Performance and Long-Term Stability of Pd/PSS and Pd/Al2O3 Membranes for Hydrogen Separation
Abstract
:1. Introduction
2. Experimental
2.1. Membrane Preparation and Membrane Module Setup


2.2. Experimental Details


3. Result and Discussion
3.1. Permeation Measurements

| ∆ p (Pa) | Pd/Al2O3 | Pd/PSS | ||
|---|---|---|---|---|
| CH4 Permeance | CO2 Permeance | CH4 Permeance | CO2 Permeance | |
| (mol/s·m2·Pa) | (mol/s·m2·Pa) | (mol/s·m2·Pa) | (mol/s·m2·Pa) | |
| 1.01 × 105 | 6.27 × 10−10 | 7.28 × 10−10 | 2.73 × 10−10 | 3.52 × 10−10 |
| 2.02 × 105 | 8.11 × 10−10 | 1.18 × 10−9 | 3.60 × 10−10 | 5.11 × 10−10 |
| 3.03 × 105 | 9.91 × 10−10 | 1.36 × 10−9 | 4.01 × 10−10 | 5.75 × 10−10 |
| 4.04 × 105 | 1.05 × 10−9 | 1.61 × 10−9 | 4.27 × 10−10 | 6.68 × 10−10 |
| 5.05 × 105 | 1.09 × 10−9 | 1.68 × 10−9 | 4.46 × 10−10 | 7.08 × 10−10 |
| 6.06 × 105 | 1.13 × 10−9 | 1.75 × 10−9 | 4.70 × 10−10 | 7.34 × 10−10 |
| 7.07 × 105 | 1.17 × 10−9 | 1.86 × 10−9 | 4.89 × 10−10 | 7.41 × 10−10 |
3.2. Hydrogen Permeation Test

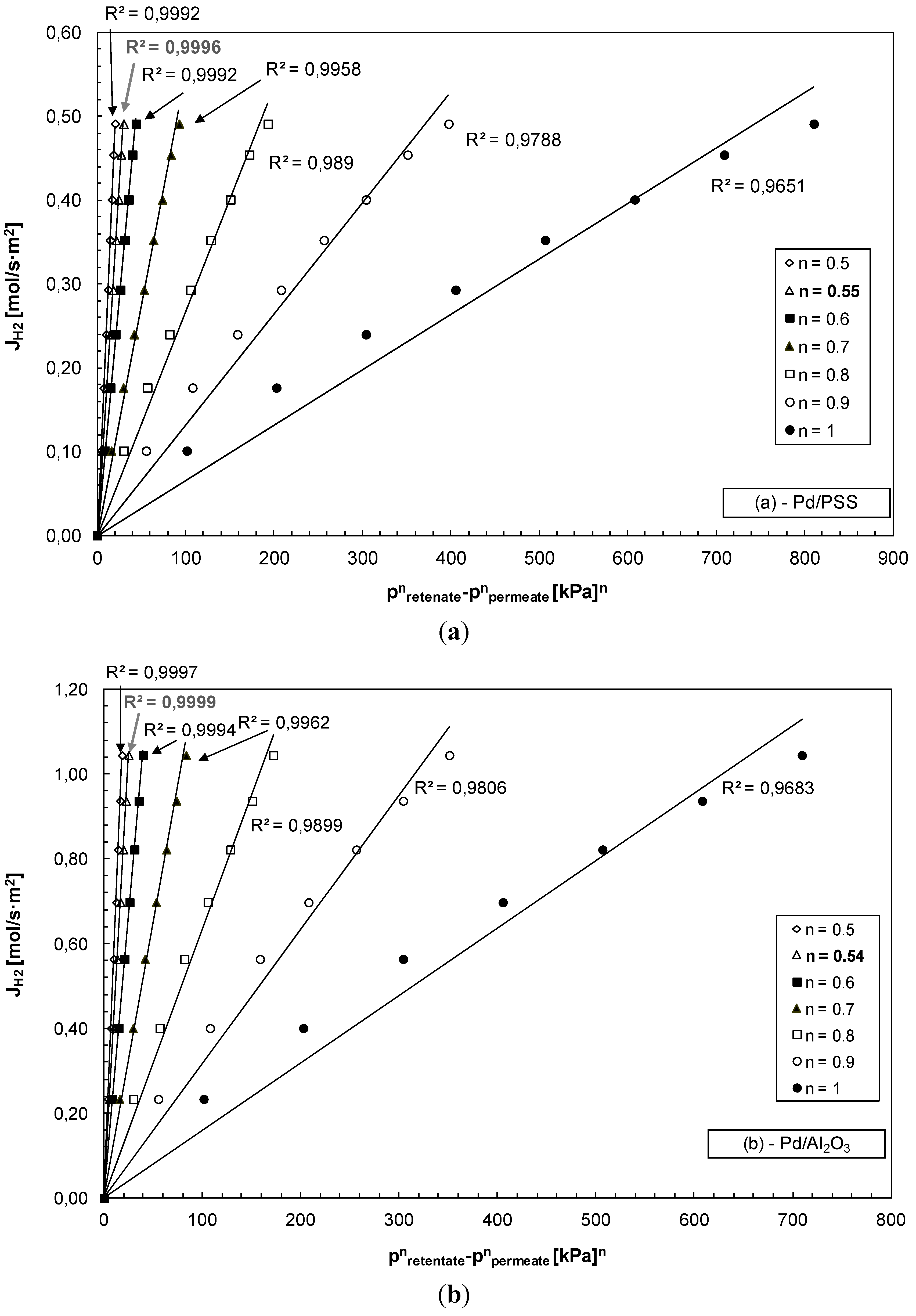

| Membrane | Layer of Pd (μm) | Ea (kJ/mol) | n | References |
|---|---|---|---|---|
| Pd/WO3/PSS | 12 | 14.7 | 0.5 | [44] |
| Pd/Al2O3 | 8.5 | 5.7 | 0.778 | [45] |
| Pd/ZrO2/PSS | 10 | 7.1 | 0.5 | [42] |
| Pd/CeO2/PSS | 6 | 17.3 | 0.85 | [43] |
| Pd/Al2O3 | 11.4 | 8.9 | 0.58 | [39] |
| Pd/SiO2/PSS | 6 | 24 a | 1 | [46] |
| Pd/FeCr/PSS | 11 | 15.03 | 0.5 | [49] |
| Pd/Al2O3 | 4.5 | 18.3 | 1 | [47] |
| Pd/Al2O3 | 5 | 7.0 | 1 | [31] |
| Pd/MPSS | 20 | 16.4 | 0.60 | [48] |
| Pd/Al2O3/PSS | 15 | 20.6 | 0.5 | [7] |
| Pd/PSS | 10 | 14.7 | 0.55 | This work |
| Pd/Al2O3 | 7 | 11.4 | 0.54 | This work |
3.3. Membrane Performance

| Membrane type | Preparation method | Pd layer (μm) | T (°C) | ∆ p (Pa) | H2 flux (mol/s·m2) | H2 permeance (mol/s·m2·Pa) | α(H2/N2) (–) | References |
|---|---|---|---|---|---|---|---|---|
| Pd/CeO2/PSS | ELP-CVD | 6 | 500 | 100,000 | 0.235 | – | 14 b | [50] |
| Pd/γ-Al2O3/Al2O3 | ELP | 5 | 300 | 400,000 | – | 1.4 × 10−6 | 1000 | [51] |
| Pd/ZrO2/PSS | ELP | 23 | 400 | 100,000 | 0.0734 | 5.2 × 10−4 a | 320 | [5] |
| Pd/γ-Al2O3/Al2O3 | ELP | 2.4 | 500 | 100,000 | – | 3.9 × 10−6 | 32,500 | [52] |
| Pd/Ni-SiO2/PSS | CVD | – | 450 | 42,000 | – | 6.4 × 10−6 | 6100 | [53] |
| Pd/YSZ/PSS | ELP | 28 | 450 | 30,000–40,000 | 0.01–0.06 | 4.5 × 10−4 a | ∞ | [54] |
| Pd/γ-Al2O3/Al2O3 | ELP | 2.6 | 370 | 400,000 | – | 4.8 × 10−7 | 3000 | [33] |
| Pd/γ-Al2O3/Al2O3 | ELP | 1 | 400 | 75,000 | – | 6.7 × 10−6 | 23 | [55] |
| Pd/SiO2/PSS | CVD | 6 | 500 | 50,000 | 0.133 | 2.7 × 10−6 | 450 | [46] |
| Pd/Al2O3 | CVD | 2 | 300 | 30,000 | – | 3.3 × 10−6 | 5000 | [56] |
| Pd/Fe2O3/PSS | ELP | 22 | 450 | 100,000 | 0.0853 | 2.7 × 10−4 a | ∞ | [13] |
| Pd/γ-Al2O3/Al2O3 | ELP | 6 | 480 | 100,000 | – | 2.6 × 10−6 | 2100 b | [31] |
| Pd/Al2O3 | CVD | 1 | 450 | 68,000 | – | 2.1 × 10−6 | 780 | [28] |
| Pd/Al2O3 | ELP | 0.9 | 460 | 199,000 | – | 3.1 × 10−6 | 1200 | [36] |
| Pd/PSS | ELP | 20 | 350 | 100,000 | – | 5 × 10−7 | 5000 | [40] |
| Pd/NaAZ/PSS | ELP | 19 | 450 | 50,000 | 0.0790 | 1.1 × 10−3 a | 608 | [57] |
| Pd/PSS | ELP | 10 | 400 | 200,000 | 0.176 | 8.7 × 10−7 | 11,800 | This work |
| Pd/Al2O3 | ELP | 7 | 400 | 100,000 | 0.233 | 2.3 × 10−6 | 7500 | This work |

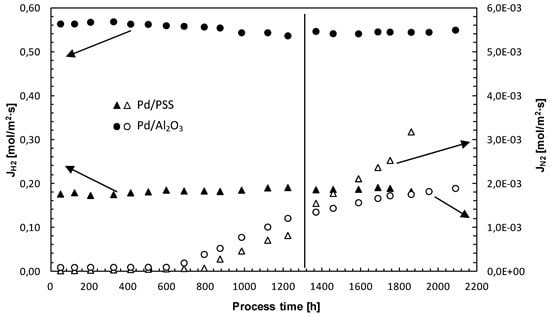
3.4. Membrane Long-Term Stability and Life Time Estimation


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zornoza, B.; Casado, C.; Navajas, A. Advances in hydrogen separation and purification with membrane technology. In Renewable Hydrogen Technologies-Production, Purification, Storage, Applications and Safety; Gandia, L.M., Arzamendi, G., Dieguez, P.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Chapter 11; pp. 245–268. [Google Scholar]
- Iulianelli, A.; Liguori, S.; Longo, T.; Basile, A. Inorganic Membrane and Membrane Reactor Technologies for Hydrogen Production. In Hydrogen Production: Prospects and Processes; Honery, D.R., Moriarty, P., Eds.; Energy Science, Engineering and Technology Nova Science Publishers, Series: Victoria, Australia, 2012; Chapter 12; pp. 377–398. [Google Scholar]
- Basile, A.; Iulianelli, A.; Longo, T.; Liguori, S.; de Falco, M. Pd-Based Selective Membrane State of the Art. In Membrane Reactors for Hydrogen Production; Marrelli, L., de Falco, M., Iaquaniello, G., Eds.; Springer: New York, NY, USA, 2011; Chapter 2; pp. 21–55. [Google Scholar]
- Hu, X.; Chen, W.; Huang, Y. Fabrication of Pd/ceramic membranes for hydrogen separation based on low-cost macroporous ceramics with pencil coating. Int. J. Hydrog. Energy 2010, 35, 7803–7808. [Google Scholar] [CrossRef]
- Huang, Y.; Dittmeyer, R. Preparation of thin palladium membranes on a porous support with rough surface. J. Membr. Sci. 2007, 302, 160–170. [Google Scholar] [CrossRef]
- Paglieri, S.N.; Foo, K.Y.; Way, J.D.; Collins, J.P.; Harper-Nixon, D.L. A new preparation technique for Pd/alumina membranes with enhanced high temperature stability. Ind. Eng. Chem. Res. 1999, 38, 1925–1936. [Google Scholar] [CrossRef]
- Li, A.W.; Grace, J.R.; Lim, C.J. Preparation of thin Pd-based composite membrane on planar metallic substrate part II. Preparation of membranes by electroless plating and characterization. J. Membr. Sci. 2007, 306, 159–165. [Google Scholar] [CrossRef]
- Yeung, K.L.; Sebastian, J.M.; Varma, A. Novel preparation of Pd/Vycor composite membranes. Catal. Today 1995, 25, 231–236. [Google Scholar] [CrossRef]
- Altinisik, O.; Dogan, M.; Dogu, G. Preparation and characterization of palladium-plated porous glass for hydrogen enrichment. Catal. Today 2005, 105, 641–646. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, H.Y.; Rui, Z.B.; Liu, P.; Li, Y.D.; Lin, Y.S. High temperature stability of palladium membranes on porous metal supports with different intermediate layers. Ind. Eng. Chem. Res. 2009, 48, 1880–1886. [Google Scholar] [CrossRef]
- Kitiwan, M.; Atong, D. Effects of porous alumina support and plating time on electroless plating of palladium membrane. J. Mater. Sci. Technol. 2010, 26, 1148–1152. [Google Scholar] [CrossRef]
- Li, A.; Liang, W.; Hughes, R. Characterization and permeation of palladium/stainless steel composite membranes. J. Membr. Sci. 1998, 149, 259–268. [Google Scholar] [CrossRef]
- Rothenberger, K.S.; Cugini, A.V.; Howard, B.H.; Killmeyer, R.P.; Ciocco, M.V.; Morreale, B.D.; Enick, R.M.; Bustamante, F.; Mardilovich, I.P.; Ma, Y.H. High pressure hydrogen permeance of porous stainless steel coated with a thin palladium film via electroless plating. J. Membr. Sci. 2004, 244, 55–68. [Google Scholar] [CrossRef]
- Chen, S.C.; Tu, G.C.; Caryat, C.Y.; Hung, C.A.; Rei, M.H. Preparation of palladium membrane by electroplating on AISI 316L porous stainless steel supports and its use for methanol steam reformer. J. Membr. Sci. 2008, 314, 5–14. [Google Scholar] [CrossRef]
- Huang, Y.; Dittmeyer, R. Preparation and characterization of composite palladium membranes on sinter-metal supports with a ceramic barrier against intermetallic diffusion. J. Membr. Sci. 2006, 282, 296–310. [Google Scholar] [CrossRef]
- Shu, J.; Adnot, A.; Grandjean, B.P.A.; Kaliaguine, S. Structurally stable composite Pd–Ag alloy membranes: Introduction of a diffusion barrier. Thin Solid Films 1996, 286, 72–79. [Google Scholar] [CrossRef]
- Nam, S.E.; Lee, K.H. Hydrogen separation by Pd alloy composite membranes: Introduction of diffusion barrier. J. Membr. Sci. 2001, 192, 177–185. [Google Scholar] [CrossRef]
- Ma, Y.H.; Mardilovich, I.P.; She, Y. Hydrogen Gas-Extraction Module and Method of Fabrication. U.S. Patent 6,152,987, 28 November 2000. [Google Scholar]
- Wei, L.; Yu, J.; Hu, X.; Huang, Y. Fabrication of H2-permeable palladium membranes based on pencil-coated porous stainless steel substrate. Int. J. Hydrog. Energy 2012, 37, 13007–13012. [Google Scholar] [CrossRef]
- Chen, W.; Hu, X.; Wang, R.; Huang, Y. On the assembling of Pd/ceramic composite membranes for hydrogen separation. Sep. Purif. Technol. 2010, 72, 92–97. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Mustafa, N.N.N. Sol-gel synthesized of nano composite palladium-alumina ceramic membrane for H2 permeability: Preparation and characterization. Int. J. Hydrog. Energy 2007, 32, 2010–2021. [Google Scholar] [CrossRef]
- Hu, X.; Yu, J.; Song, J.; Wang, X.; Huang, Y. Toward low-cost Pd/ceramic composite membranes for hydrogen separation: A case study on reuse of the recycled porous Al2O3 substrates in membrane fabrication. Int. J. Hydrog. Energy 2011, 36, 15794–15802. [Google Scholar] [CrossRef]
- Chi, Y.H.; Yen, P.S.; Jeng, M.S.; Ko, S.T.; Lee, T.C. Preparation of thin Pd membrane on porous stainless steel tubes modified by a two-step method. Int. J. Hydrog. Energy 2010, 35, 6303–6310. [Google Scholar] [CrossRef]
- Sari, R.; Yaakob, Z.; Ismail, M.; Daud, W.R.W.; Hakim, L. Palladium-alumina composite membrane for hydrogen separator fabricated by combined sol-gel, and electroless plating technique. Ceram. Int. 2013, 39, 3211–3219. [Google Scholar] [CrossRef]
- Checchetto, R.; Bazzanella, N.; Patton, B.; Miotello, A. Palladium membranes prepared by r.f. magnetron sputtering for hydrogen purification. Surf. Coat. Technol. 2004, 177–178, 73–79. [Google Scholar] [CrossRef]
- Li, Z.Y.; Maeda, H.; Kusakabe, K.; Morooka, S.; Anzai, H.; Akiyama, S. Preparation of palladium-silver alloy membranes for hydrogen separation by the spray pyrolysis method. J. Membr. Sci. 1993, 78, 247–254. [Google Scholar] [CrossRef]
- Yam, S.; Maeda, H.; Kusakabe, K.; Morooka, S. Thin palladium films formed in support pores by metal-organic chemical vapour deposition method and application to hydrogen separation. Ind. Eng. Chem. Res. 1994, 33, 616–622. [Google Scholar]
- Jun, C.S.; Lee, K.H. Palladium and palladium alloy composite membranes prepared by metal-organic chemical vapour deposition method (cold-wall). J. Membr. Sci. 2000, 176, 121–130. [Google Scholar] [CrossRef]
- Broglia, M.; Pinacci, P.; Radaelli, M.; Bottino, A.; Capannelli, G.; Comite, A.; Vanacore, G.; Zani, M. Synthesis and characterization of Pd membranes on alumina-modified porous stainless steel supports. Desalination 2009, 245, 508–515. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Yeung, K.L. Effects of electroless plating chemistry on the synthesis of palladium membranes. J. Membr. Sci. 2001, 182, 195–203. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, G.; Yang, W. A modified electroless plating technique for thin dense palladium composite membranes with enhanced stability. J. Membr. Sci. 2008, 314, 67–84. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, H.; Rui, Z.; Lin, Y.; Li, Y. Preparation of thin palladium composite membranes and application to hydrogen/nitrogen separation. Chin. J. Chem. Eng. 2007, 15, 643–647. [Google Scholar]
- Nair, B.K.R.; Choi, J.; Harold, M.P. Electroless plating and permeation features of Pd and Pd/Ag hollow fiber composite membranes. J. Membr. Sci. 2007, 288, 67–84. [Google Scholar] [CrossRef]
- Peters, T.A.; Tucho, W.M.; Ramachandran, A.; Stange, M.; Walmsley, J.C.; Holmestad, R.; Borg, A.; Bredesen, R. Thin Pd-23%Ag/stainless steel composite membranes: Long-term stability, life-time estimation and post-process characterization. J. Membr. Sci. 2009, 326, 572–581. [Google Scholar] [CrossRef]
- Kulprathipanja, A.; Alptekin, G.O.; Falconer, J.L.; Way, J.D. Effects of water gas shift gases on Pd–Cu alloy membrane surface morphology and separation properties. Ind. Eng. Chem. Res. 2004, 43, 4188–4198. [Google Scholar] [CrossRef]
- Yun, S.; Ko, J.H.; Oyama, S.T. Ultrathin palladium membranes prepared by a novel electric field assisted activation. J. Membr. Sci. 2011, 369, 482–489. [Google Scholar] [CrossRef]
- Pinacci, P.; Broglia, M.; Drago, F. Development of Pd Composite Membranes for Hydrogen Production in Membrane Reactors. In Proceedings of the European Fuel Cell (EFC), Rome, Italy, 14–16 December 2011.
- Pinacci, P.; Broglia, M.; Valli, C.; Capannelli, G.; Comite, A. Evaluation of the water gas shift reaction in a palladium membrane reactor. Catal. Today 2010, 156, 165–172. [Google Scholar] [CrossRef]
- Collins, J.P.; Way, J.D. Preparation and characterization of a composite palladium-ceramic membrane. Ind. Eng. Chem. Res. 1993, 32, 3006–3013. [Google Scholar] [CrossRef]
- Mardilovich, P.P.; She, Y.; Ma, Y.H.; Rei, M.H. Defect-free palladium membranes on porous stainless-steel support. AIChE J. 1998, 44, 310–322. [Google Scholar] [CrossRef]
- Guazzone, F.; Engwall, E.E.; Ma, Y.H. Effect of surface activity, defects and mass transfer on hydrogen permeance and n-value in composite palladium-porous stainless steel membranes. Catal. Today 2006, 118, 24–31. [Google Scholar]
- Wang, D.; Tong, J.; Xu, H.; Matsumura, Y. Preparation of palladium membrane over porous stainless steel tube modified with zirconium oxide. Catal. Today 2004, 93–95, 689–693. [Google Scholar] [CrossRef]
- Tong, J.; Matsumura, Y.; Suda, H.; Haraya, K. Thin and dense Pd/ CeO2/MPSS composite membrane for hydrogen separation and steam reforming of methane. Sep. Purif. Technol. 2005, 46, 1–10. [Google Scholar] [CrossRef]
- Zahedi, M.; Afra, B.; Mobarake, M.D.; Bahmani, M. Preparation of a Pd membrane on a WO3 modified porous stainless steel for hydrogen separation. J. Membr. Sci. 2009, 333, 45–49. [Google Scholar] [CrossRef]
- Ilias, S.; Su, N.; Udo-Aka, U.I.; King, F.G. Application of electroless deposition thin film palladium composite membrane in hydrogen separation. Sep. Sci. Technol. 1997, 32, 487–504. [Google Scholar]
- Su, C.; Jin, T.; Kuraoka, K. Thin palladium film supported on SiO2-modified porous stainless steel for a high-hydrogen-flux membrane. Ind. Eng. Chem. Res. 2005, 44, 3053–3058. [Google Scholar] [CrossRef]
- Chen, H.; Chu, C.; Huang, T. Comprehensive characterization and permeation analysis of thin Pd/Al2O3 composite membranes prepared by suction-assisted electroless deposition. Sep. Sci. Technol. 2005, 39, 1461–1483. [Google Scholar] [CrossRef]
- Mardilovich, I.P.; Engwall, E.; Ma, Y.H. Dependence of hydrogen flux on the pore size and plating surface topology of asymmetric Pd-porous stainless steel membranes. Desalination 2002, 144, 85–89. [Google Scholar] [CrossRef]
- Sanz, R.; Calles, J.A.; Alique, D.; Furones, L. New synthesis method of Pd membranes over tubular PSS supports via “pore-plating” for hydrogen separation processes. Int. J. Hydrog. Energy 2012, 37, 18476–18485. [Google Scholar] [CrossRef]
- Tong, J.; Sua, C.; Kuraoka, K.; Suda, H.; Matsumura, Y. Preparation of thin Pd membrane on CeO2-modified porous metal by a combined method of electroless plating and chemical vapor deposition. J. Membr. Sci. 2006, 269, 101–108. [Google Scholar] [CrossRef]
- Tanaka, D.A.P.; Tanco, M.A.L.; Okazaki, T.N.J.; Wakui, Y.; Mizukami, F.; Suzuki, T.M. Fabrication of hydrogen-permeable composite membranes packed with palladium nanoparticles. Adv. Mater. 2006, 18, 630–632. [Google Scholar] [CrossRef]
- Li, H.; Goldbach, A.; Li, W.; Xu, H. PdC formation in ultra-thin Pd membranes during separation of H2/CO mixtures. J. Membr. Sci. 2007, 299, 130–137. [Google Scholar]
- Lee, D.W.; Lee, Y.G.; Nam, S.E.; Ihm, S.K.; Lee, K.H. Study on the variation of morphology and separation behavior of the stainless steel supported membranes at high temperature. J. Membr. Sci. 2003, 220, 137–153. [Google Scholar] [CrossRef]
- Sanz, R.; Calles, J.A.; Alique, D.; Furones, L.; Ordonez, S.; Marìn, P.; Corengia, P.; Fernandez, E. Preparation, testing and modelling of a hydrogen selective Pd/YSZ/SS composite membrane. Int. J. Hydrog. Energy 2011, 36, 15783–15793. [Google Scholar] [CrossRef]
- Zhao, H.B.; Pflanz, K.; Gu, J.H.; Li, A.W.; Stroh, N.; Brunner, H.; Xiong, G.X. Preparation of palladium composite membranes by modified electroless plating procedure. J. Membr. Sci. 1998, 142, 147–157. [Google Scholar] [CrossRef]
- Itoh, N.; Akiha, T.; Sato, T. Preparation of thin palladium composite membrane tube by a CVD technique and its hydrogen permselectivity. Catal. Today 2005, 104, 231–237. [Google Scholar] [CrossRef]
- Bosko, M.L.; Ojeda, F.; Lombardo, E.A.; Cornaglia, L.M. NaA zeolite as an effective diffusion barrier in composite Pd/PSS membranes. J. Membr. Sci. 2009, 331, 57–65. [Google Scholar] [CrossRef]
- Heidari, M.; Zamaniyan, A.; Kordi, A.S.; Babakhani, E.G.; Amanipour, M. Effect of sintering temperature on microstructure and hydrogen permeation properties of perovskite membrane. J. Mater. Sci. Technol. 2013, 29, 137–141. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Yoshino, Y.; Nomura, M.; Nair, B.N.; Nakao, S.I. A hybrid processing method for high performance hydrogen-selective silica membranes. J. Membr. Sci. 2007, 297, 5–9. [Google Scholar] [CrossRef]
- Lee, D.W.; Lee, Y.G.; Nam, S.E.; Sea, B.; Lee, K.H. Preparation and characterization of SiO2 composite membrane for purification of hydrogen from methanol steam reforming as an energy carrier system for PEMFC. Sep. Purif. Technol. 2003, 32, 45–50. [Google Scholar] [CrossRef]
- Lie, J.A.; Hagg, M.B. Carbon membranes from cellulose and metal loaded cellulose. Carbon 2005, 43, 2600–2607. [Google Scholar] [CrossRef]
- Separation Technology R&D Needs for Hydrogen Production in the Chemical and Petrochemical Industries. Available online: http://www.rom-innovation.com/pdf/separation-need-in-chemical2020-and-hydrogen.pdf (accessed on 18 February 2014).
- Wang, D.L.; Li, K.; Teo, W.K. Effects of temperature and pressure on gas permselection properties in asymmetric membranes. J. Membr. Sci. 1995, 105, 89–115. [Google Scholar] [CrossRef]
- Pinnau, I.; Toy, L.G. Gas and vapor transport properties of amorphous perfluorinated copolymer membranes based on 2,2-bistrifluoromethyl-4,5-difluoro-1,3-dioxole/tetrafluoroethylene. J. Membr. Sci. 1996, 109, 125–133. [Google Scholar] [CrossRef]
- Sen, D.; Kalipcilar, H.; Yilmaz, L. Development of zeolite filled polycarbonate mixed matrix gas separation membranes. Desalination 2006, 200, 222–224. [Google Scholar] [CrossRef]
- Wang, D.; Flanagan, T.B.; Shanahan, K.L. Permeation of Hydrogen through Pre-oxidized Pd Membranes in the absence and Presence of CO. J. Alloys Compd. 2004, 372, 158–164. [Google Scholar] [CrossRef]
- Pakizeh, M.; Omidkhah, M.R.; Zarringhalam, A. Synthesis and characterization of new silica membranes using template–sol–gel technology. Int. J. Hydrog. Energy 2007, 32, 1825–1836. [Google Scholar]
- Gu, Y.; Oyama, S.T. Ultrathin, hydrogen-selective silica membranes deposited on alumina-graded structures prepared from size-controlled boehmite sols. J. Membr. Sci. 2007, 306, 216–227. [Google Scholar] [CrossRef]
- Hatori, H.; Takagi, H.; Yamada, Y. Gas separation properties of molecular sieving carbon membranes with nanopore channels. Carbon 2004, 42, 1169–1173. [Google Scholar] [CrossRef]
- Shaoa, L.; Low, B.T.; Chunga, T.S.; Greenberg, A.R. Polymeric membranes for the hydrogen economy: Contemporary approaches and prospects for the future. J. Membr. Sci. 2009, 327, 18–31. [Google Scholar] [CrossRef]
- Hong, M.; Li, S.; Falconer, J.L.; Noble, D.R. Hydrogen purification using a SAPO-34 membrane. J. Membr. Sci. 2008, 307, 277–283. [Google Scholar]
- Li, Y.; Chung, T.S. Highly selective sulfonated polyethersulfone (SPES)-based membranes with transition metal counterions for hydrogen recovery and natural gas separation. J. Membr. Sci. 2008, 308, 128–135. [Google Scholar] [CrossRef]
- Guazzone, F.; Ma, Y.H. Leak growth mechanism in composite Pd membranes prepared by the electroless deposition method. AIChE J. 2008, 54, 487–492. [Google Scholar] [CrossRef]
- Augustine, A.S.; Mardilovich, I.P.; Kazantzis, N.K.; Ma, Y.H. Durability of PSS-supported Pd-membranes under mixed gas and water–gas shift conditions. J. Membr. Sci. 2012, 415–416, 213–220. [Google Scholar] [CrossRef]
- Pinacci, A.B.P.; Iulianelli, A.; Broglia, M.; Drago, F.; Liguori, S.; Longo, T.; Calabrò, V. Ethanol steam reforming reaction in a porous stainless steel supported palladium membrane reactor. Int. J. Hydrog. Energy 2011, 36, 2029–2037. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liguori, S.; Iulianelli, A.; Dalena, F.; Pinacci, P.; Drago, F.; Broglia, M.; Huang, Y.; Basile, A. Performance and Long-Term Stability of Pd/PSS and Pd/Al2O3 Membranes for Hydrogen Separation. Membranes 2014, 4, 143-162. https://doi.org/10.3390/membranes4010143
Liguori S, Iulianelli A, Dalena F, Pinacci P, Drago F, Broglia M, Huang Y, Basile A. Performance and Long-Term Stability of Pd/PSS and Pd/Al2O3 Membranes for Hydrogen Separation. Membranes. 2014; 4(1):143-162. https://doi.org/10.3390/membranes4010143
Chicago/Turabian StyleLiguori, Simona, Adolfo Iulianelli, Francesco Dalena, Pietro Pinacci, Francesca Drago, Maria Broglia, Yan Huang, and Angelo Basile. 2014. "Performance and Long-Term Stability of Pd/PSS and Pd/Al2O3 Membranes for Hydrogen Separation" Membranes 4, no. 1: 143-162. https://doi.org/10.3390/membranes4010143