Effect of Cross-Linking on the Mechanical and Thermal Properties of Poly(amidoamine) Dendrimer/Poly(vinyl alcohol) Hybrid Membranes for CO2 Separation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials

2.2. Membrane Preparation
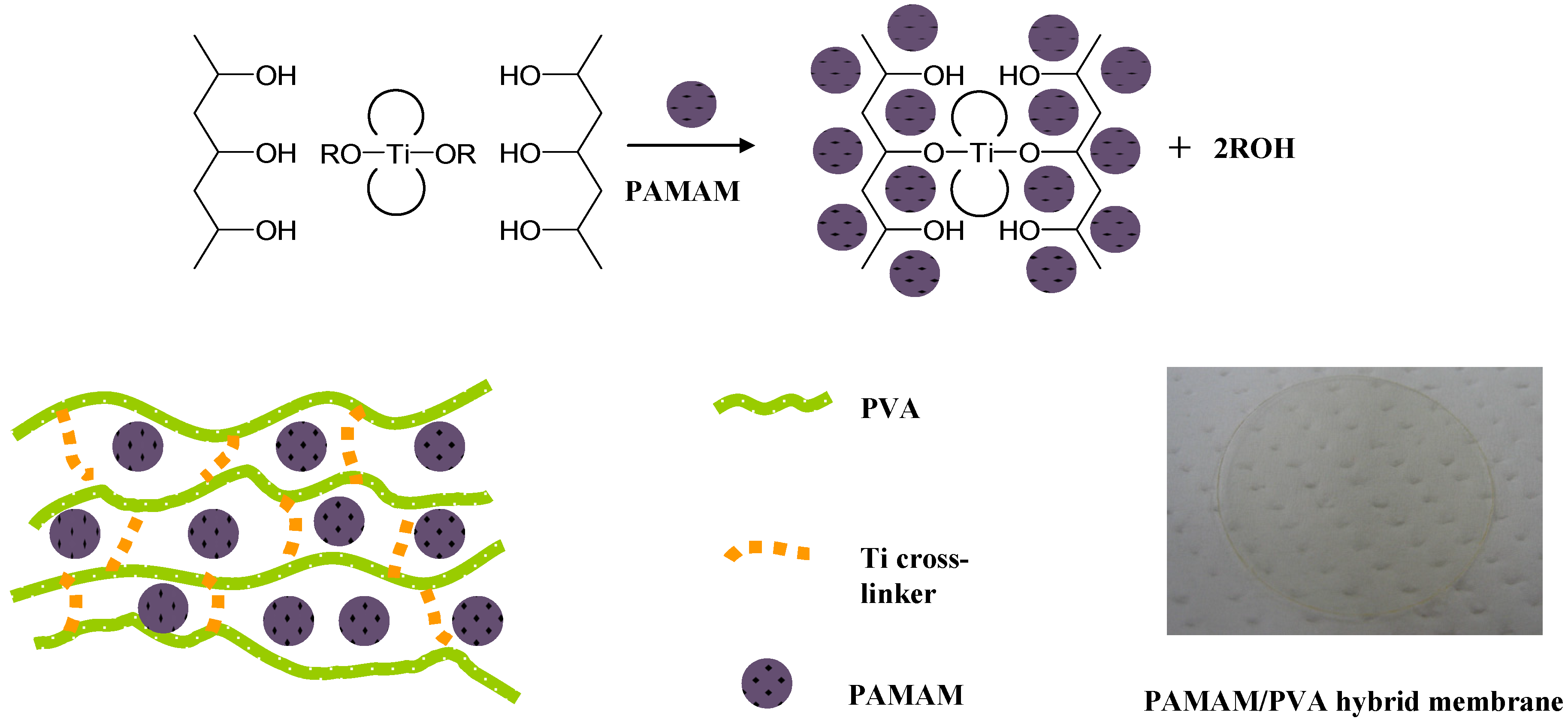
2.3. Permeation Experiments
2.4. Membrane Characterization
3. Results and Discussion
3.1. Nanoindentation Analysis

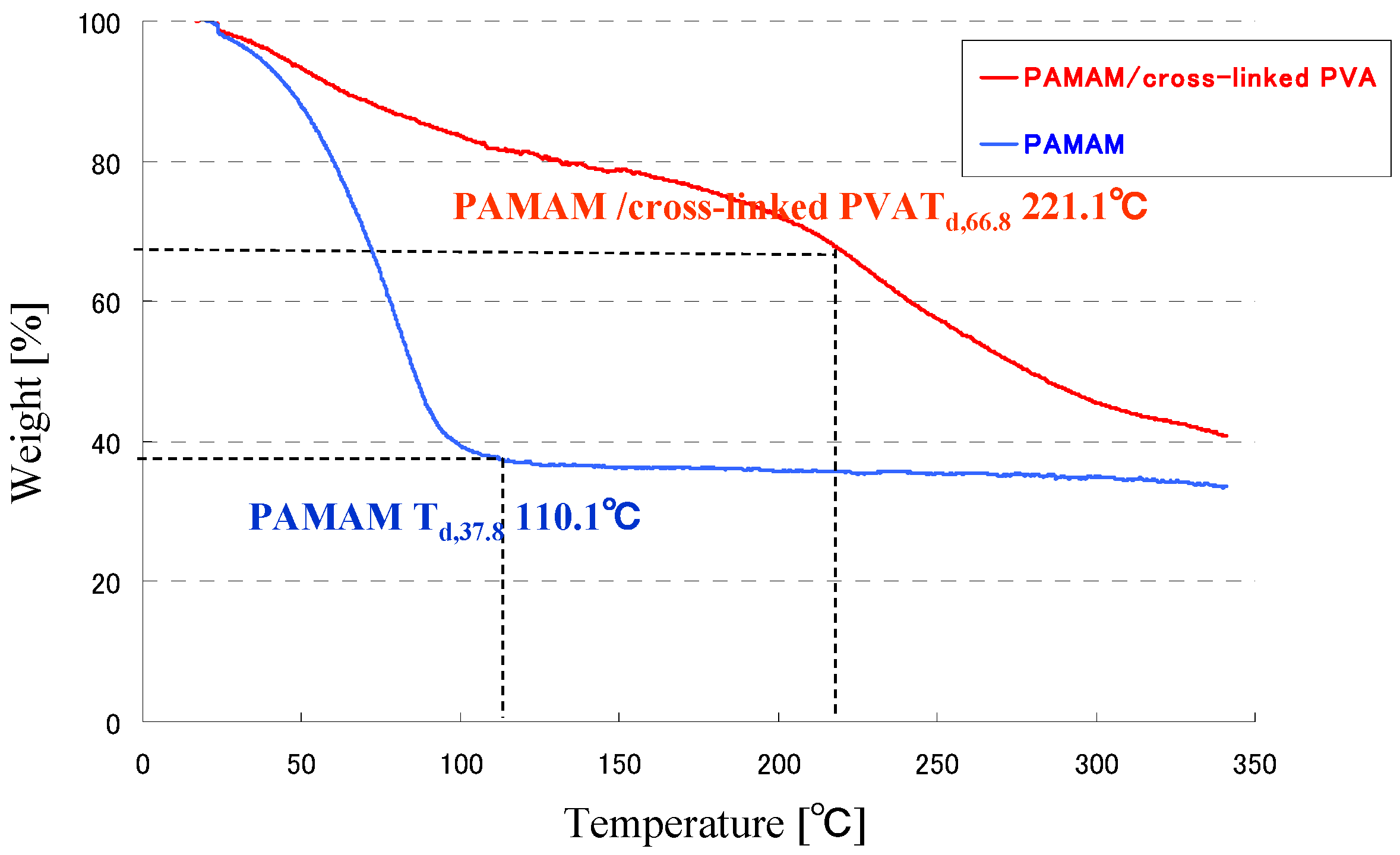
3.2. TG Analysis
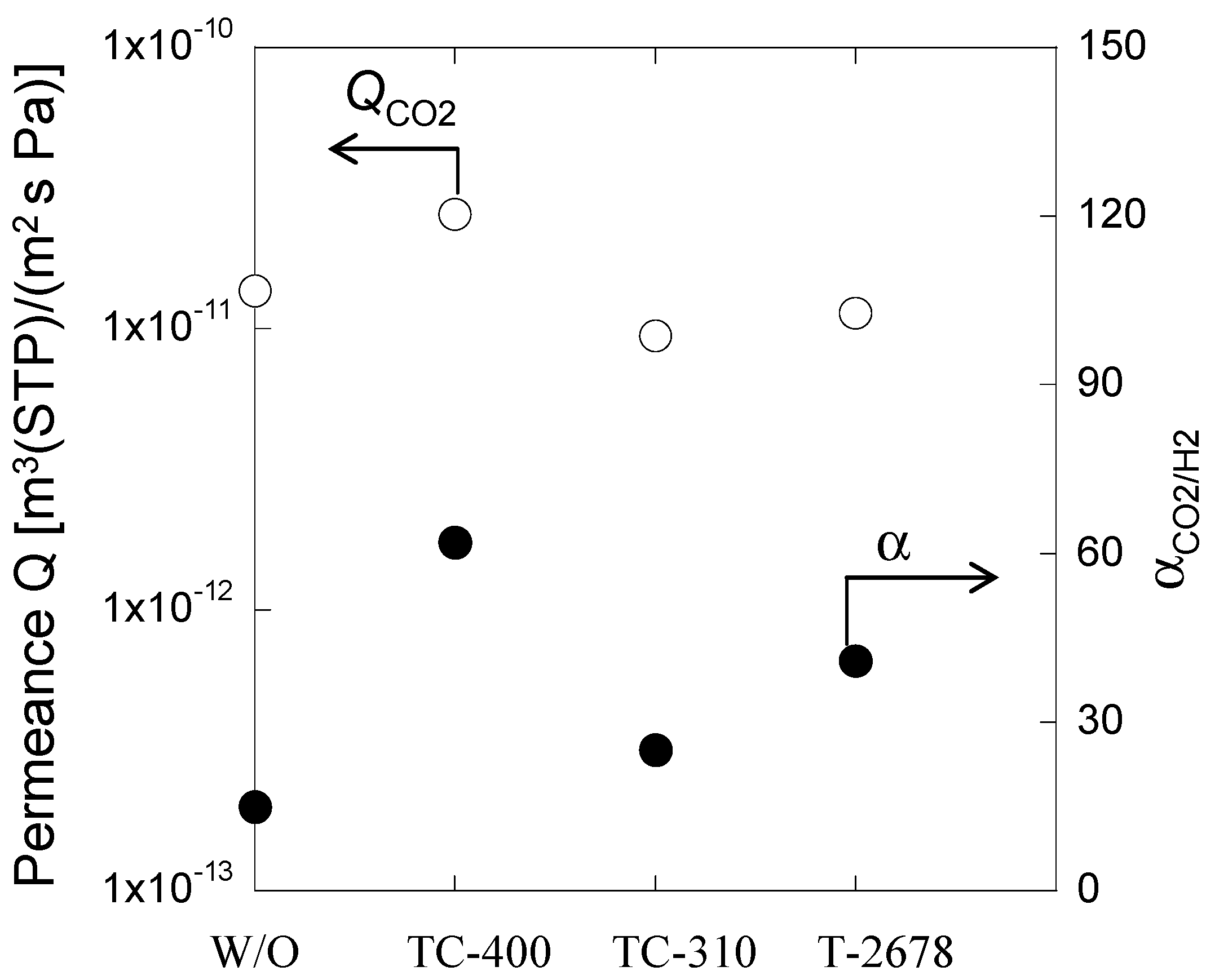
3.3. Effect of Cross-Linker on CO2 Separation Properties


3.4. Effect of CO2 Partial Pressure on CO2 Separation Properties

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, H.; Liang, X. Strategy for promoting low-carbon technology transfer to developing countries: The case of CCS. Energy Policy 2011, 39, 3106–3116. [Google Scholar] [CrossRef]
- Meisen, A.; Shuai, X. Research and development issues in CO2 capture. Energy Convers. Manag. 1997, 38, S37–S42. [Google Scholar] [CrossRef]
- Feron, P.H.M. CO2 Capture: The Characterisation of Gas Separation/Removal Membrane Systems Applied to the Treatment of Flue Gases Arising from Power Generation Using Fossil Fuel; IEA greenhouse gas R & D programme: Cheltenham, UK, 1992. [Google Scholar]
- Nagumo, R.; Kazama, S.; Fujioka, Y. Techno-economic evaluation of the coal-based integrated gasification combined cycle with CO2 capture and storage technology. Energy Proced. 2009, 1, 4089–4093. [Google Scholar] [CrossRef]
- Kovvali, A.S.; Chen, H.; Sirkar, K.K. Dendrimer membranes: A CO2-selective molecular gate. J. Am. Chem. Soc. 2000, 122, 7594–7595. [Google Scholar] [CrossRef]
- Kovvali, A.S.; Sirkar, K.K. Dendrimer liquid membranes: CO2 separation from gas mixtures. Ind. Eng. Chem. Res. 2001, 40, 2502–2511. [Google Scholar] [CrossRef]
- Kovvali, A.S.; Sirkar, K.K. Carbon dioxide separation with novel solvents as liquid membranes. Ind. Eng. Chem. Res. 2002, 41, 2287–2295. [Google Scholar] [CrossRef]
- Duan, S.; Kouketsu, T.; Kazama, S.; Yamada, K. Development of PAMAM dendrimer composite membranes for CO2 separation. J. Membr. Sci. 2006, 283, 2–6. [Google Scholar] [CrossRef]
- Kouketsu, T.; Duan, S.; Kai, T.; Kazama, S.; Yamada, K. PAMAM dendrimer composite membrane for CO2 separation: Formation of a chitosan gutter layer. J. Membr. Sci. 2007, 287, 51–59. [Google Scholar] [CrossRef]
- Kai, T.; Kouketsu, T.; Duan, S.; Kazama, S.; Yamada, K. Development of commercial-sized dendrimer composite membrane modules for CO2 removal from flue gas. Sep. Purif. Technol. 2008, 63, 524–530. [Google Scholar] [CrossRef]
- Taniguchi, I.; Duan, S.; Kazama, S.; Fujioka, Y. Facile fabrication of a novel high performance CO2 separation membrane: immobilization of poly(amidoamine) dendrimers in poly(ethyleneglycol) networks. J. Membr. Sci. 2008, 322, 277–280. [Google Scholar] [CrossRef]
- Taniguchi, I.; Kai, T.; Duan, S.; Kazama, S. PAMAM Dendrimer Containing Polymeric Membrane for Preferential CO2 Separation Over H2—Interplay between CO2 Separation Properties and Morphology. Energy Proced. 2013, 37, 1067–1075. [Google Scholar] [CrossRef]
- Taniguchi, I.; Urai, H.; Kai, T.; Duan, S.; Kazama, S. A CO2-selective molecular gate of poly(amidoamine) dendrimer immobilized in a poly(ethylene glycol) network. J. Membr. Sci. 2013, 444, 96–100. [Google Scholar] [CrossRef]
- Zou, J.; Ho, W.S. CO2-selective polymeric membranes containing amines in crosslinked poly(vinyl alcohol). J. Membr. Sci. 2006, 286, 310–321. [Google Scholar] [CrossRef]
- Huang, J.; Zou, J.; Ho, W.S. Carbon Dioxide Capture Using a CO2-Selective Facilitated Transport Membrane. Ind. Eng. Chem. Res. 2008, 47, 1261–1267. [Google Scholar] [CrossRef]
- Matsuyama, H.; Terada, A.; Nakagawara, T.; Kitamura, Y.; Teramoto, M. Facilitated transport of CO2 through polyethylenimine/poly(vinyl alcohol) blend membrane. J. Membr. Sci. 1999, 163, 221–227. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, Z.; Yi, C.; Bai, Y.; Wang, J.; Wang, S. Gas transport property of polyallylamine-poly(vinyl alcohol)/polysulfone composite membranes. J. Membr. Sci. 2008, 310, 184–196. [Google Scholar] [CrossRef]
- Duan, S.; Taniguchi, I.; Kai, T.; Kazama, S. Poly(amidoamine) dendrimer/poly(vinyl alcohol) hybrid membranes for CO2 capture. J. Membr. Sci. 2012, 423, 107–112. [Google Scholar]
- Duan, S.; Kai, T.; Taniguchi, I.; Kazama, S. Development of Poly(amidoamine) Dendrimer/Poly(vinyl alcohol) Hybrid Membranes for CO2 Separation. Desalin. Water Treat. 2013, 51, 5337–5342. [Google Scholar] [CrossRef]
- Duan, S.; Taniguchi, I.; Kai, T.; Kazama, S. Development of poly(amidoamine) dendrimer/polyvinyl alcohol hybrid membranes for CO2 capture at elevated pressures. Energy Proced. 2013, 37, 924–931. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Duan, S.; Kai, T.; Saito, T.; Yamazaki, K.; Ikeda, K. Effect of Cross-Linking on the Mechanical and Thermal Properties of Poly(amidoamine) Dendrimer/Poly(vinyl alcohol) Hybrid Membranes for CO2 Separation. Membranes 2014, 4, 200-209. https://doi.org/10.3390/membranes4020200
Duan S, Kai T, Saito T, Yamazaki K, Ikeda K. Effect of Cross-Linking on the Mechanical and Thermal Properties of Poly(amidoamine) Dendrimer/Poly(vinyl alcohol) Hybrid Membranes for CO2 Separation. Membranes. 2014; 4(2):200-209. https://doi.org/10.3390/membranes4020200
Chicago/Turabian StyleDuan, Shuhong, Teruhiko Kai, Takashi Saito, Kota Yamazaki, and Kenichi Ikeda. 2014. "Effect of Cross-Linking on the Mechanical and Thermal Properties of Poly(amidoamine) Dendrimer/Poly(vinyl alcohol) Hybrid Membranes for CO2 Separation" Membranes 4, no. 2: 200-209. https://doi.org/10.3390/membranes4020200




