Structure and Nanomechanics of Model Membranes by Atomic Force Microscopy and Spectroscopy: Insights into the Role of Cholesterol and Sphingolipids
Abstract
:1. Introduction
2. AFM: Topographical and Mechanical Characterization of SLBs
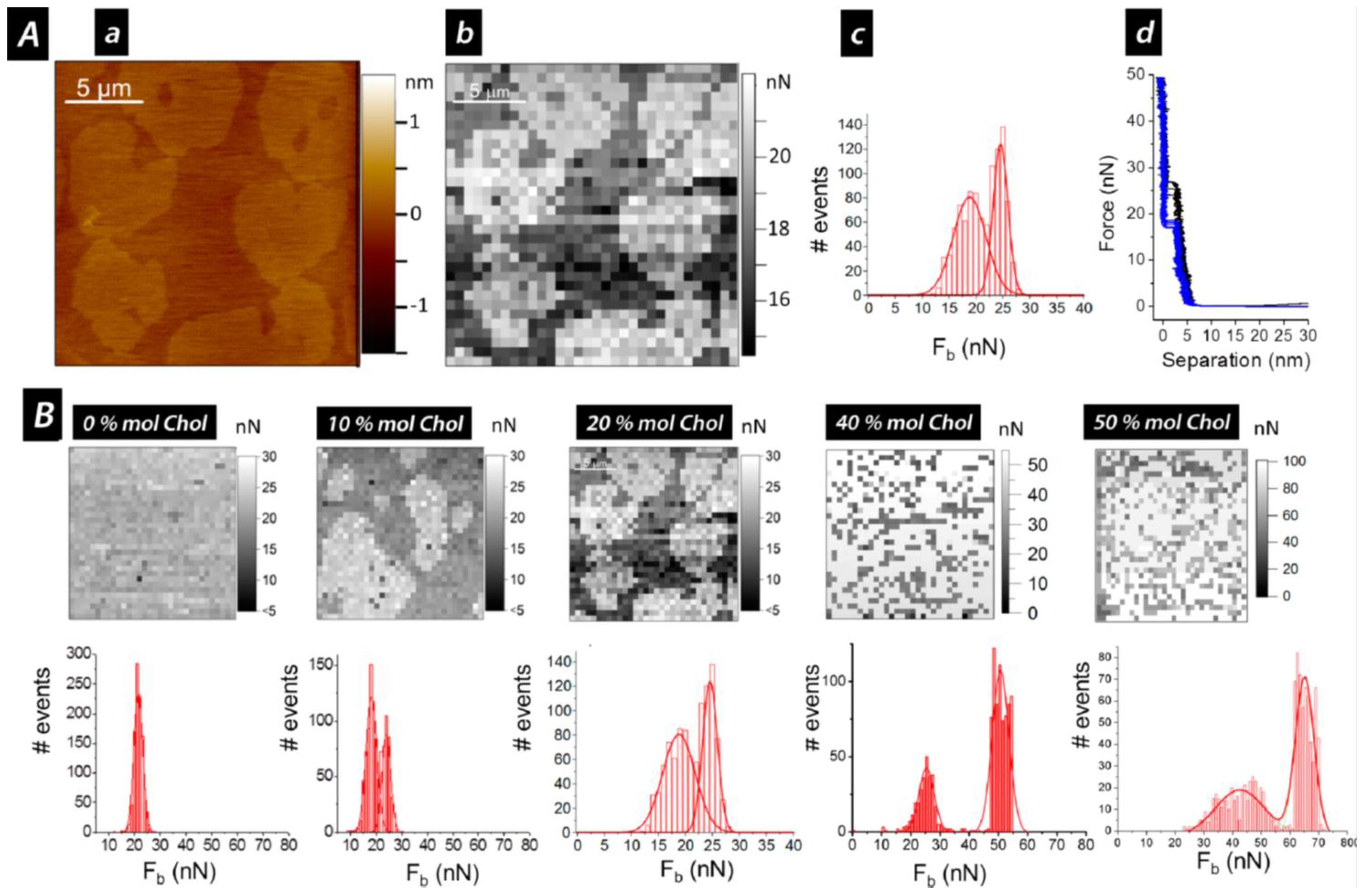
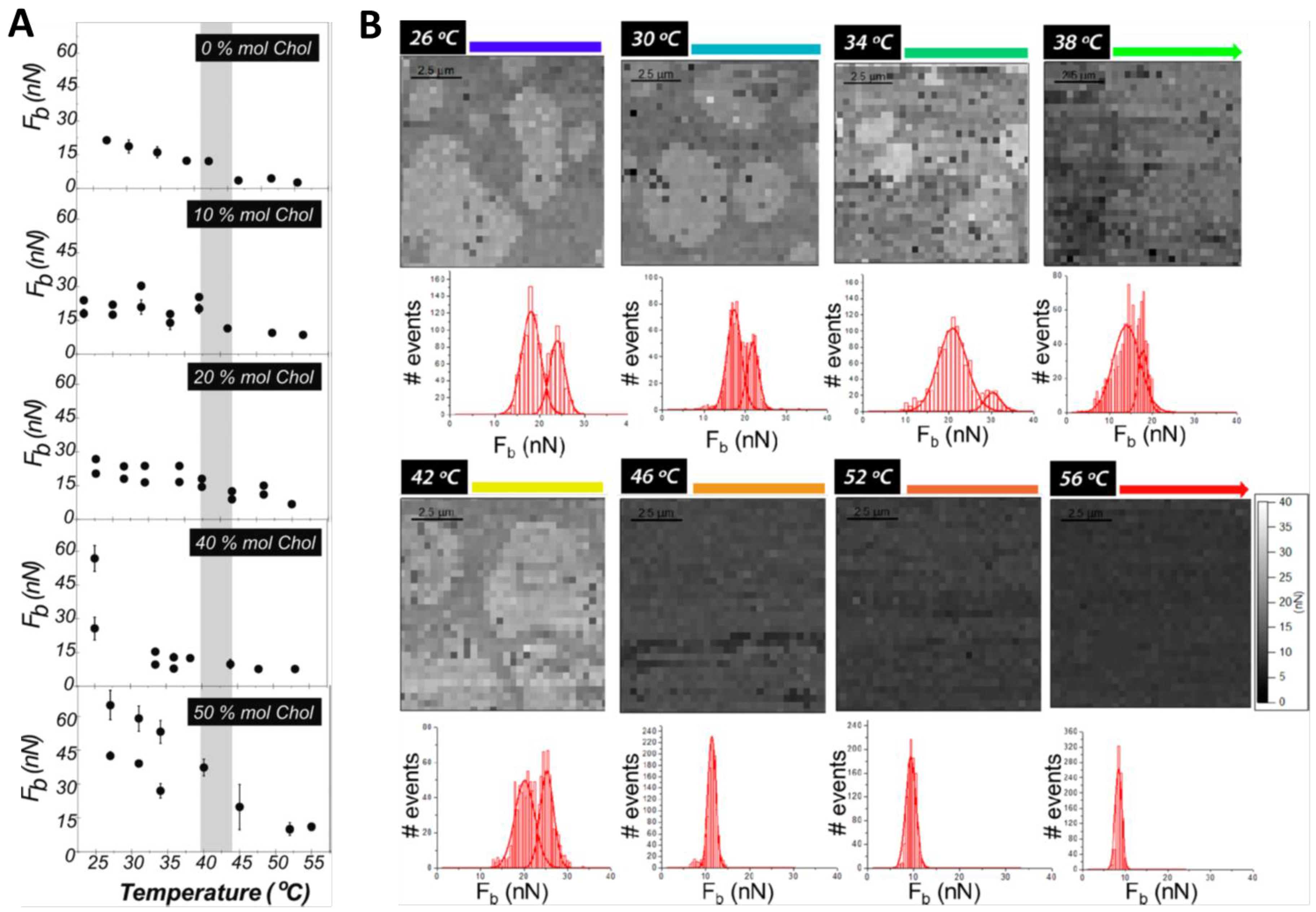
3. Cholesterol’s Effect on Phosphatidylcholine SLBs
4. Sphingolipids and Chol in Model SLBs
4.1. Topography and Nanomechanical Stability by AFM
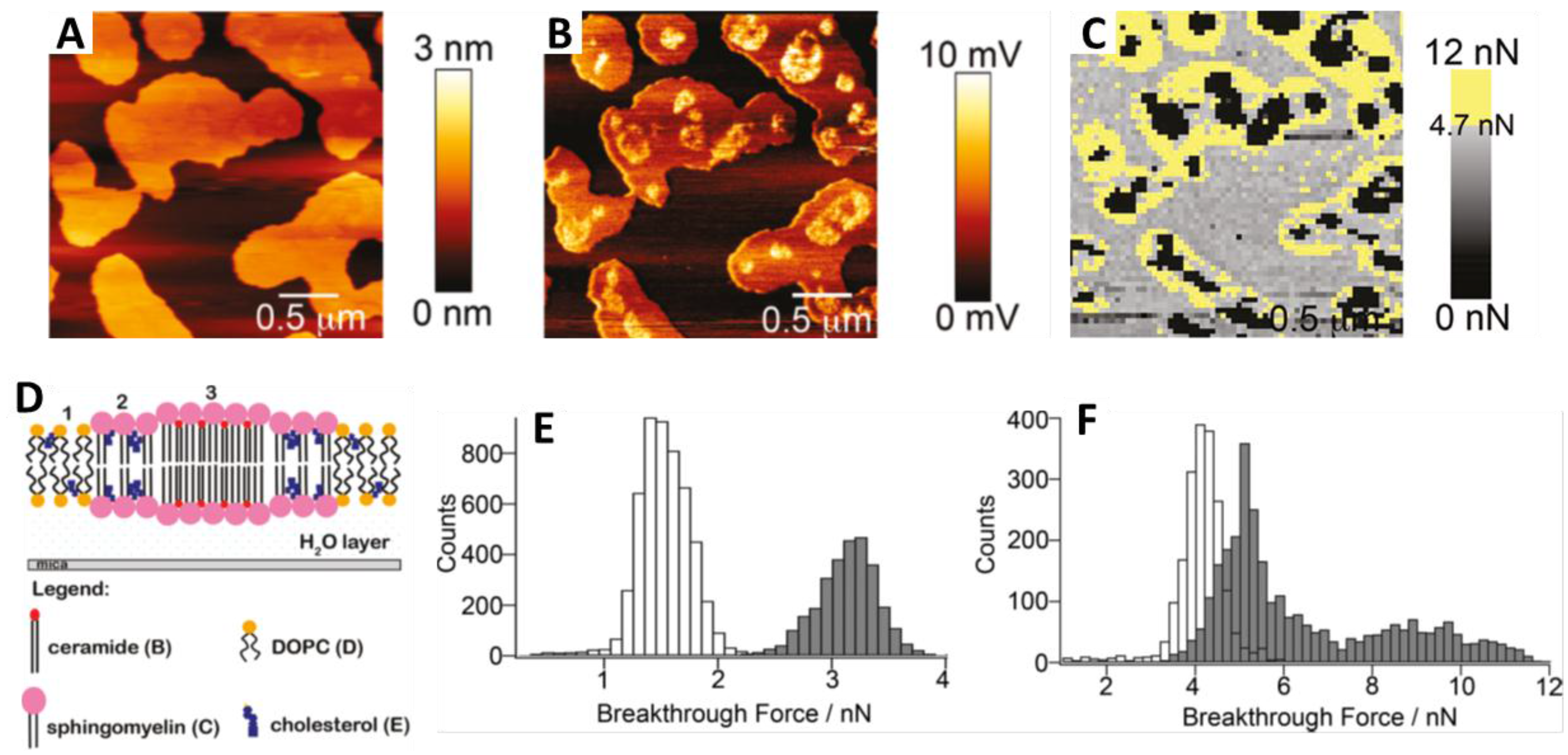
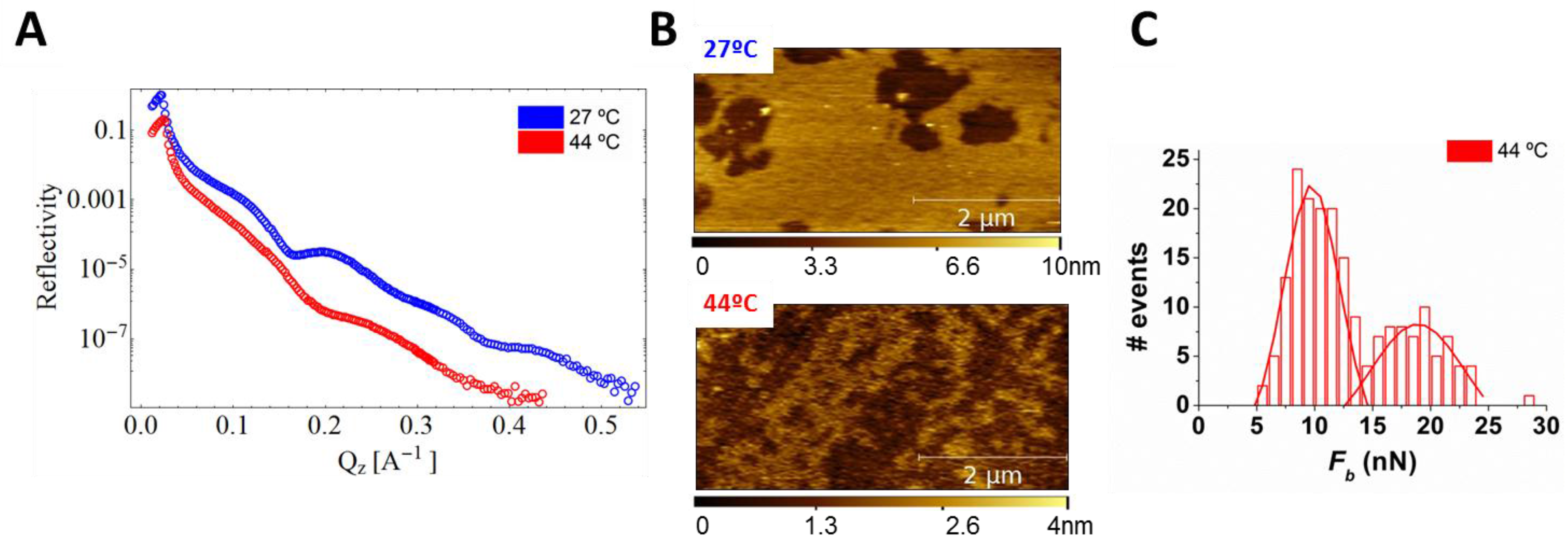
4.1.1. Sphingomyelin
4.1.2. Ceramide
4.1.3. Galactosylceramide
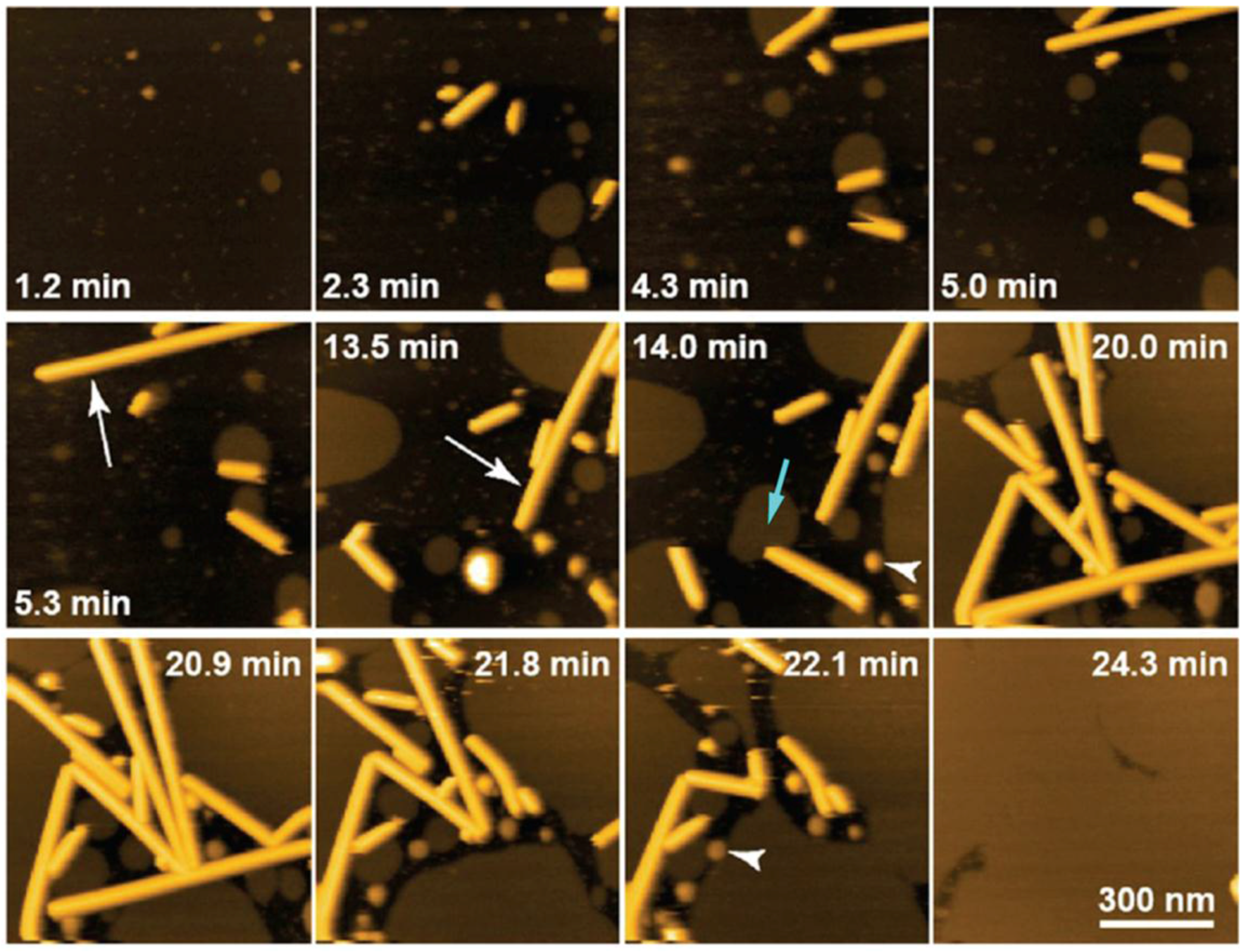
5. Forthcoming Steps: Coupling AFM with X-Ray Techniques
6. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Janmey, P.A.; Kinnunen, P.K.J. Biophysical properties of lipids and dynamic membranes. Trends Cell Biol. 2006, 16, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Maxfield, F.R.; Mondal, M. Sterol and lipid trafficking in mammalian cells. Biochem. Soc. 2006, 34, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, D.; Simons, K. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Vaz, W.L.C. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. 2004, 33, 269–295. [Google Scholar] [CrossRef] [PubMed]
- Chiantia, S.; Kahya, N.; Schwille, P. Raft domain reorganization driven by short- and long-chain ceramide: A combined AFM and FCS study. Langmuir 2007, 23, 7659–7665. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, J.; Rowat, A.C.; Brief, E.; Hsueh, Y.W.; Thewalt, J.L.; Zuckermann, M.J.; Ipsen, J.H. Universal behavior of membranes with sterols. Biophys. J. 2006, 90, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.J.; Mills, T.T.; Tristram-Nagle, S.; Nagle, J.F. Cholesterol perturbs lipid bilayers nonuniversally. Phys. Rev. Lett. 2008, 100. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Morata, L.; Giannotti, M.I.; Sanz, F. Influence of cholesterol on the phase transition of lipid bilayers: A temperature-controlled force spectroscopy study. Langmuir 2012, 28, 12851–12860. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Morata, L.; Giannotti, M.I.; Sanz, F. Structural impact of cations on lipid bilayer models: Nanomechanical properties by afm-force spectroscopy. Mol. Membr. Biol. 2014, 31, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Rog, T.; Pasenkiewicz-Gierula, M.; Vattulainen, I.; Karttunen, M. Ordering effects of cholesterol and its analogues. BBA-Biomembranes 2009, 1788, 97–121. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-C.; Lee, M.-T.; Chen, F.-Y.; Huang, H.W. The condensing effect of cholesterol in lipid bilayers. Biophys. J. 2007, 92, 3960–3967. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.J.; Tristram-Nagle, S.; Nagle, J.F. Effect of cholesterol on structural and mechanical properties of membranes depends on lipid chain saturation. Phys. Rev. E 2009, 80. [Google Scholar] [CrossRef] [PubMed]
- Kucerka, N.; Perlmutter, J.D.; Pan, J.; Tristram-Nagle, S.; Katsaras, J.; Sachs, J.N. The effect of cholesterol on short- and long-chain monounsaturated lipid bilayers as determined by molecular dynamics simulations and X-ray scattering. Biophys. J. 2008, 95, 2792–2805. [Google Scholar] [CrossRef] [PubMed]
- Mingeot-Leclercq, M.-P.; Deleu, M.; Brasseur, R.; Dufrene, Y.F. Atomic force microscopy of supported lipid bilayers. Nat. Protoc. 2008, 3, 1654–1659. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Morata, L.; Giannotti, M.I.; Sanz, F. Stability of Lipid Bilayers as Model Membranes: Atomic Force Microscopy and Spectroscopy Approach. In Atomic force Microscopy in Liquid; Baró, A.M., Reifenberger, R.G., Eds.; Wiley-VCH Verlag GmbH & Co.KGaA: Weinheim, Germany, 2012. (In Press) [Google Scholar]
- Castellana, E.T.; Cremer, P.S. Solid supported lipid bilayers: From biophysical studies to sensor design. Surf. Sci. Rep. 2006, 61, 429–444. [Google Scholar] [CrossRef]
- Giocondi, M.C.; Vie, V.; Lesniewska, E.; Milhiet, P.E.; Zinke-Allmang, M.; le Grimellec, C. Phase topology and growth of single domains in lipid bilayers. Langmuir 2001, 17, 1653–1659. [Google Scholar] [CrossRef]
- Giocondi, M.C.; Yamamoto, D.; Lesniewska, E.; Milhiet, P.E.; Ando, T.; le Grimellec, C. Surface topography of membrane domains. BBA-Biomembranes 2010, 1798, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Talham, D.R.; Yamamoto, T.; Meisel, M.W. Langmuir-blodgett films of molecular organic materials. J. Phys. Condens. Matter 2008, 20. [Google Scholar] [CrossRef]
- Mennicke, U.; Salditt, T. Preparation of solid-supported lipid bilayers by spin-coating. Langmuir 2002, 18, 8172–8177. [Google Scholar] [CrossRef]
- Reimhult, E.; Hook, F.; Kasemo, B. Intact vesicle adsorption and supported biomembrane formation from vesicles in solution: Influence of surface chemistry, vesicle size, temperature, and osmotic pressure. Langmuir 2003, 19, 1681–1691. [Google Scholar] [CrossRef]
- Tamm, L.K.; McConnell, H.M. Supported phospholipid-bilayers. Biophys. J. 1985, 47, 105–113. [Google Scholar] [CrossRef]
- Redondo-Morata, L.; Giannotti, M.I.; Sanz, F. Afm-based force-clamp monitors lipid bilayer failure kinetics. Langmuir 2012, 28, 6403–6410. [Google Scholar] [CrossRef] [PubMed]
- Slotte, J.P. Direct observation of the action of cholesterol oxidase in monolayers. BBA Lipid Lipid Metab. 1995, 1259, 180–186. [Google Scholar] [CrossRef]
- Schram, V.; Lin, H.N.; Thompson, T.E. Topology of gel-phase domains and lipid mixing properties in phase-separated two-component phosphatidylcholine bilayers. Biophys. J. 1996, 71, 1811–1822. [Google Scholar] [CrossRef]
- Honig, D.; Mobius, D. Direct visualization of monolayers at the air-water-interface by brewster-angle microscopy. J. Phys. Chem. 1991, 95, 4590–4592. [Google Scholar] [CrossRef]
- Fragneto, G.; Charitat, T.; Daillant, J. Floating lipid bilayers: Models for physics and biology. Eur. Biophys. J. 2012, 41, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Nagle, J.F.; Tristram-Nagle, S. Structure of lipid bilayers. BBA Rev. Biomembr. 2000, 1469, 159–195. [Google Scholar]
- Kucerka, N.; Heberle, F.A.; Pan, J.J.; Katsaras, J. Structural significance of lipid diversity as studied by small angle neutron and X-ray scattering. Membranes 2015, 5, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Gliss, C.; Randel, O.; Casalta, H.; Sackmann, E.; Zorn, R.; Bayerl, T. Anisotropic motion of cholesterol in oriented dppc bilayers studied by quasielastic neutron scattering: The liquid-ordered phase. Biophys. J. 1999, 77, 331–340. [Google Scholar] [CrossRef]
- Fragneto, G. Neutrons and model membranes. Eur. Phys. J. Spec. Top. 2012, 213, 327–342. [Google Scholar] [CrossRef]
- Evans, E.; Heinrich, V.; Ludwig, F.; Rawicz, W. Dynamic tension spectroscopy and strength of biomembranes. Biophys. J. 2003, 85, 2342–2350. [Google Scholar] [CrossRef]
- Garcia-Manyes, S.; Sanz, F. Nanomechanics of lipid bilayers by force spectroscopy with AFM: A perspective. BBA-Biomembranes 2010, 1798, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Picas, L.; Milhiet, P.E.; Hernandez-Borrell, J. Atomic force microscopy: A versatile tool to probe the physical and chemical properties of supported membranes at the nanoscale. Chem. Phys. Lipids 2012, 165, 845–860. [Google Scholar] [CrossRef] [PubMed]
- El Kirat, K.; Morandat, S.; Dufrene, Y.F. Nanoscale analysis of supported lipid bilayers using atomic force microscopy. BBA-Biomembranes 2010, 1798, 750–765. [Google Scholar] [CrossRef] [PubMed]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic force microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Parot, P.; Dufrene, Y.F.; Hinterdorfer, P.; le Grimellee, C.; Navajas, D.; Pellequer, J.L.; Scheuring, S. Past, present and future of atomic force microscopy in life sciences and medicine. J. Mol. Recognit. 2007, 20, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Uchihashi, T.; Kodera, N. High-speed atomic force microscopy. Jpn. J. Appl. Phys. 2012, 51. [Google Scholar] [CrossRef]
- Eghiaian, F.; Rico, F.; Colom, A.; Casuso, I.; Scheuring, S. High-speed atomic force microscopy: Imaging and force spectroscopy. FEBS Lett. 2014, 588, 3631–3638. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Uchihashi, T.; Scheuring, S. Filming biomolecular processes by high-speed atomic force microscopy. Chem. Rev. 2014, 114, 3120–3188. [Google Scholar] [CrossRef] [PubMed]
- Scheuring, S.; Dufrene, Y.F. Atomic force microscopy: Probing the spatial organization, interactions and elasticity of microbial cell envelopes at molecular resolution. Mol. Microbiol. 2010, 75, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Giannotti, M.I.; Vancso, G.J. Interrogation of single synthetic polymer chains and polysaccharides by AFM-based force spectroscopy. Chemphyschem 2007, 8, 2290–2307. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, S.G.; Colton, R.J.; Lilleodden, E.T.; Gerberich, W.W. Anomalous plastic deformation at surfaces: Nanoindentation of gold single crystals. Phys. Rev. B 1997, 55, 16057–16060. [Google Scholar] [CrossRef]
- Giannotti, M.I.; Rinaudo, M.; Vancso, G.J. Force spectroscopy of hyaluronan by atomic force microscopy: From hydrogen-bonded networks toward single-chain behavior. Biomacromolecules 2007, 8, 2648–2652. [Google Scholar] [CrossRef] [PubMed]
- Rico, F.; Gonzalez, L.; Casuso, I.; Puig-Vidal, M.; Scheuring, S. High-speed force spectroscopy unfolds titin at the velocity of molecular dynamics simulations. Science 2013, 342, 741–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, T.E.; Oberhauser, A.F.; Carrion-Vazquez, M.; Marszalek, P.E.; Fernandez, J.M. The study of protein mechanics with the atomic force microscope. Trends Biochem. Sci. 1999, 24, 379–384. [Google Scholar] [CrossRef]
- Giannotti, M.I.; de Vaca, I.C.; Artes, J.M.; Sanz, F.; Guallar, V.; Gorostiza, P. Direct measurement of the nanomechanical stability of a redox protein active site and its dependence upon metal binding. J. Phys. Chem. B 2015, 119, 12050–12058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.U.; Chrisey, L.A.; Colton, R.J. Direct measurement of the forces between complementary strands of DNA. Science 1994, 266. [Google Scholar] [CrossRef]
- Alessandrini, A.; Facci, P. Nanoscale mechanical properties of lipid bilayers and their relevance in biomembrane organization and function. Micron 2012, 43, 1212–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Manyes, S.; Redondo-Morata, L.; Oncins, G.; Sanz, F. Nanomechanics of lipid bilayers: Heads or tails? J. Am. Chem. Soc. 2010, 132, 12874–12886. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manyes, S.; Oncins, G.; Sanz, F. Effect of ion-binding and chemical phospholipid structure on the nanomechanics of lipid bilayers studied by force spectroscopy. Biophys. J. 2005, 89, 1812–1826. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manyes, S.; Oncins, G.; Sanz, F. Effect of temperature on the nanomechanics of lipid bilayers studied by force spectroscopy. Biophys. J. 2005, 89, 4261–4274. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manyes, S.; Oncins, G.; Sanz, F. Effect of ph and ionic strength on phospholipid nanomechanics and on deposition process onto hydrophilic surfaces measured by AFM. Electrochim. Acta 2006, 51, 5029–5036. [Google Scholar] [CrossRef]
- Li, J.K.; Sullan, R.M.A.; Zou, S. Atomic force microscopy force mapping in the study of supported lipid bilayers. Langmuir 2011, 27, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Abdulreda, M.H.; Moy, V.T. Atomic force microscope studies of the fusion of floating lipid bilayers. Biophys. J. 2007, 92, 4369–4378. [Google Scholar] [CrossRef] [PubMed]
- Butt, H.J.; Franz, V. Rupture of molecular thin films observed in atomic force microscopy. I. Theory. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2002, 66. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Sun, G.; Franz, V.; Butt, H.-J. Rupture of molecular thin films observed in atomic force microscopy. II. Experiment. Phys. Rev. E 2002, 66. [Google Scholar] [CrossRef] [PubMed]
- Franz, V.; Loi, S.; Muller, H.; Bamberg, E.; Butt, H.H. Tip penetration through lipid bilayers in atomic force microscopy. Colloid Surf. B 2002, 23, 191–200. [Google Scholar] [CrossRef]
- Sullan, R.M.A.; Li, J.K.; Hao, C.; Walker, G.C.; Zou, S. Cholesterol-dependent nanomechanical stability of phase-segregated multicomponent lipid bilayers. Biophys. J. 2010, 99, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, A.; Seeger, H.M.; Caramaschi, T.; Facci, P. Dynamic force spectroscopy on supported lipid bilayers: Effect of temperature and sample preparation. Biophys. J. 2012, 103, 38–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gumi-Audenis, B.; Sanz, F.; Giannotti, M.I. Impact of galactosylceramides on the nanomechanical properties of lipid bilayer models: An AFM-force spectroscopy study. Soft Matter 2015, 11, 5447–5454. [Google Scholar] [CrossRef] [PubMed]
- Oberhauser, A.F.; Hansma, P.K.; Carrion-Vazquez, M.; Fernandez, J.M. Stepwise unfolding of titin under force-clamp atomic force microscopy. Proc. Nat. Acad. Sci. USA 2001, 98, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Arrhenius, S. On the reaction rate of the inversion of non-refined sugar upon souring. Z. Phys. Chem. 1889, 4, 226–248. [Google Scholar]
- Bell, G.I. Models for specific adhesion of cells to cells. Science 1978, 200, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, A.; Facci, P. Phase transitions in supported lipid bilayers studied by AFM. Soft Matter 2014, 10, 7145–7164. [Google Scholar] [CrossRef] [PubMed]
- Murthy, A.V.R.; Guyomarc’h, F.; Lopez, C. The temperature-dependent physical state of polar lipids and their miscibility impact the topography and mechanical properties of bilayer models of the milk fat globule membrane. BBA-Biomembranes 2016, 1858, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Miyagi, A.; Redondo-Morata, L.; Scheuring, S. Temperature-controlled high-speed AFM: Real-time observation of ripple phase transitions. Small 2016, 12, 6106–6113. [Google Scholar] [CrossRef] [PubMed]
- Leonenko, Z.V.; Finot, E.; Ma, H.; Dahms, T.E.S.; Cramb, D.T. Investigation of temperature-induced phase transitions in DOPC and DPPC phospholipid bilayers using temperature-controlled scanning force microscopy. Biophys. J. 2004, 86, 3783–3793. [Google Scholar] [CrossRef] [PubMed]
- Seeger, H.M.; di Cerbo, A.; Alessandrini, A.; Facci, P. Supported lipid bilayers on mica and silicon oxide: Comparison of the main phase transition behavior. J. Phys. Chem. B 2010, 114, 8926–8933. [Google Scholar] [CrossRef] [PubMed]
- McMullen, T.P.W.; McElhaney, R.N. New aspects of the interaction of cholesterol with dipalmitoylphosphatidylcholine bilayers as revealed by high-sensitivity differential scanning calorimetry. BBA-Biomembranes 1995, 1234, 90–98. [Google Scholar] [CrossRef]
- Karmakar, S.; Raghunathan, V.A.; Mayor, S. Phase behaviour of dipalmitoyl phosphatidylcholine (DPPC)-cholesterol membranes. J. Phys. Condens. Matter 2005, 17, S1177–S1182. [Google Scholar] [CrossRef]
- Chiang, Y.-W.; Costa, A.J.; Freed, J.H. Dynamic molecular structure and phase diagram of DPPC-cholesterol binary mixtures: A 2D-ELDOR study. J. Phys. Chem. B 2007, 111, 11260–11270. [Google Scholar] [CrossRef] [PubMed]
- Marsh, D. Liquid-ordered phases induced by cholesterol: A compendium of binary phase diagrams. BBA-Biomembranes 2010, 1798, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.L.; Marquardt, D.; Dies, H.; Kucerka, N.; Yamani, Z.; Harroun, T.A.; Katsaras, J.; Shi, A.C.; Rheinstadter, M.C. The observation of highly ordered domains in membranes with cholesterol. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Vist, M.R.; Davis, J.H. Phase-equilibria of cholesterol dipalmitoylphosphatidylcholine mixtures—h-2 nuclear magnetic-resonance and differential scanning calorimetry. Biochemistry 1990, 29, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, A.I.; Tristram-Nagle, S.; Nagle, J.F. Partial molecular volumes of lipids and cholesterol. Chem. Phys. Lipids 2006, 143, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Goni, F.M.; Alonso, A. Biophysics of sphingolipids I. Membrane properties of sphingosine, ceramides and other simple sphingolipids. BBA-Biomembranes 2006, 1758, 1902–1921. [Google Scholar] [CrossRef] [PubMed]
- Bollinger, C.R.; Teichgraber, V.; Gulbins, E. Ceramide-enriched membrane domains. BBA Mol. Cell. Res. 2005, 1746, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Curatolo, W. The physical-properties of glycolipids. Biochim. Biophys. Acta 1987, 906, 111–136. [Google Scholar] [CrossRef]
- Longo, M.L.; Blanchette, C.D. Imaging cerebroside-rich domains for phase and shape characterization in binary and ternary mixtures. BBA-Biomembranes 2010, 1798, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Han, X.L.; Cheng, H. Characterization and direct quantitation of cerebroside molecular species from lipid extracts by shotgun lipidomics. J. Lipid Res. 2005, 46, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.M.; King, I.A.; Yardley, H.J. Plasma-membrane of granular cells from pig epidermis: Isolation and lipid and protein-composition. J. Investig. Dermatol. 1978, 71, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.C. The subcellular-localization of the glycosphingolipids in the epithelial-cells of rat small-intestine. Biochim. Biophys. Acta 1983, 733, 295–299. [Google Scholar] [CrossRef]
- Blanchette, C.D.; Lin, W.C.; Ratto, T.V.; Longo, M.L. Galactosylceramide domain microstructure: Impact of cholesterol and nucleation/growth conditions. Biophys. J. 2006, 90, 4466–4478. [Google Scholar] [CrossRef] [PubMed]
- Chiantia, S.; Ries, J.; Kahya, N.; Schwille, P. Combined AFM and two-focus SFCS study of raft-exhibiting model membranes. Chemphyschem 2006, 7, 2409–2418. [Google Scholar] [CrossRef] [PubMed]
- Guyomarc’h, F.; Zou, S.; Chen, M.H.; Milhiet, P.E.; Godefroy, C.; Vie, V.; Lopez, C. Milk sphingomyelin domains in biomimetic membranes and the role of cholesterol: Morphology and nanomechanical properties investigated using AFM and force spectroscopy. Langmuir 2014, 30, 6516–6524. [Google Scholar] [CrossRef] [PubMed]
- Murthy, A.V.R.; Guyomarc’h, F.; Lopez, C. Cholesterol decreases the size and the mechanical resistance to rupture of sphingomyelin rich domains, in lipid bilayers studied as a model of the milk fat globule membrane. Langmuir 2016, 32, 6757–6765. [Google Scholar] [CrossRef] [PubMed]
- Chiantia, S.; Kahya, N.; Ries, J.; Schwille, P. Effects of ceramide on liquid-ordered domains investigated by simultaneous AFM and FCS. Biophys. J. 2006, 90, 4500–4508. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Johnston, L.J. Ceramide-enriched microdomains in planar membranes. Curr. Opin. Colloid Interface 2010, 15, 489–498. [Google Scholar] [CrossRef]
- Sullan, R.M.A.; Li, J.K.; Zou, S. Direct correlation of structures and nanomechanical properties of multicomponent lipid bilayers. Langmuir 2009, 25, 7471–7477. [Google Scholar] [CrossRef] [PubMed]
- Sullan, R.M.A.; Li, J.K.; Zou, S. Quantification of the nanomechanical stability of ceramide-enriched domains. Langmuir 2009, 25, 12874–12877. [Google Scholar] [CrossRef] [PubMed]
- London, M.; London, E. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts)—Implications for lipid raft structure and function. J. Biol. Chem. 2004, 279, 9997–10004. [Google Scholar]
- Ali, M.R.; Cheng, K.H.; Huang, J. Ceramide drives cholesterol out of the ordered lipid bilayer phase into the crystal phase in 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine/cholesterol/ceramide ternary mixtures. Biochemistry 2006, 45, 12629–12638. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Feigenson, G.W. A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys. J. 1999, 76, 2142–2157. [Google Scholar] [CrossRef]
- Veatch, S.L.; Keller, S.L. Organization in lipid membranes containing cholesterol. Phys. Rev. Lett. 2002, 89. [Google Scholar] [CrossRef] [PubMed]
- Fidorra, M.; Heimburg, T.; Bagatolli, L.A. Direct visualization of the lateral structure of porcine brain cerebrosides/popc mixtures in presence and absence of cholesterol. Biophys. J. 2009, 97, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.S.; Dhez, O.; le Denmat, S.; Chevrier, J.; Felici, R.; Comin, F. Local detection of X-ray spectroscopies with an in-situ atomic force microscope. J. Instrum. 2008, 3. [Google Scholar] [CrossRef]
- Scheler, T.; Rodrigues, M.; Cornelius, T.W.; Mocuta, C.; Malachias, A.; Magalhaes-Paniago, R.; Comin, F.; Chevrier, J.; Metzger, T.H. Probing the elastic properties of individual nanostructures by combining in situ atomic force microscopy and micro-X-ray diffraction. Appl. Phys. Lett. 2009, 94. [Google Scholar] [CrossRef]
- Pilet, N.; Raabe, J.; Stevenson, S.E.; Romer, S.; Bernard, L.; McNeill, C.R.; Fink, R.H.; Hug, H.J.; Quitmann, C. Nanostructure characterization by a combined X-ray absorption/scanning force microscopy system. Nanotechonlogy 2012, 23. [Google Scholar] [CrossRef] [PubMed]
- Leclere, C.; Cornelius, T.W.; Ren, Z.; Davydok, A.; Micha, J.S.; Robach, O.; Richter, G.; Belliard, L.; Thomas, O. In situ bending of an au nanowire monitored by micro laue diffraction. J. Appl. Crystallogr. 2015, 48, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Mastropietro, F.; Davydok, A.; Langlais, S.; Richard, M.I.; Furter, J.J.; Thomas, O.; Dupraz, M.; Verdier, M.; Beutier, G.; et al. Scanning force microscope for in situ nanofocused X-ray diffraction studies. J. Synchrotron Radiat. 2014, 21, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Rodrigues, M.S. Combined X-ray-atomic force microscopy tools at the ESRF: The first 10 years. Synchrotron Radiat. News 2016, 29, 3–7. [Google Scholar] [CrossRef]
- Vitorino, M.V.; Fuchs, Y.; Dane, T.; Rodrigues, M.S.; Rosenthal, M.; Panzarella, A.; Bernard, P.; Hignette, O.; Dupuy, L.; Burghammer, M.; et al. An in situ atomic force microscope for normal-incidence nanofocus X-ray experiments. J. Synchrotron Radiat. 2016, 23, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Gumi-Audenis, B.; Carla, F.; Vitorino, M.V.; Panzarella, A.; Porcar, L.; Boilot, M.; Guerber, S.; Bernard, P.; Rodrigues, M.S.; Sanz, F.; et al. Custom afm for X-ray beamlines: In situ biological investigations under physiological conditions. J. Synchrotron Radiat. 2015, 22, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.E.; Majewski, J.; Gog, T.; Kuhl, T.L. Characterization of biological thin films at the solid-liquid interface by X-ray reflectivity. Phys. Rev. Lett. 2005, 94. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gumí-Audenis, B.; Costa, L.; Carlá, F.; Comin, F.; Sanz, F.; Giannotti, M.I. Structure and Nanomechanics of Model Membranes by Atomic Force Microscopy and Spectroscopy: Insights into the Role of Cholesterol and Sphingolipids. Membranes 2016, 6, 58. https://doi.org/10.3390/membranes6040058
Gumí-Audenis B, Costa L, Carlá F, Comin F, Sanz F, Giannotti MI. Structure and Nanomechanics of Model Membranes by Atomic Force Microscopy and Spectroscopy: Insights into the Role of Cholesterol and Sphingolipids. Membranes. 2016; 6(4):58. https://doi.org/10.3390/membranes6040058
Chicago/Turabian StyleGumí-Audenis, Berta, Luca Costa, Francesco Carlá, Fabio Comin, Fausto Sanz, and Marina I. Giannotti. 2016. "Structure and Nanomechanics of Model Membranes by Atomic Force Microscopy and Spectroscopy: Insights into the Role of Cholesterol and Sphingolipids" Membranes 6, no. 4: 58. https://doi.org/10.3390/membranes6040058





