Preparation and Characterization of Hydrophilically Modified PVDF Membranes by a Novel Nonsolvent Thermally Induced Phase Separation Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hydrophilically-Modified PVDF Membranes
2.3. Membrane Characterization
2.4. Antifouling Performance and Membrane Fouling Resistance Analysis
3. Results and Discussion
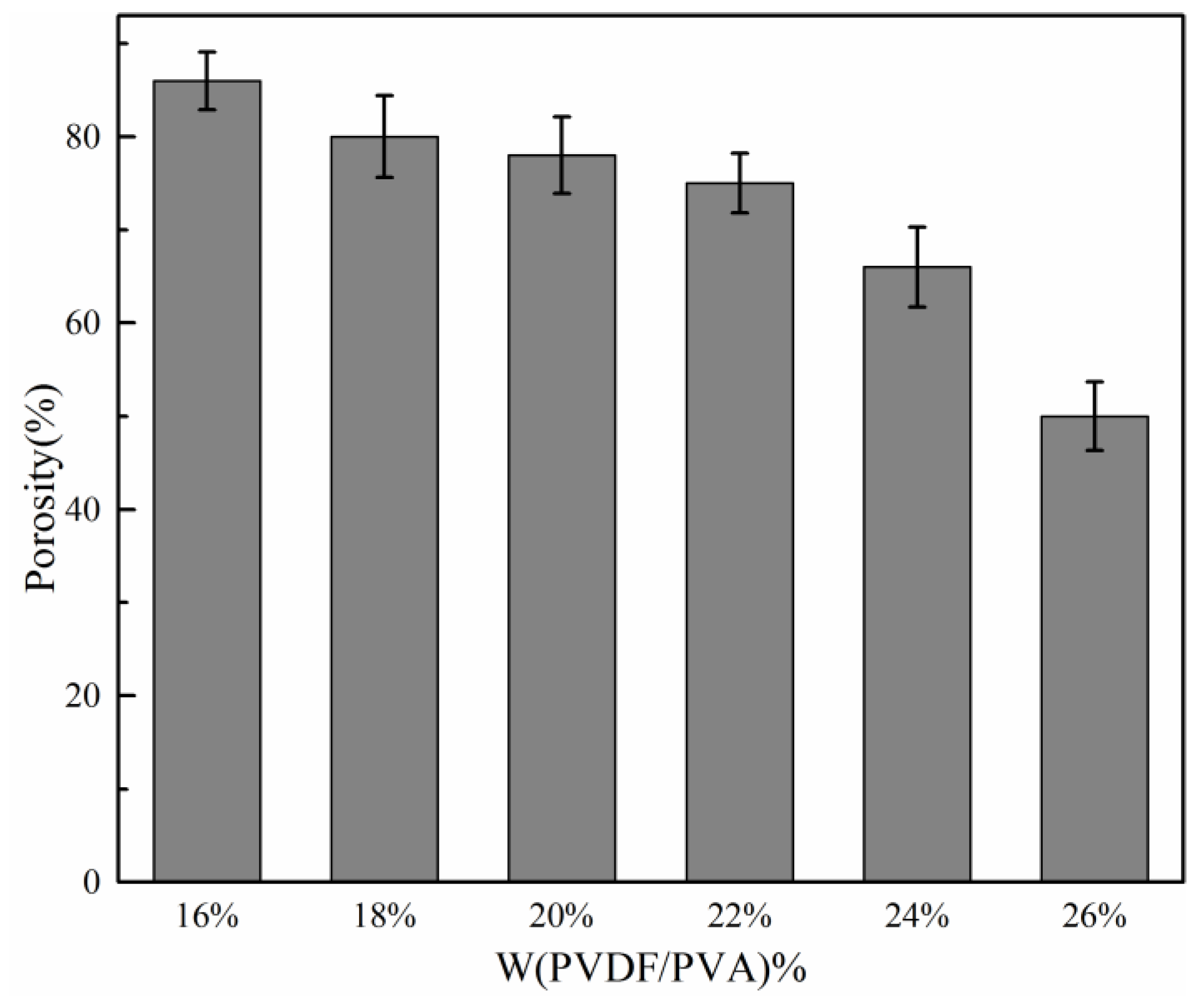
3.1. Investigation on Total Polymer Concentration
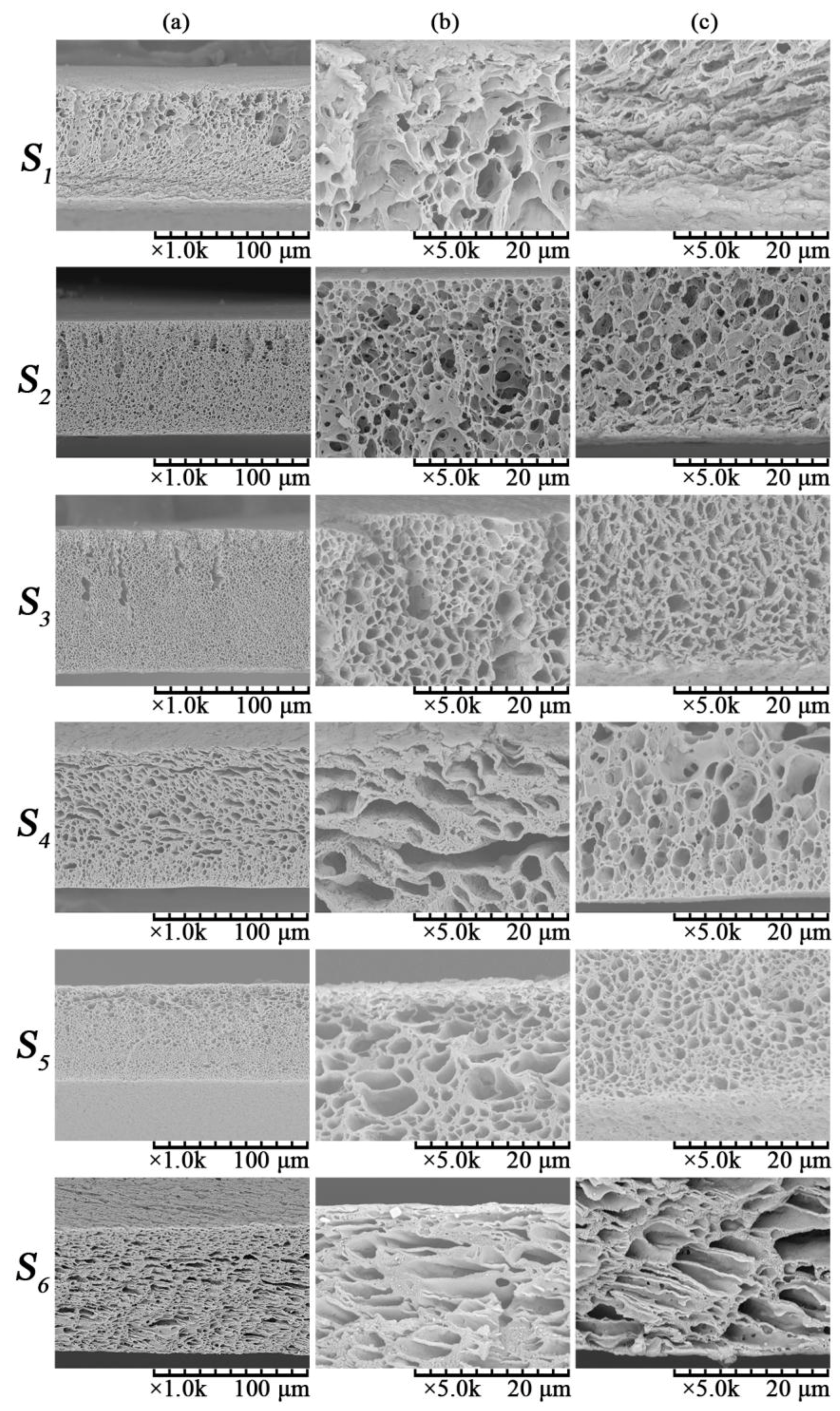
3.1.1. Effect of Total Polymer Concentration on Membrane Morphology
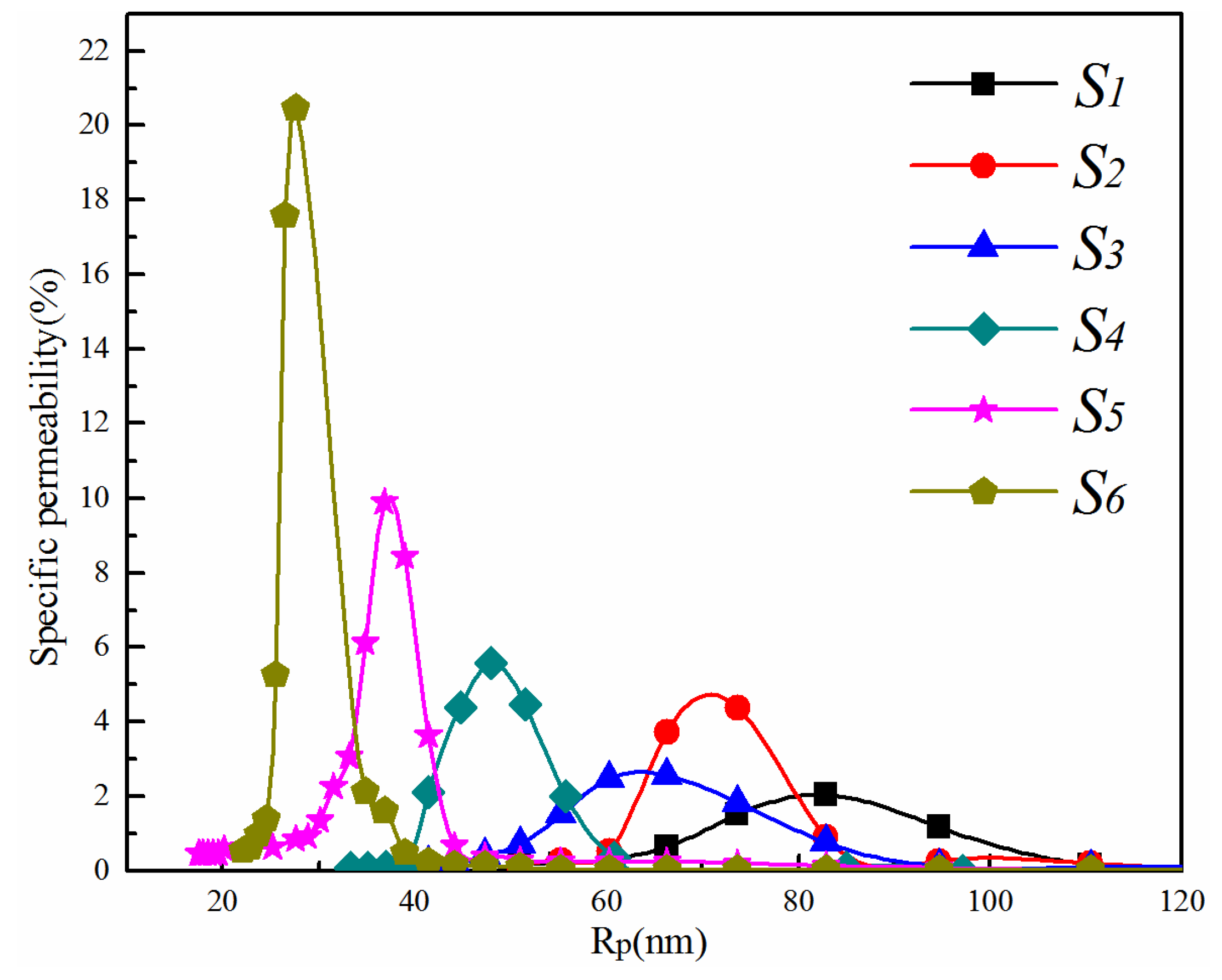
3.1.2. Effect of Total Polymer Concentration on Membrane Pore Size and Pore Size Distribution
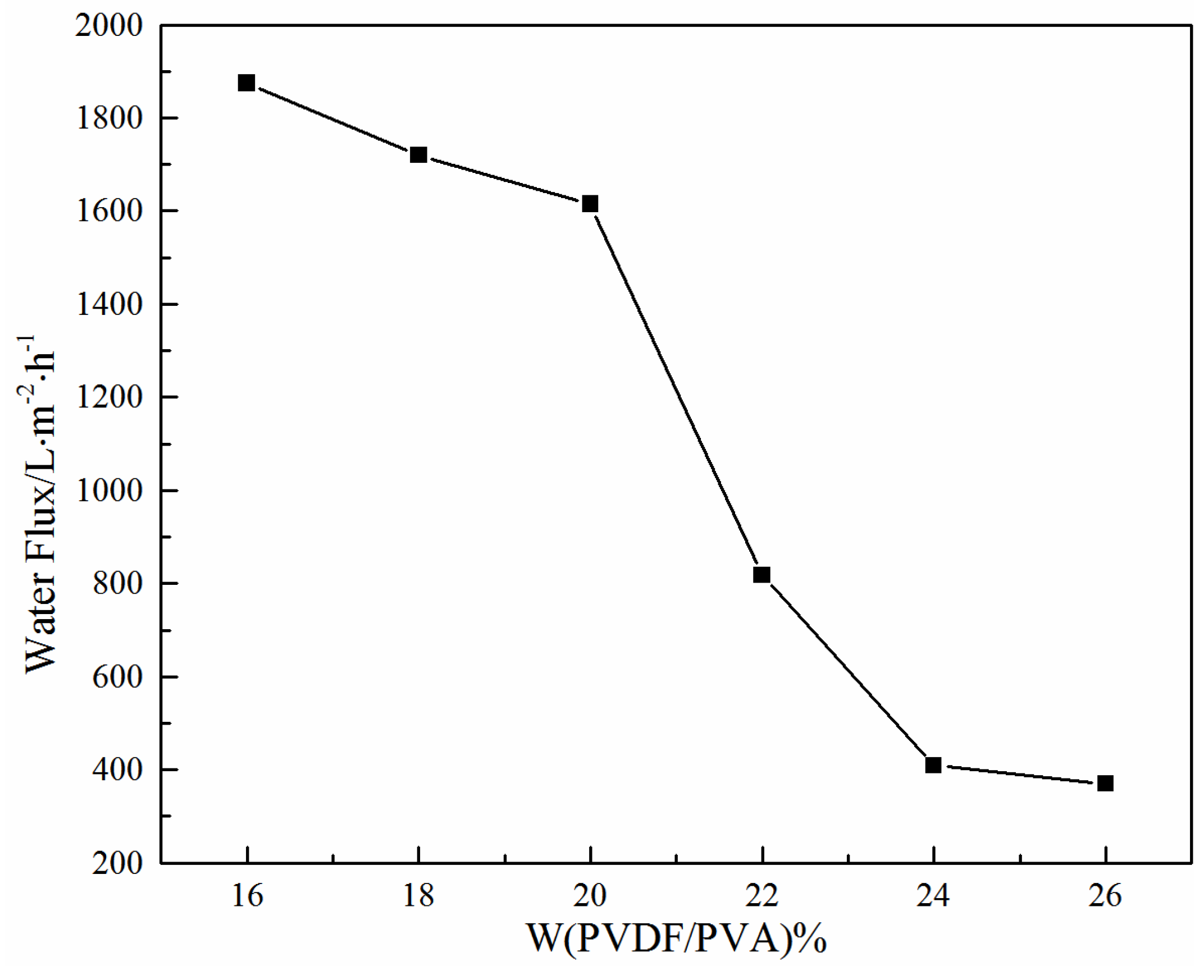
3.1.3. Effect of Total Polymer Concentration on Water Flux
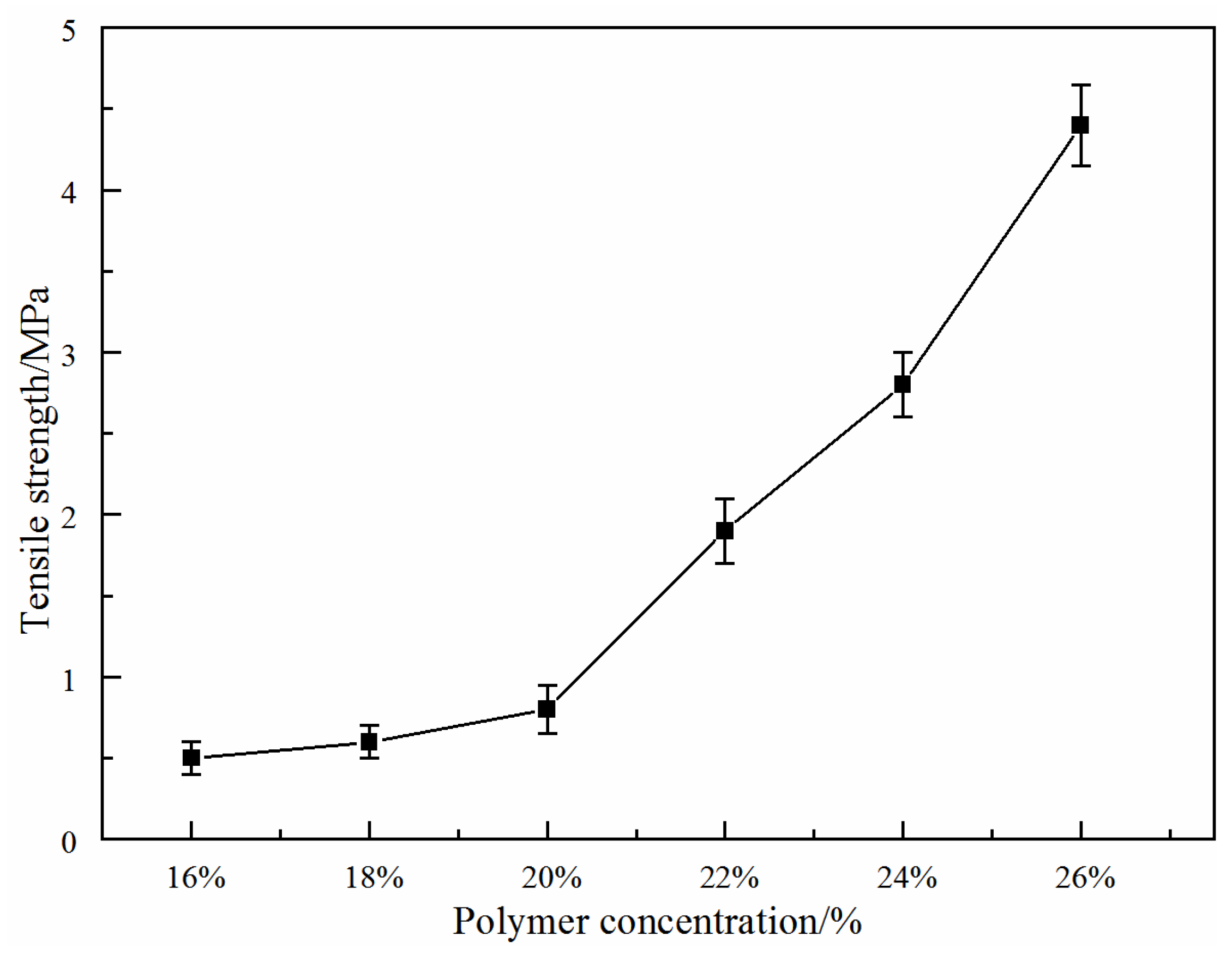
3.1.4. Effect of Total Polymer Concentration on Membrane Mechanical Properties
3.2. Investigation on the PVDF/PVA Blend Ratio
3.2.1. Effect of PVDF/PVA Blend Ratio on Membrane Morphology
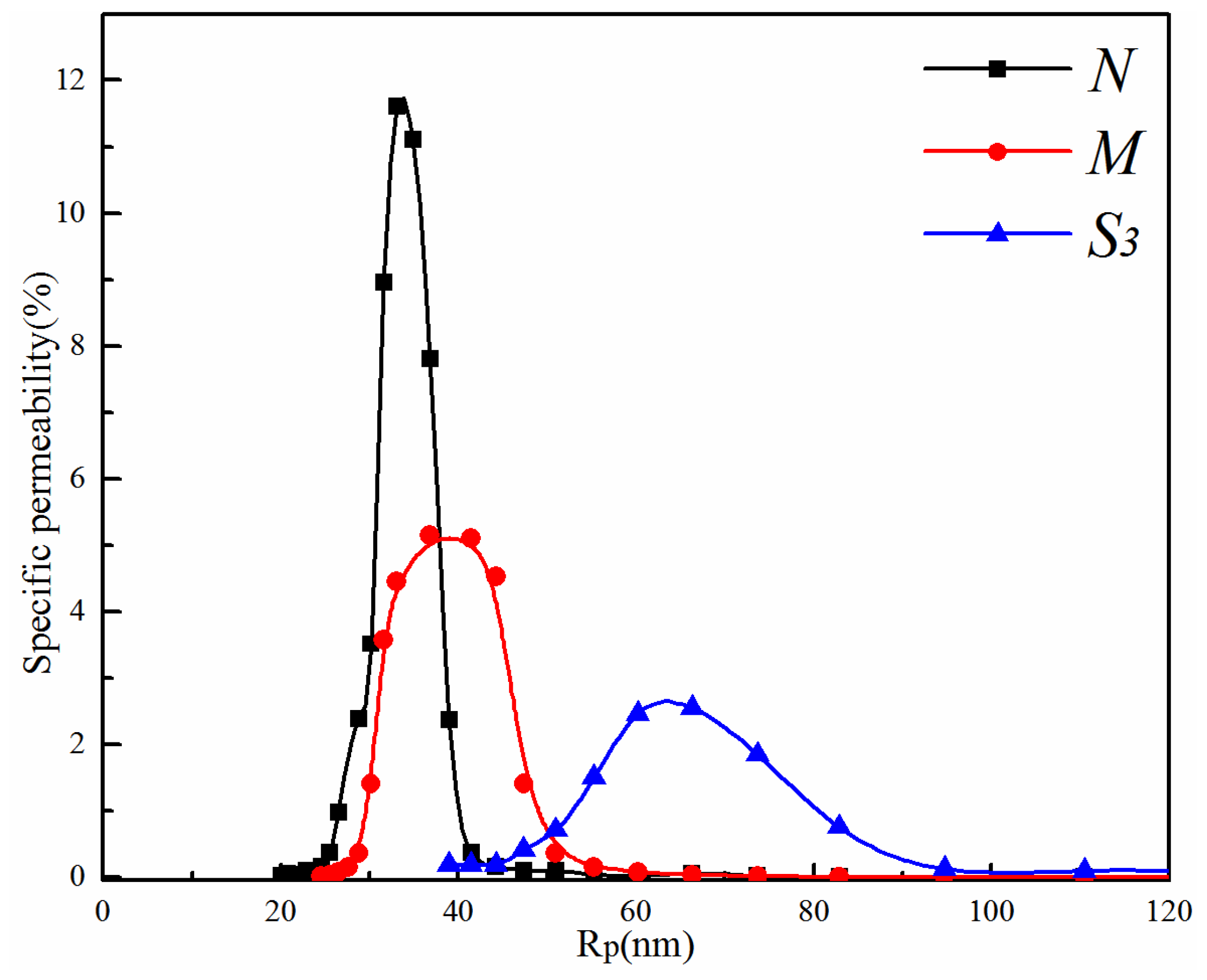
3.2.2. Effect of the PVDF/PVA Blend Ratio on Membrane Pore Size and Pore Size Distribution
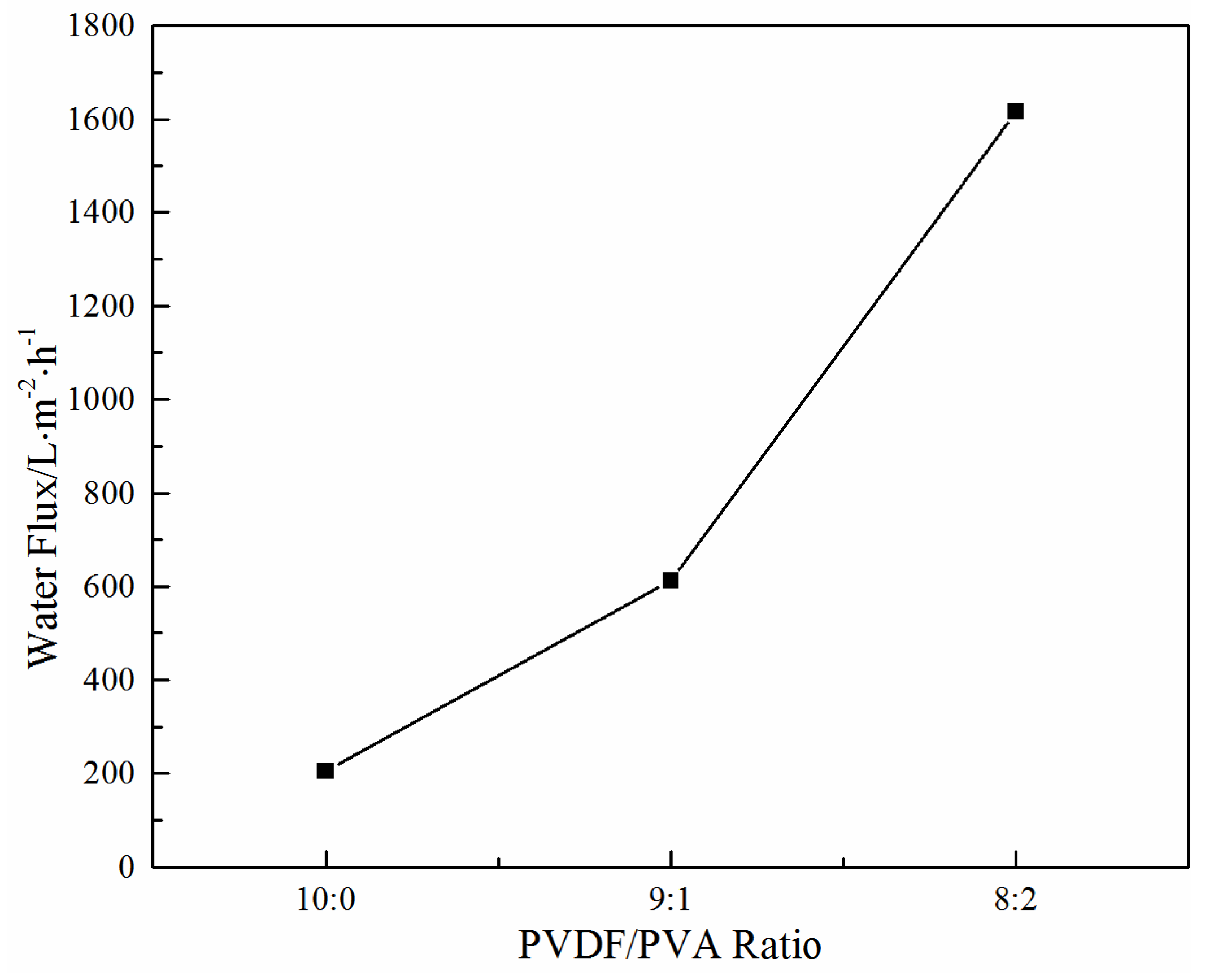
3.2.3. Effect of the PVDF/PVA Blend Ratio on Water Flux
3.2.4. Hydrophilicity of PVDF/PVA Blend Membranes
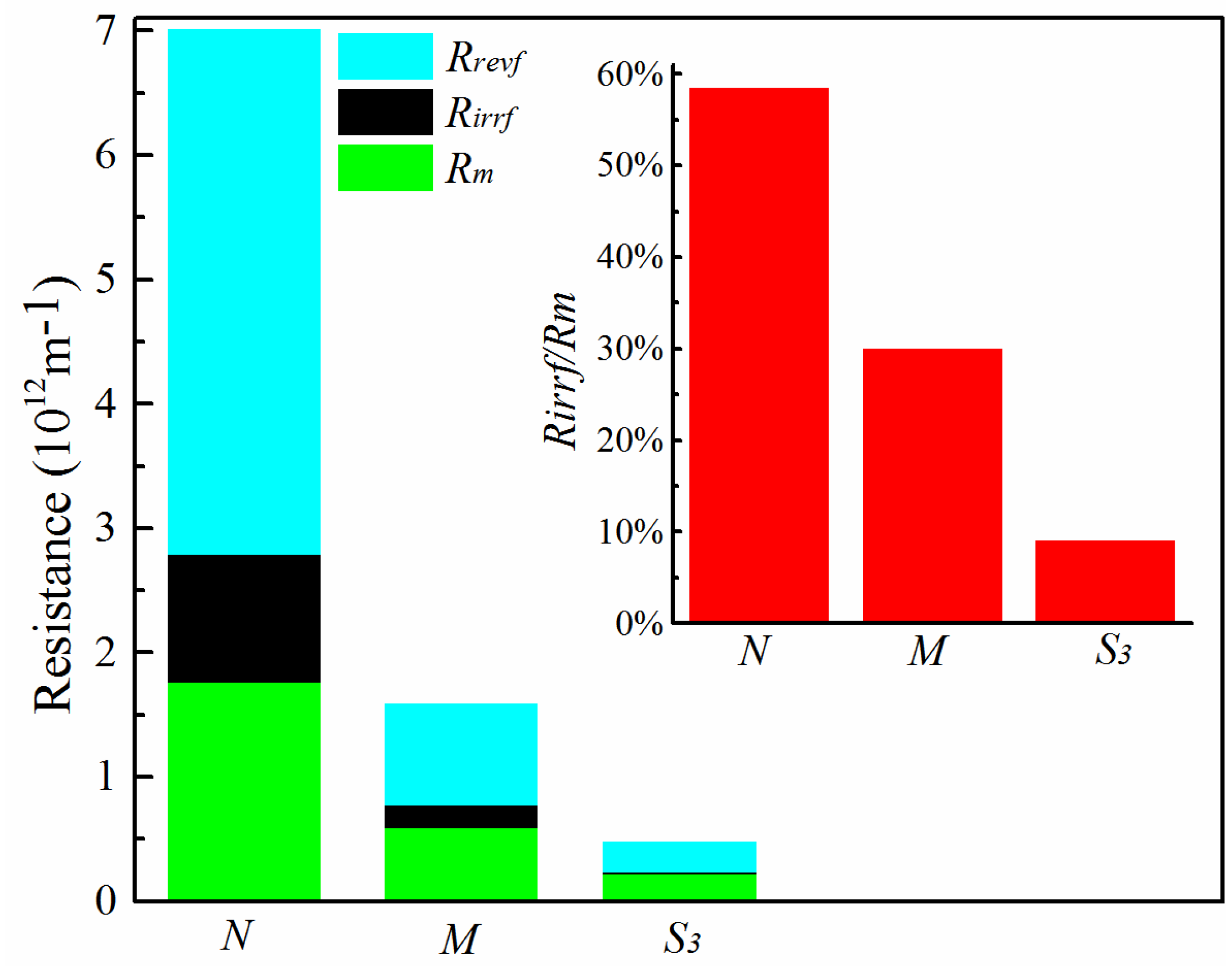
3.3. Antifouling Performance and Membrane Resistance Analysis
4. Conclusions
- (1)
- A novel nonsolvent thermally-induced phase separation (NTIPS) method was successfully employed to prepare hydrophilically-modified PVDF membranes. The PVDF/PVA blend membranes exhibited improved hydrophilicity, higher water permeability and enhanced fouling propensity.
- (2)
- As the total polymer concentrations increased, the pore size, porosity and water flux of the PVDF/PVA blend membranes reduced, and the mechanical strength was improved. The membrane pore size could be deliberately tuned to meet separation requirements.
- (3)
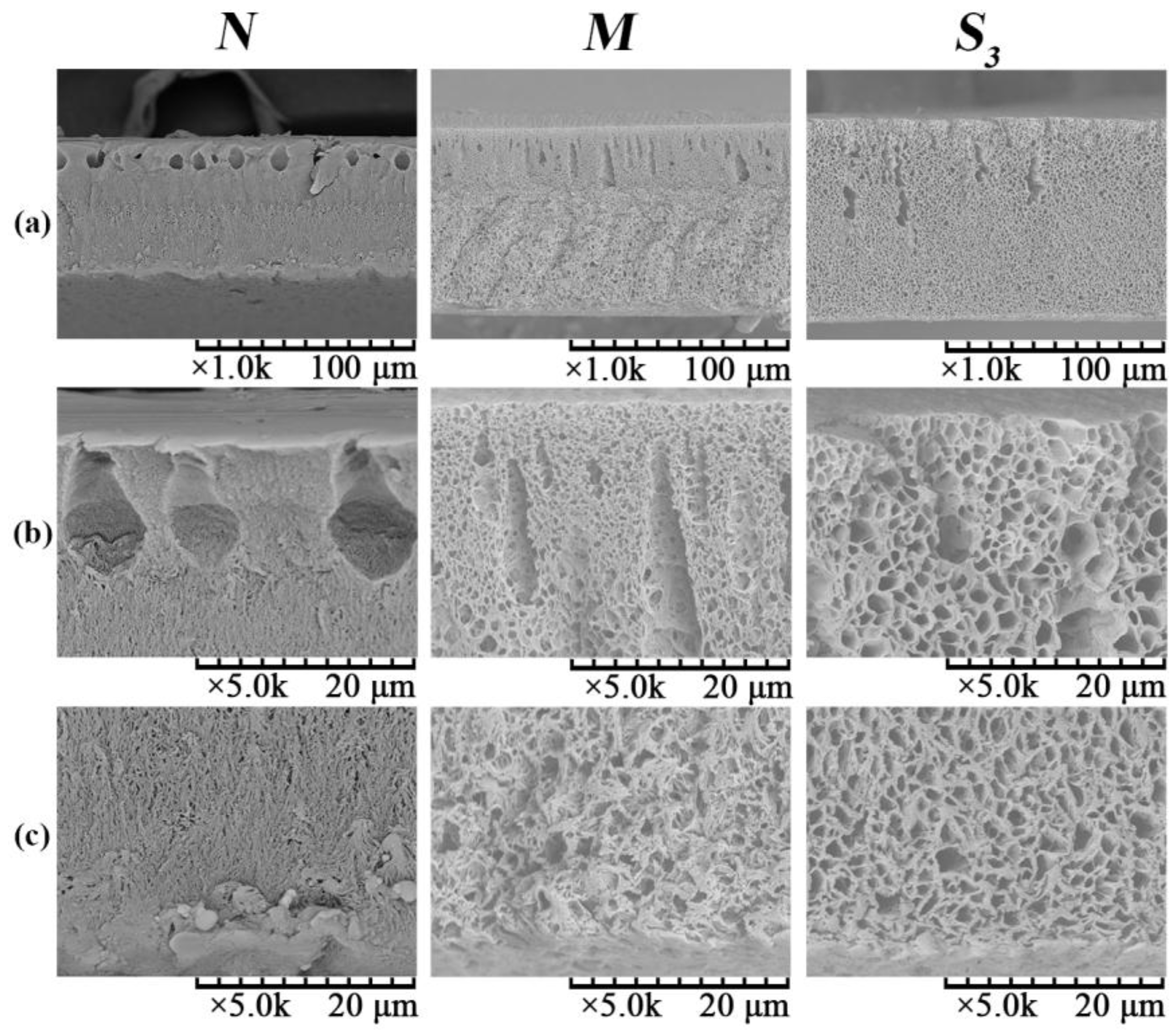
- Both surface and cross-sectional morphologies suggested that the formation of the hydrophilically-modified PVDF/PVA blend membranes was due to NTIPS mechanisms. Different from the top surface structure, which was mainly formed via the NIPS mechanism, the bottom surface of all membranes exhibited a bicontinuous network induced by TIPS.
- (4)
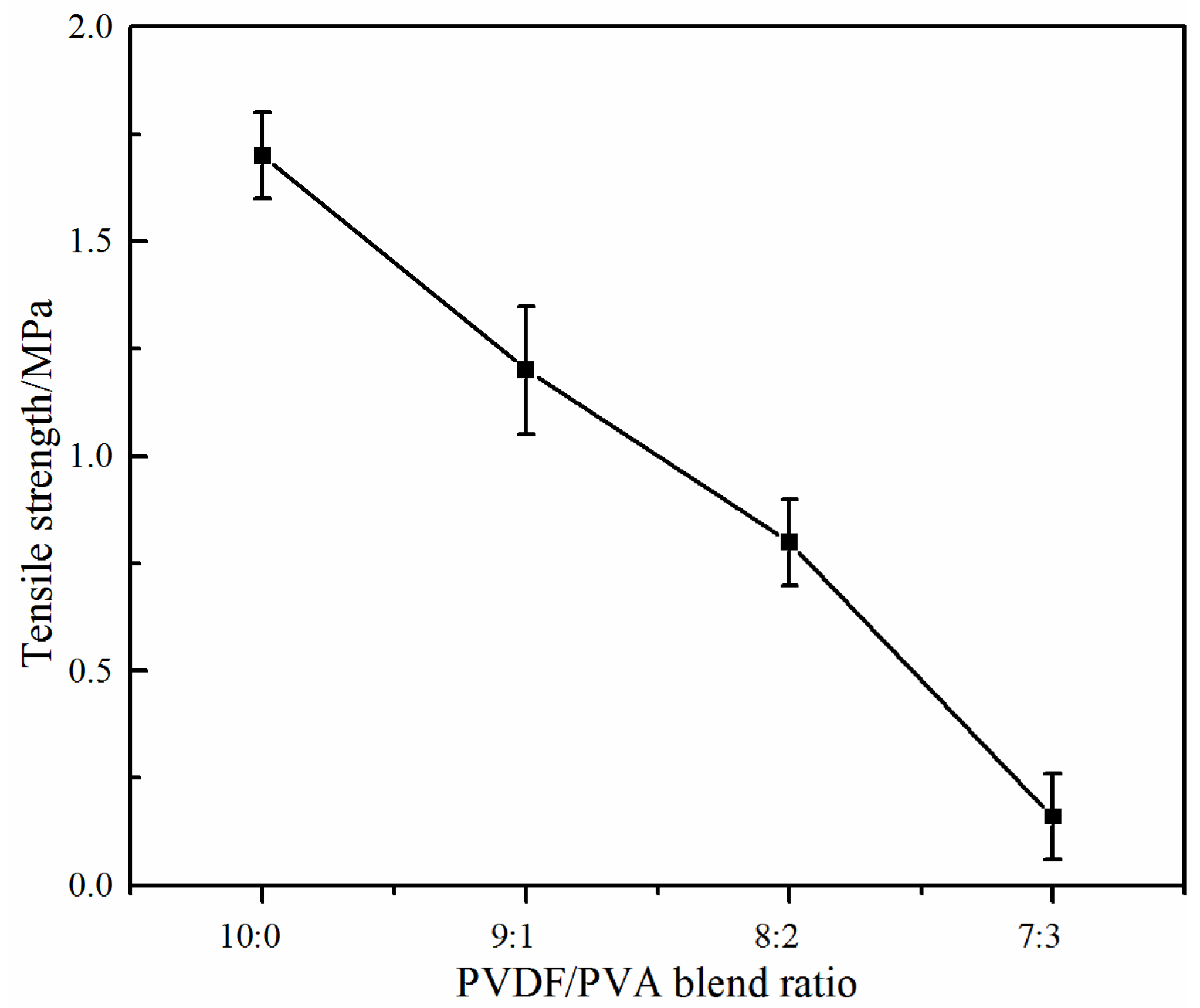
- The dynamic water contact angle of the modified membrane dropped more rapidly indicating improved hydrophilicity with the addition of PVA. However, the ratio of PVA to PVDF should be carefully chosen with the considerations of membrane mechanical strength and filtration performance.
- (5)
- Membrane resistance analysis revealed that the hydrophilically-modified PVDF membranes had lower total resistance of mass transfer (hence, higher permeability) and showed great potential for mitigating irreversible fouling. High performance MF/UF membranes with the desired pore size can be achieved by optimizing fabrication parameters in NTIPS. In future research, surface functionalization could be incorporated into this work to obtain advanced composite membranes with further improved hydrophilicity and antifouling properties for wastewater treatment.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Le-Clech, P.; Chen, V.; Fane, T.A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Bruggen, B.V.D.; Vandecasteele, C.; Gestel, T.V.; Doyen, W.; Leysen, R. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 2003, 22, 46–56. [Google Scholar] [CrossRef]
- Sukitpaneenit, P.; Chung, T.S. Molecular design of the morphology and pore size of PVDF hollow fiber membranes for ethanol-water separation employing the modified pore-flow concept. J. Membr. Sci. 2011, 374, 67–82. [Google Scholar] [CrossRef]
- Kang, G.D.; Cao, Y.M. Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Kyllönen, H.; Pirkonen, P.; Nyström, M.; Nuortila-Jokinen, J.; Grönroos, A. Experimental aspects of ultrasonically enhanced cross-flow membrane filtration of industrial wastewater. Ultrason. Sonochem. 2006, 13, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Argüello, M.A.; Álvarez, S.; Riera, F.A.; Álvarez, R. Enzymatic cleaning of inorganic ultrafiltration membranes used for whey protein fractionation. J. Membr. Sci. 2003, 216, 121–134. [Google Scholar] [CrossRef]
- Chen, S.C.; Fu, X.Z.; Chung, T.S. Fouling behaviors of polybenzimidazole (PBI)-polyhedral oligomericsilsesquioxane (POSS)/polyacrylonitrile (PAN) hollow fiber membranes for engineering osmosis processes. Desalination 2014, 335, 17–26. [Google Scholar] [CrossRef]
- Juang, R.S.; Lin, K.H. Flux recovery in the ultrafiltration of suspended solutions with ultrasound. J. Membr. Sci. 2004, 243, 115–124. [Google Scholar] [CrossRef]
- Muthukumaran, S.; Kentish, S.E.; Ashokkumar, M.; Stevens, G.W. Mechanisms for the ultrasonic enhancement of dairy whey ultrafiltration. J. Membr. Sci. 2005, 258, 106–114. [Google Scholar] [CrossRef]
- Muthukumaran, S.; Kentish, S.; Lalchandani, S.; Ashokkumar, M.; Mawson, R.; Stevens, G.W.; Grieser, F. The optimisation of ultrasonic cleaning procedures for dairy fouled ultrafiltration membranes. Ultrason. Sonochem. 2005, 12, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Li, N.N.; Xiao, C.F.; An, S.L.; Hu, X.Y. Preparation and properties of PVDF/PVA hollow fiber membranes. Desalination 2010, 250, 530–537. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface modifications for antifouling membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef] [PubMed]
- Bonyadi, S.; Chung, T.S.; Krantz, W.B. Investigation of corrugation phenomenon in the inner contour of hollow fibers during the non-solvent induced phase-separation process. J. Membr. Sci. 2007, 299, 200–210. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Li, K. Effect of ethanol composition in water coagulation bath on morphology of PVDF hollow fibre membranes. J. Membr. Sci. 1998, 150, 75–85. [Google Scholar] [CrossRef]
- Edwie, F.; Teoh, M.M.; Chung, T.S. Effects of additives on dual-layer hydrophobic–hydrophilic PVDF hollow fiber membranes for membrane distillation and continuous performance. Chem. Eng. Sci. 2012, 68, 567–578. [Google Scholar] [CrossRef]
- Young, T.H.; Cheng, L.P.; Lin, D.J.; Fane, L.; Chuang, W.Y. Mechanisms of PVDF membrane formation by immersion-precipitation in soft (1-octanol) and harsh (water) nonsolvents. Polymer 1999, 40, 5315–5323. [Google Scholar] [CrossRef]
- Gu, M.H.; Zhang, J.; Wang, X.L.; Tao, H.J.; Ge, L.T. Formation of poly (vinylidene fluoride) (PVDF) membranes via thermally induced phase separation. Desalination 2006, 192, 160–167. [Google Scholar] [CrossRef]
- Li, D.M.; Krantz, W.B.; Greenberg, A.R.; Sani, R.L. Membrane formation via thermally induced phase separation (TIPS): Model development and validation. J. Membr. Sci. 2006, 279, 50–60. [Google Scholar] [CrossRef]
- Li, X.F.; Wang, Y.G.; Lu, X.L.; Xiao, C.F. Morphology changes of polyvinylidene fluoride membrane under different phase separation mechanisms. J. Membr. Sci. 2008, 320, 477–482. [Google Scholar] [CrossRef]
- Lin, D.J.; Chang, H.H.; Chen, T.C.; Lee, Y.C.; Cheng, L.P. Formation of porous poly(vinylidene fluoride) membranes with symmetric or asymmetric morphology by immersion precipitation in the water/TEP/PVDF system. Eur. Polym. J. 2006, 42, 1581–1594. [Google Scholar] [CrossRef]
- Van de Witte, P.; Dijkstra, P.J.; Van den Berg, J.W.A.; Feijen, J. Phase separation processes in polymer solutions in relation to membrane formation. J. Membr. Sci. 1996, 117, 1–31. [Google Scholar] [CrossRef]
- Atkinson, P.M.; Lloyd, D.R. Anisotropic flat sheet membrane formation via TIPS: Atmospheric convection and polymer molecular weight effects. J. Membr. Sci. 2000, 175, 225–238. [Google Scholar] [CrossRef]
- Ji, G.L.; Zhu, B.K.; Cui, Z.Y.; Zhang, C.F.; Xu, Y.Y. PVDF porous matrix with controlled microstructure prepared by TIPS process as polymer electrolyte for lithium ion battery. Polymer 2007, 48, 6415–6425. [Google Scholar] [CrossRef]
- Holland, B.J.; Hay, J.N. The thermal degradation of poly(vinyl alcohol). Polymer 2001, 42, 6775–6783. [Google Scholar] [CrossRef]
- Xiao, T.H.; Wang, P.; Yang, X.; Cai, X.H.; Lu, J. Fabrication and characterization of novel asymmetric polyvinylidene fluoride (PVDF) membranes by the nonsolvent thermally induced phase separation (NTIPS) method for membrane distillation applications. J. Membr. Sci. 2015, 489, 160–174. [Google Scholar] [CrossRef]
- Zhang, L.P.; Chen, G.W.; Tang, H.W.; Cheng, Q.Z.; Wang, S.Q. Preparation and Characterization of composite membranes of Polysulfone and Microcrystalline Cellulos. J. Appl. Polym. Sci. 2009, 112, 550–556. [Google Scholar] [CrossRef]
- Calvo, J.I.; Bottino, A.; Capannelli, G.; Hernández, A. Comparison of liquid-liquid displacement porosimetry and scanning electron microscopy image analysis to characterize ultrafiltration track-etched membranes. J. Membr Sci. 2004, 239, 189–197. [Google Scholar] [CrossRef]
- Sanz, J.M.; Jardines, D.; Bottino, A.; Capannelli, G.; Hernández, A.; Calvo, J.I. Liquid–liquid porometry for an accurate membrane characterization. Desalination 2006, 200, 195–197. [Google Scholar] [CrossRef]
- Calvo, J.I.; Peinador, R.I.; Prádanos, P.; Palacio, L.; Bottino, A.; Capannelli, G.; Hernández, A. Liquid-liquid displacement porometry to estimate the molecular weight cut-off of ultrafiltration membranes. Desalination 2011, 268, 174–181. [Google Scholar] [CrossRef]
- Peinador, R.I.; Calvo, J.I.; Prádanos, P.; Palacio, L.; Hernández, A. Characterisation of polymeric UF membranes by liquid–liquid displacement porosimetry. J. Membr. Sci. 2010, 348, 238–244. [Google Scholar] [CrossRef]
- An, Q.F.; Li, F.; Ji, Y.L.; Chen, H.L. Influence of polyvinyl alcohol on the surface morphology, separation and anti-fouling performance of the composite polyamide nanofiltration membranes. J. Membr. Sci. 2011, 367, 158–165. [Google Scholar] [CrossRef]
- Gohari, R.J.; Lau, W.J.; Matsuura, T.; Ismail, A.F. Effect of surface pattern formation on membrane fouling and its control in phase inversion process. J. Membr. Sci. 2013, 446, 326–331. [Google Scholar] [CrossRef]
- Mi, B.X.; Elimelech, M. Organic fouling of forward osmosis membranes: Fouling reversibility and cleaning without chemical reagents. J. Membr. Sci. 2010, 348, 337–345. [Google Scholar] [CrossRef]
- Liang, S.; Kang, Y.; Tiraferri, A.; Giannelis, E.P.; Huang, X.; Elimelech, M. Highly hydrophilic polyvinylidene fluoride (PVDF) ultrafiltration membranes via postfabrication grafting of surface-tailored silica nanoparticles. Appl. Mater. Interfaces 2013, 5, 6694–6703. [Google Scholar] [CrossRef] [PubMed]
- Mulder, J. Basic Principles of Membrane Technology; Springer: Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; pp. 281–311. [Google Scholar]
- Luján-Facundo, M.J.; Mendoza-Roca, J.A.; Cuartas-Uribe, B.; Álvarez-Blanco, S. Ultrasonic cleaning of ultrafiltration membranes fouled with BSA solution. Sep. Purif. Technol. 2013, 120, 275–281. [Google Scholar] [CrossRef]
- Wei, D.Q.; Tao, Y.; Zhang, Z.H.; Zhang, X.H. Effect of pre-ozonation on mitigation of ceramic UF membrane fouling caused by algal extracellular organic matters. Chem. Eng. J. 2016, 294, 157–166. [Google Scholar] [CrossRef]
- Shang, M.; Matsuyama, H.; Maki, T.; Teramoto, M.; Lloyd, D.R. Preparation and characterization of poly (ethylene-co-vinyl alcohol) membranes via thermally induced liquid–liquid phase separation. Appl. Polym. Sci. 2003, 87, 853–860. [Google Scholar] [CrossRef]
- Wang, L.; Huang, D.X.; Wang, X.D.; Meng, X.R.; Lv, Y.T.; Wang, X.; Miao, R. Preparation of PVDF membranes via the low-temperature TIPS method with diluent mixtures: The role of coagulation conditions and cooling rate. Desalination 2015, 361, 25–37. [Google Scholar] [CrossRef]



| Membrane ID | Total Polymer (PVDF/PVA) Concentration (wt %) | PVDF/PVA Blend Ratio | CPL Concentration (wt %) |
|---|---|---|---|
| S1 | 16 | 8:2 | 84 |
| S2 | 18 | 8:2 | 82 |
| S3 | 20 | 8:2 | 80 |
| S4 | 22 | 8:2 | 78 |
| S5 | 24 | 8:2 | 76 |
| S6 | 26 | 8:2 | 74 |
| L | 20 | 7:3 | 80 |
| M | 20 | 9:1 | 80 |
| N | 20 | 10:0 | 80 |
| Membrane ID (Polymer Concentration) | S1 16 wt % | S2 18 wt % | S3 20 wt % | S4 22 wt % | S5 24 wt % | S6 26 wt % |
|---|---|---|---|---|---|---|
| Mean pore size (nm) | 81.7 | 73.6 | 64.6 | 48.0 | 36.2 | 27.6 |
| Membrane ID (PVDF:PVA Blend Ratio) | N (10:0) | M (9:1) | S3 (8:2) |
|---|---|---|---|
| Mean pore size (nm) | 34.3 | 41.6 | 64.6 |
| Local Resistance | N | M | S3 |
|---|---|---|---|
| Rm (1012 m−1) | 1.76 | 0.60 | 0.22 |
| Rirrf (1012 m−1) | 1.03 | 0.18 | 0.02 |
| Rrevf (1012 m−1) | 4.22 | 0.81 | 0.24 |
| Rt (1012 m−1) | 7.02 | 1.59 | 0.49 |
| Rirrf/Rm | 58.5% | 30.0% | 9.1% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, N.; Xiao, T.; Cai, X.; Ding, L.; Fu, Y.; Yang, X. Preparation and Characterization of Hydrophilically Modified PVDF Membranes by a Novel Nonsolvent Thermally Induced Phase Separation Method. Membranes 2016, 6, 47. https://doi.org/10.3390/membranes6040047
Hu N, Xiao T, Cai X, Ding L, Fu Y, Yang X. Preparation and Characterization of Hydrophilically Modified PVDF Membranes by a Novel Nonsolvent Thermally Induced Phase Separation Method. Membranes. 2016; 6(4):47. https://doi.org/10.3390/membranes6040047
Chicago/Turabian StyleHu, Ningen, Tonghu Xiao, Xinhai Cai, Lining Ding, Yuhua Fu, and Xing Yang. 2016. "Preparation and Characterization of Hydrophilically Modified PVDF Membranes by a Novel Nonsolvent Thermally Induced Phase Separation Method" Membranes 6, no. 4: 47. https://doi.org/10.3390/membranes6040047







