fuel Cells?" Membranes 2, no. 3: 478-492. https://doi.org/10.3390/membranes2030478