Effect of Preparation Methods on Crystallization Behavior and Tensile Strength of Poly(vinylidene fluoride) Membranes
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of NIPS Membranes
2.3. Preparation of Melt Spinning Membranes
2.4. Preparation of Solution-Cast Membranes
| Membrane label | PVDF concentration in casting solution (wt %) | PG concentration in casting solution (wt %) | Evaporation temperature (°C) |
|---|---|---|---|
| F5-60 | 5 | 0 | 60 |
| F5-140 | 5 | 0 | 140 |
| F5-7-60 | 5 | 7 | 60 |
| F5-7-140 | 5 | 7 | 140 |
2.5. Membrane Characterization




3. Results and Discussion
3.1. Crystalline Structures in the Surface Layers
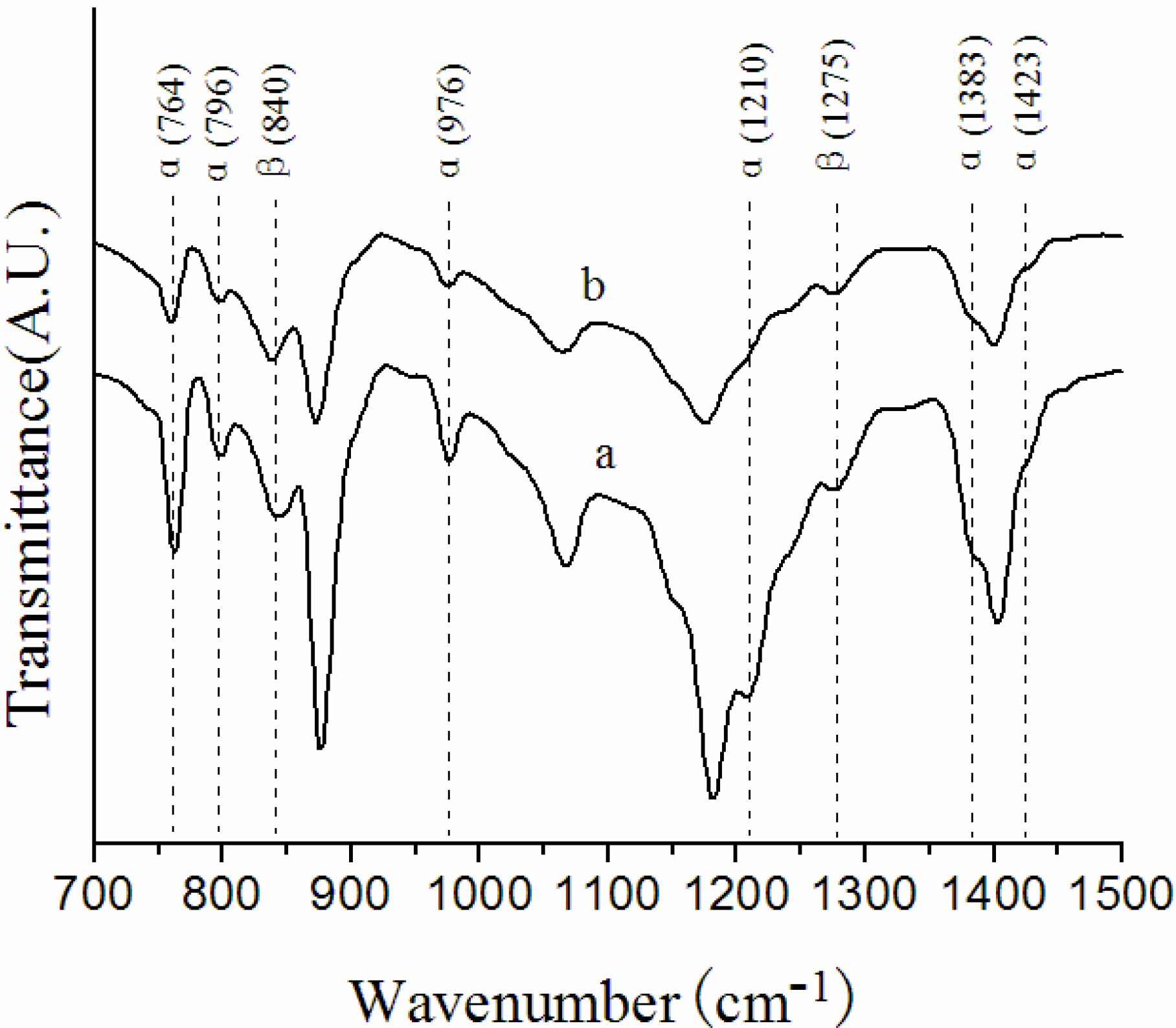
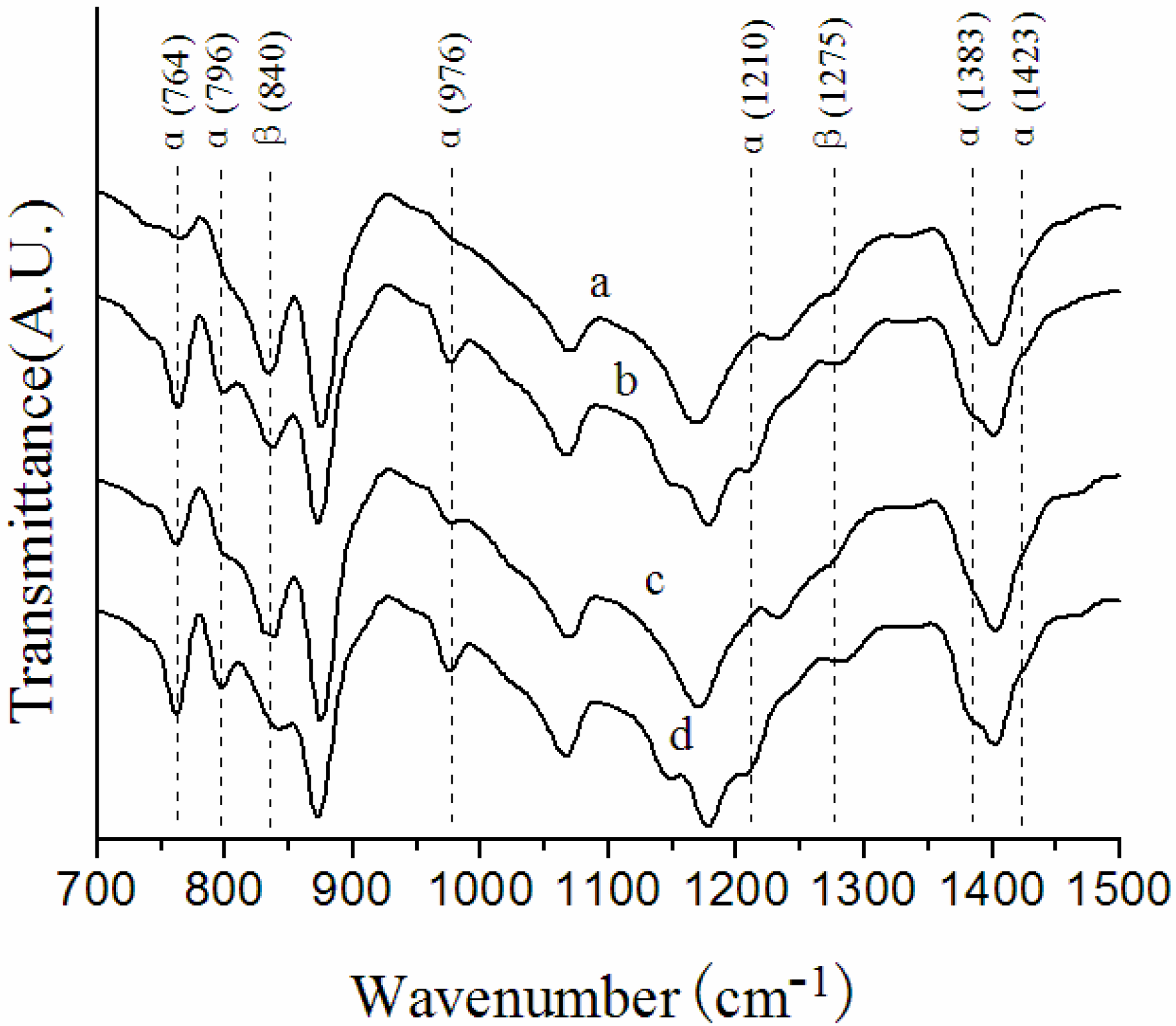
| Membrane label | α/β |
|---|---|
| NIPS membrane | 1.30 |
| Melt spinning membrane | 0.88 |
| F5-60 | 0.33 |
| F5-140 | 1.10 |
| F5-7-60 | 0.56 |
| F5-7-140 | 1.14 |
3.2. Crystalline Structures of the Overall Membranes
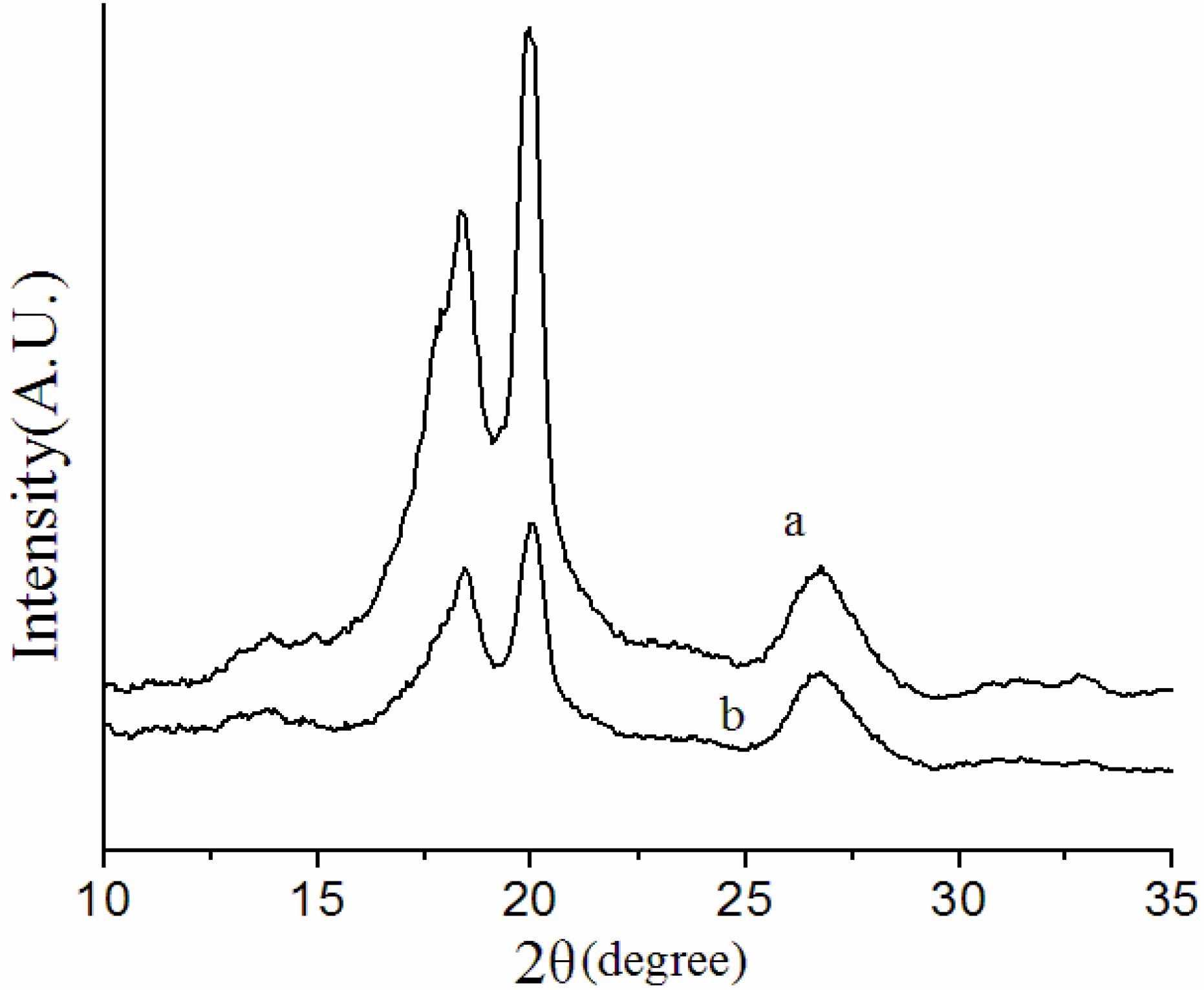

3.3. Thermal Behavior of the Membranes

| Membrane label | ∆Hf(J/g) | Xc(%) |
|---|---|---|
| NIPS membrane | 62.8 | 60.0 |
| Melt spinning membrane | 51.0 | 48.7 |
| F5-60 | 73.3 | 70.0 |
| F5-140 | 66.0 | 63.0 |
| F5-7-60 | 65.8 | 62.8 |
| F5-7-140 | 57.9 | 55.3 |
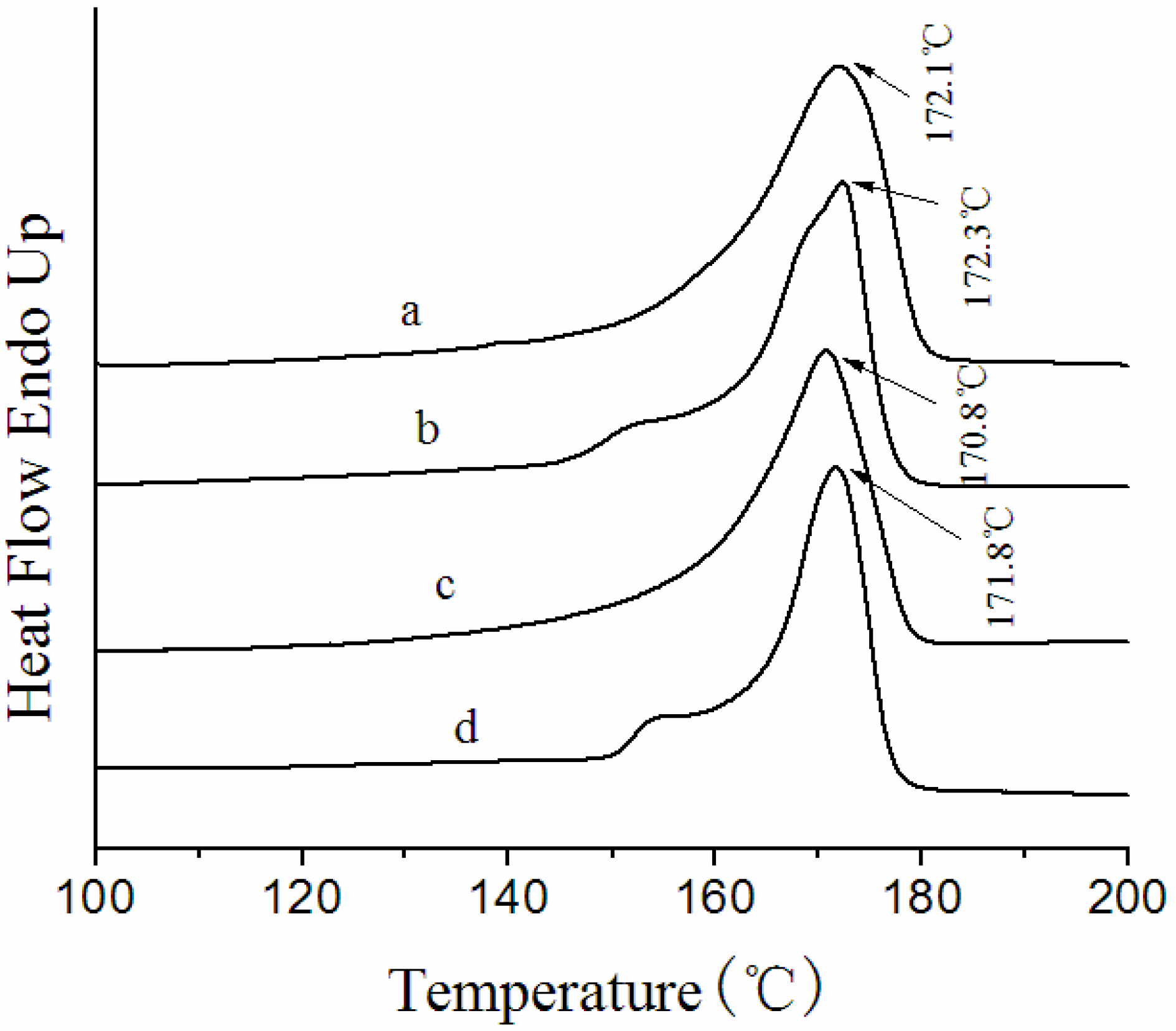
3.4. Crystalline Morphology


3.5. Tensile Strength of the Membranes

4. Conclusions
- (1)
- The NIPS PVDF membrane had a mixture of α and β phases in the outer skin and was predominantly of α phase in the overall membrane, total crystallinity was 60.0% with no spherulite;
- (2)
- The melt spinning PVDF membrane had a mixture of α and β phases in the outer skin and was predominantly of α phase in the overall membrane, total crystallinity was 48.7% with perfect spherulites;
- (3)
- The crystallization behavior of the solution-cast membranes was related to the evaporation temperature and additive: when the evaporation temperature was lower (at 60 °C), total crystallinity increased and PVDF formed the β phase more favorably; As the soluble additive was added into the dope solution, the total crystallinity decreased, the α phase in the outer skin increased, and the crystal conformation of the overall membranes had no significant change. When the evaporation temperature was 140 °C with a soluble additive in the dope solution, obvious spherulites were observed;
- (4)
- Among the PVDF membranes prepared by various methods, it was crystalline morphology rather than crystal form and total crystallinity that determined the tensile strength. The difference in crystalline phase and amorphous-crystalline interfacial region induced by different crystalline morphologies exerted a great influence on the tensile strengths of the membranes, which were much higher with perfect spherulites.
Acknowledgments
Conflicts of Interest
References
- Li, M.Z.; Li, J.H.; Shao, X.S.; Miao, J.; Wang, J.B.; Zhang, Q.Q.; Xu, X.P. Grafting zwitterionic brush on the surface of PVDF membrane using physisorbed free radical grafting technique. J. Membr. Sci. 2012, 405–406, 141–148. [Google Scholar]
- Wang, K.Y.; Chung, T.S.; Gryta, M. Hydrophobic PVDF hollow fiber membranes with narrow pore size distribution and ultra-thin skin for the freshwater production through membrane distillation. Chem. Eng. Sci. 2008, 63, 2587–2594. [Google Scholar] [CrossRef]
- Teoh, M.M.; Chung, T.S.; Yeo, Y.S. Dual-layer PVDF/PTFE composite hollow fibers with a thin macrovoid-free selective layer for water production via membrane distillation. Chem. Eng. J. 2011, 171, 684–691. [Google Scholar] [CrossRef]
- Atchariyawut, S.; Feng, C.S.; Wang, R.; Jiraratananon, R.; Liang, D.T. Effect of membrane structure on mass-transfer in the membrane gas-liquid contacting process using microporous PVDF hollow fibers. J. Membr. Sci. 2006, 285, 272–281. [Google Scholar] [CrossRef]
- Hou, D.; Wang, J.; Sun, X.C.; Ji, Z.G.; Luan, Z.K. Preparation and properties of PVDF composite hollow fiber membranes for desalination through direct contact membrane distillation. J. Membr. Sci. 2012, 405–406, 185–200. [Google Scholar]
- Benz, M.; Euler, W.B. Determination of the crystalline phases of poly(vinylidene fluoride) under different preparation conditions using differential scanning calorimetry and infrared spectroscopy. J. Appl. Polym. Sci. 2003, 89, 1093–1100. [Google Scholar]
- Gregorio, R., Jr. Determination of the α, β and γ crystalline phases of poly(vinylidene fluoride) films prepared at different conditions. J. Appl. Polym. Sci. 2006, 100, 3272–3279. [Google Scholar] [CrossRef]
- Mohajir, B.E.E.; Heymans, N. Changes in structural and mechanical behaviour of PVDF with processing and thermomechanical treatments. 1. Change in structure. Polymer 2001, 42, 5661–5667. [Google Scholar] [CrossRef]
- Mohajir, B.E.E.; Heymans, N. Changes in structural and mechanical behaviour of PVDF with processing or thermal treatment. 2. Evolution of mechanical behavior. Polymer 2001, 42, 7017–7023. [Google Scholar] [CrossRef]
- Vijayakumar, R.P.; Khakhar, D.V.; Misra, A. Studies on α to β phase transformations in mechanically deformed PVDF films. J. Appl. Polym. Sci. 2010, 117, 3491–3497. [Google Scholar]
- Teyssedre, G.; Bernes, A.; Lacabanne, C. Influence of the crystalline phase on the molecular mobility of PVDF. J. Polym. Sci. B Polym. Phys. 1993, 31, 2027–2034. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Ideris, N.; Ooi, B.S.; Low, S.C.; Ismail, A. Morphology and polymorph study of a polyvinylidene fluoride (PVDF) membrane for protein binding: Effect of the dissolving temperature. Desalination 2011, 278, 318–324. [Google Scholar] [CrossRef]
- Sukitpaneenit, P.; Chung, T.S. Molecular elucidation of morphology and mechanical properties of PVDF hollow fiber membranes from aspects of phase inversion, crystallization and rheology. J. Membr. Sci. 2009, 340, 192–205. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, A.Q.; Zhu, B.K.; Du, C.H.; Xu, Y.Y. Polymorphism in porous poly(vinylidene fluoride) membranes formed via immersion precipitation process. J. Membr. Sci. 2008, 319, 169–175. [Google Scholar] [CrossRef]
- Choi, S.H.; Tasselli, F.; Jansen, J.C.; Barbieri, G.; Drioli, E. Effect of the preparation conditions on the formation of asymmetric poly(vinylidene fluoride) hollow fibre membranes with a dense skin. Eur. Polym. J. 2010, 46, 1713–1725. [Google Scholar]
- Buonomenna, M.G.; Macchi, P.; Davoli, M.; Drioli, E. Poly(vinylidene fluoride) membranes by phase inversion: The role the casting and coagulation conditions play in their morphology, crystalline structure and properties. Eur. Polym. J. 2007, 43, 1557–1572. [Google Scholar] [CrossRef]
- Cheng, L.P. Effect of temperature on the formation of microporous PVDF membranes by precipitation from 1-octanol/DMF/PVDF and water/DMF/PVDF systems. Macromolecules 1999, 32, 6668–6674. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Sun, D.; An, Q.; Chen, H. Formation mechanism and crystallization of poly(vinylidene fluoride) membrane via immersion precipitation method. Desalination 2009, 236, 170–178. [Google Scholar] [CrossRef]
- Gregorio, R., Jr.; Capitao, R.C. Morphology and phase transition of high melt temperature crystallized poly(vinylidene fluoride). J. Mater. Sci. 2000, 35, 299–306. [Google Scholar] [CrossRef]
- Gregorio, R., Jr.; Cestari, M. Effect of crystallization temperature on the crystalline phase content and morphology of poly(vinylidene Fluoride). J. Polym. Sci. B Polym. Phys. 1994, 32, 859–870. [Google Scholar] [CrossRef]
- Prest, W.M.; Luca, D.J. The morphology and thermal response of high-temperature crystallized poly(vinylidene fluoride). J. Appl. Phys. 1975, 46, 4136–4143. [Google Scholar] [CrossRef]
- Salimi, A.; Yousefi, A.A. Conformational changes and phase transformation mechanisms in PVDF solution-cast membranes. J. Polym. Sci. B Polym. Phys. 2004, 42, 3487–3495. [Google Scholar]
- Gregorio, R., Jr.; Borges, D.S. Effect of crystallization rate on the formation of the polymorphs of solution cast poly(vinylidene fluoride). Polymer 2008, 49, 4009–4016. [Google Scholar] [CrossRef]
- Mohammadi, B.; Yousefi, A.A.; Bellah, S.M. Effect of tensile strain rate and elongation on crystalline structure and piezoelectric properties of PVDF thin membranes. Polym. Test. 2007, 26, 42–50. [Google Scholar] [CrossRef]
- Du, C.H.; Zhu, B.K.; Xu, Y.Y. Effects of stretching on crystalline phase structure and morphology of hard elastic PVDF fibers. J. Appl. Polym. Sci. 2007, 104, 2254–2259. [Google Scholar] [CrossRef]
- Salimi, A.; Yousefi, A.A. FTIR studies of β-phase crystal formation in stretched PVDF membranes. Polym. Test. 2003, 22, 699–704. [Google Scholar] [CrossRef]
- Ozkazanc, E.; Guney, H.Y. The variation of the dielectric constant and loss index with temperature and draw ratio in α-PVDF. J. Appl. Polym. Sci. 2009, 112, 2482–2485. [Google Scholar]
- Liu, F.; Hashim, N.A.; Liu, Y.T.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Liu, J.; Lu, X.L.; Wu, C.R. Effect of annealing conditions on crystallization behavior and mechanical properties of NIPS poly(vinylidene fluoride) hollow fiber membranes. J. Appl. Polym. Sci. 2013, 129, 1417–1425. [Google Scholar] [CrossRef]
- Gregorio, R., Jr.; Nocit, N.C.P.S. Effect of PMMA addition on the solution crystallization of the α and β phases of poly(viny1idene fluoride) (PVDF). J. Phys. D Appl. Phys. 1995, 28, 432–436. [Google Scholar] [CrossRef]
- Ma, W.Z.; Zhang, J.; Wang, X.L.; Wang, S.M. Effect of PMMA on crystallization behavior and hydrophilicity of poly(vinylidene fluoride)/poly(methyl methacrylate) blend prepared in semi-dilute solutions. Appl. Surf. Sci. 2007, 253, 8377–8388. [Google Scholar] [CrossRef]
- Hodge, R.M.; Edward, G.H.; Simon, G.P. Water absorption and states of water in semicrystalline poly(vinyl alcohol) films. Polymer 1996, 3, 1371–1376. [Google Scholar] [CrossRef]
- Nakagawa, K.; Ishida, Y. Annealing effects in poly(viny1idene fluoride) as revealed by specific volume measurements, differential scanning calorimetry, and electron microscopy. J. Polym. Sci. 1973, 11, 2153–2171. [Google Scholar]
- Nasir, M.; Matsumoto, H.; Danno, T.; Minagawa, M.; Irisawa, T.; Shioya, M.; Tanioka, A. Control of diameter, morphology, and structure of PVDF nanofiber fabricated by electrospray deposition. J. Polym. Sci. B Polym. Phys. 2006, 44, 779–786. [Google Scholar]
- Wallner, G.M.; Major, Z.; Maier, G.A.; Lang, R.W. Fracture analysis of annealed PVDF films. Polym. Test. 2008, 27, 392–402. [Google Scholar] [CrossRef]
- Hobbs, J.K.; Mcmaster, T.J.; Miles, M.J. Cracking in spherulites of poly(hydroxybutyrate). Polymer 1996, 37, 3241–3246. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, J.; Lu, X.; Wu, C. Effect of Preparation Methods on Crystallization Behavior and Tensile Strength of Poly(vinylidene fluoride) Membranes. Membranes 2013, 3, 389-405. https://doi.org/10.3390/membranes3040389
Liu J, Lu X, Wu C. Effect of Preparation Methods on Crystallization Behavior and Tensile Strength of Poly(vinylidene fluoride) Membranes. Membranes. 2013; 3(4):389-405. https://doi.org/10.3390/membranes3040389
Chicago/Turabian StyleLiu, Jie, Xiaolong Lu, and Chunrui Wu. 2013. "Effect of Preparation Methods on Crystallization Behavior and Tensile Strength of Poly(vinylidene fluoride) Membranes" Membranes 3, no. 4: 389-405. https://doi.org/10.3390/membranes3040389




