Concentration of Tea Extracts by Osmotic Evaporation: Optimisation of Process Parameters and Effect on Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Modelling Water Flux and Mass Transfer Resistance
2.2. Osmotic Evaporation Set-Up and Experimental Procedure
2.3. Extraction Process of Teas
2.4. Total Phenolic Content
2.5. Antioxidant Activity
3. Results and Discussion
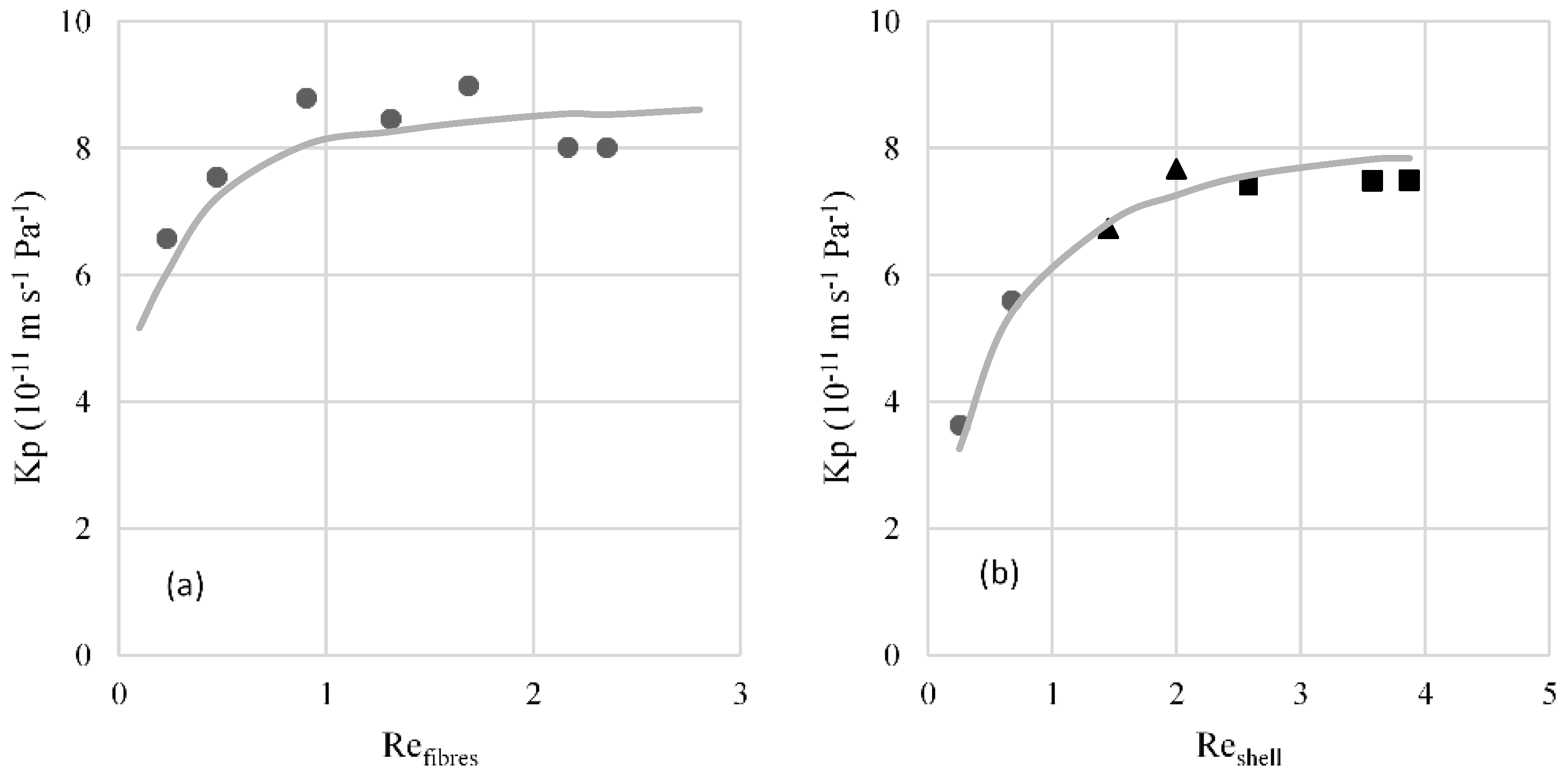
3.1. Hydrodynamic Conditions in the Fibres and Shell Sides
3.2. Mass Transfer Correlations
3.3. Concentration Process by Osmotic Evaporation
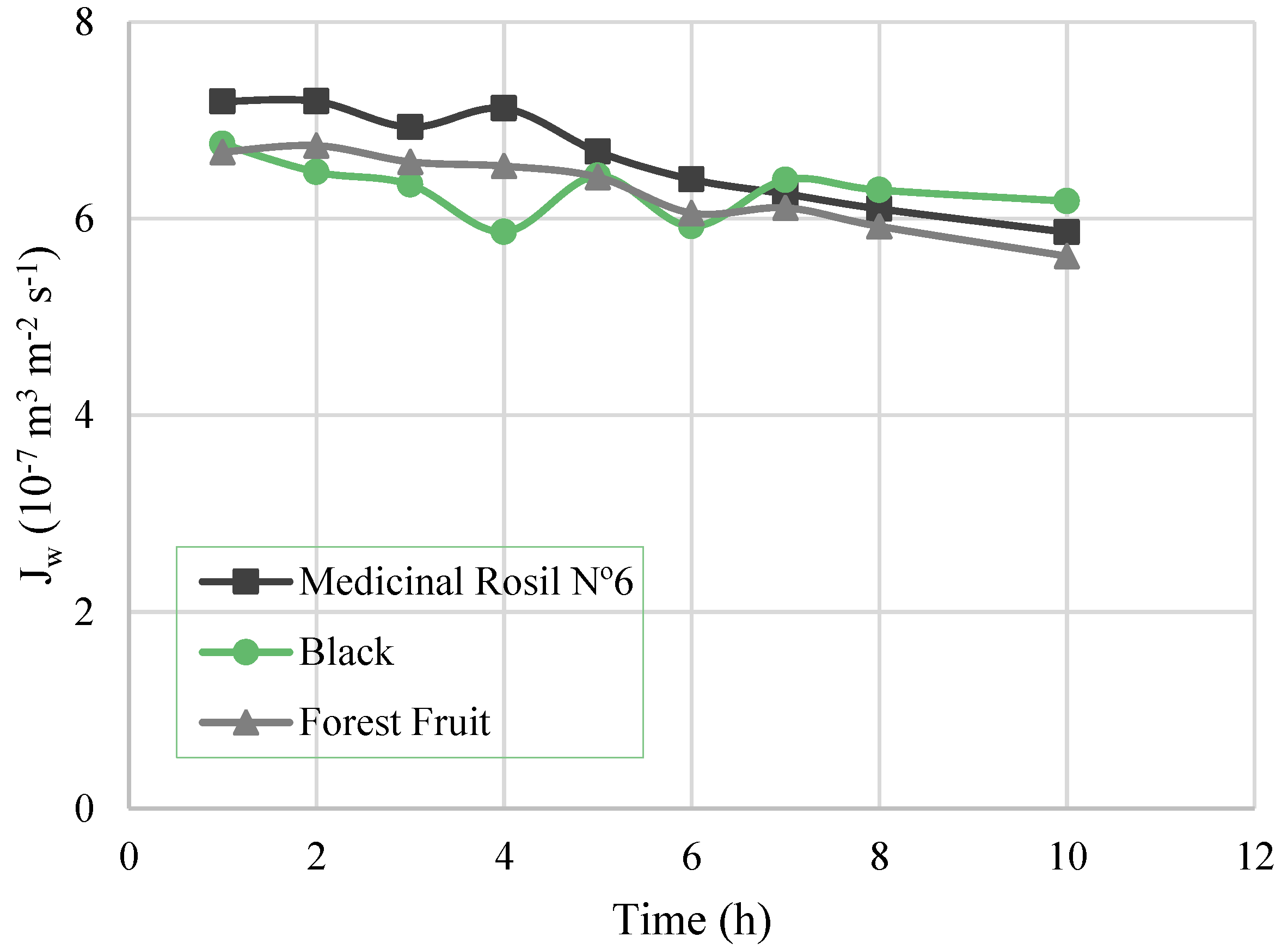
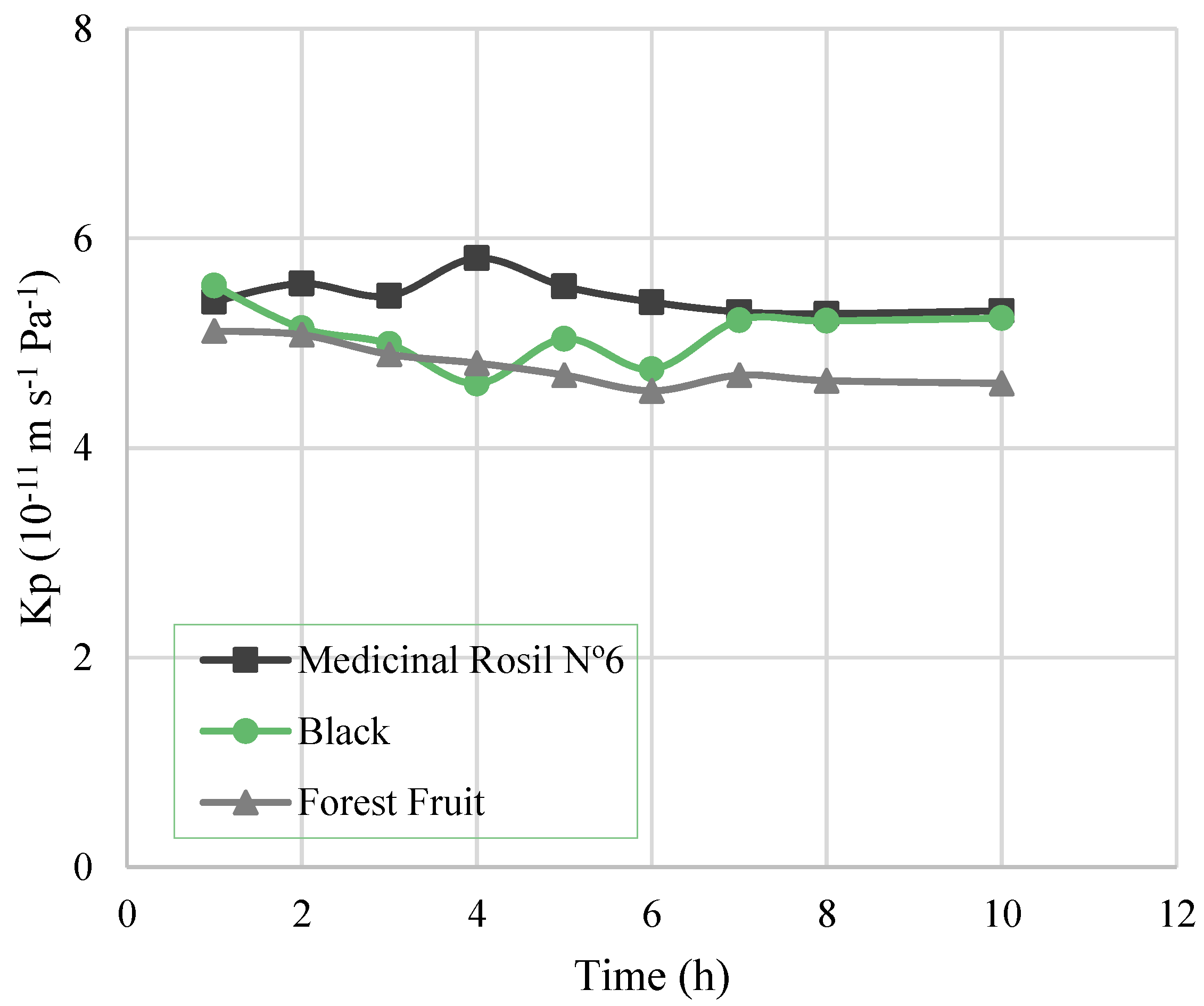
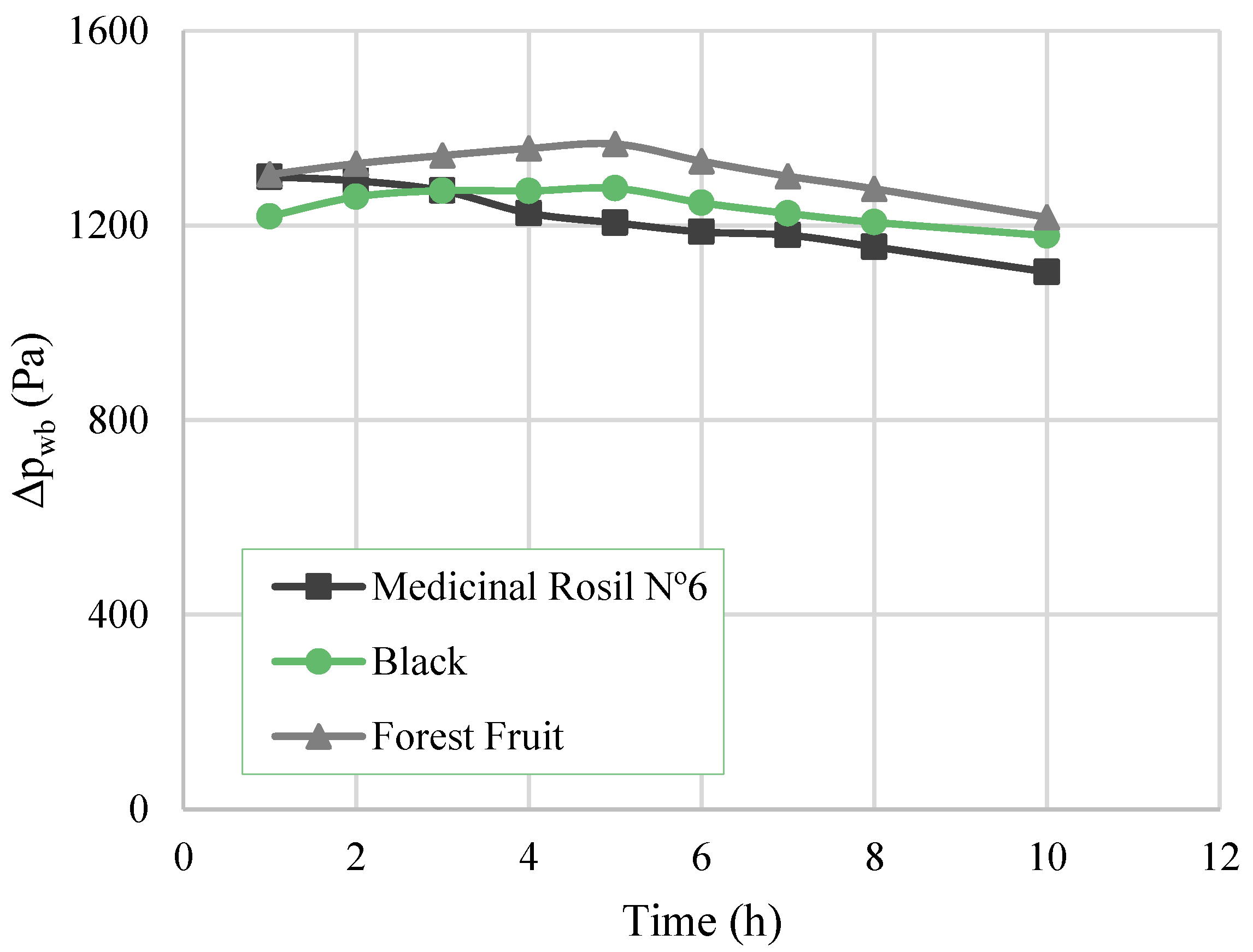
3.3.1. Water Flux, Overall Mass Transfer Coefficient, and Driving Force
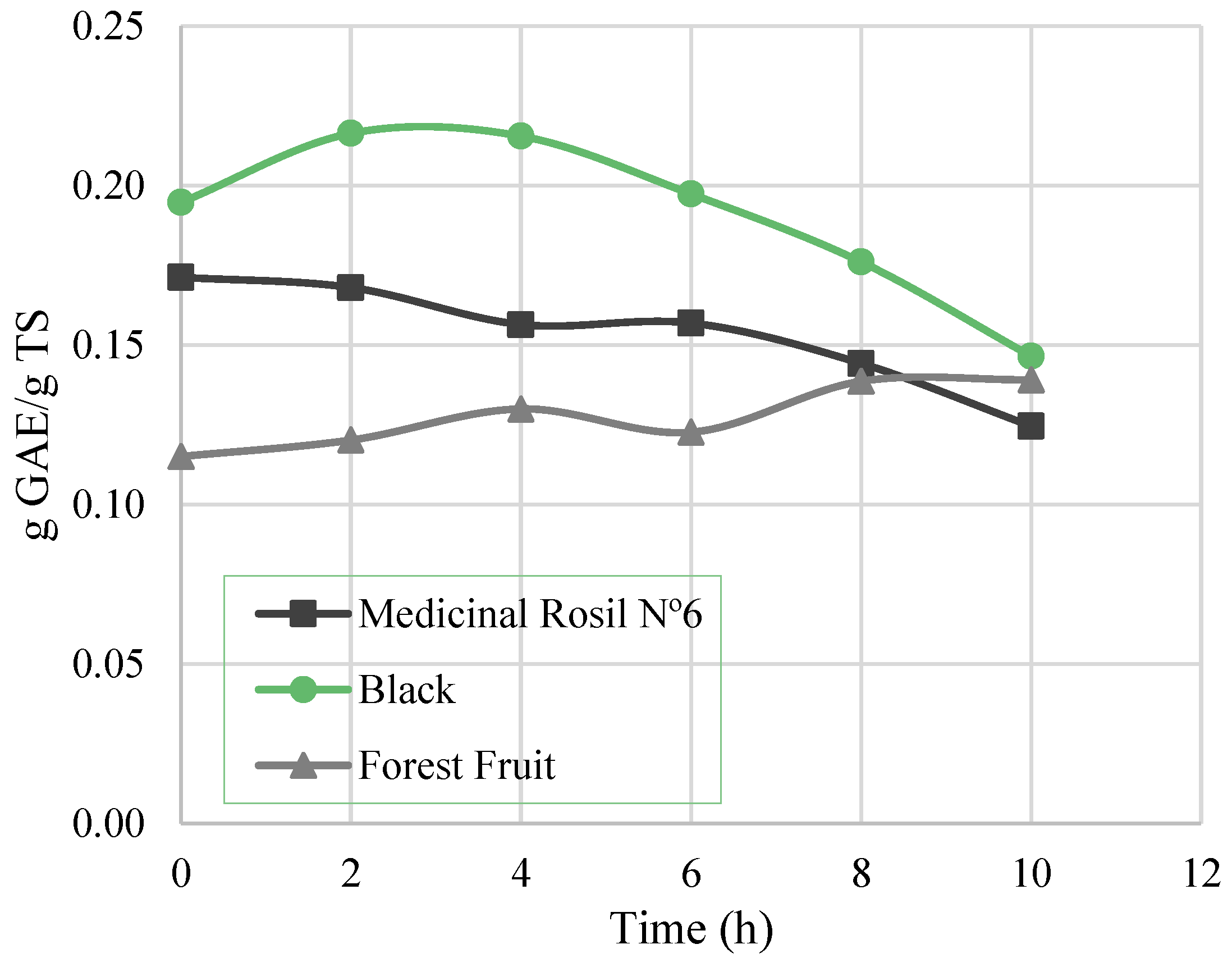
3.3.2. Total Phenolic Content
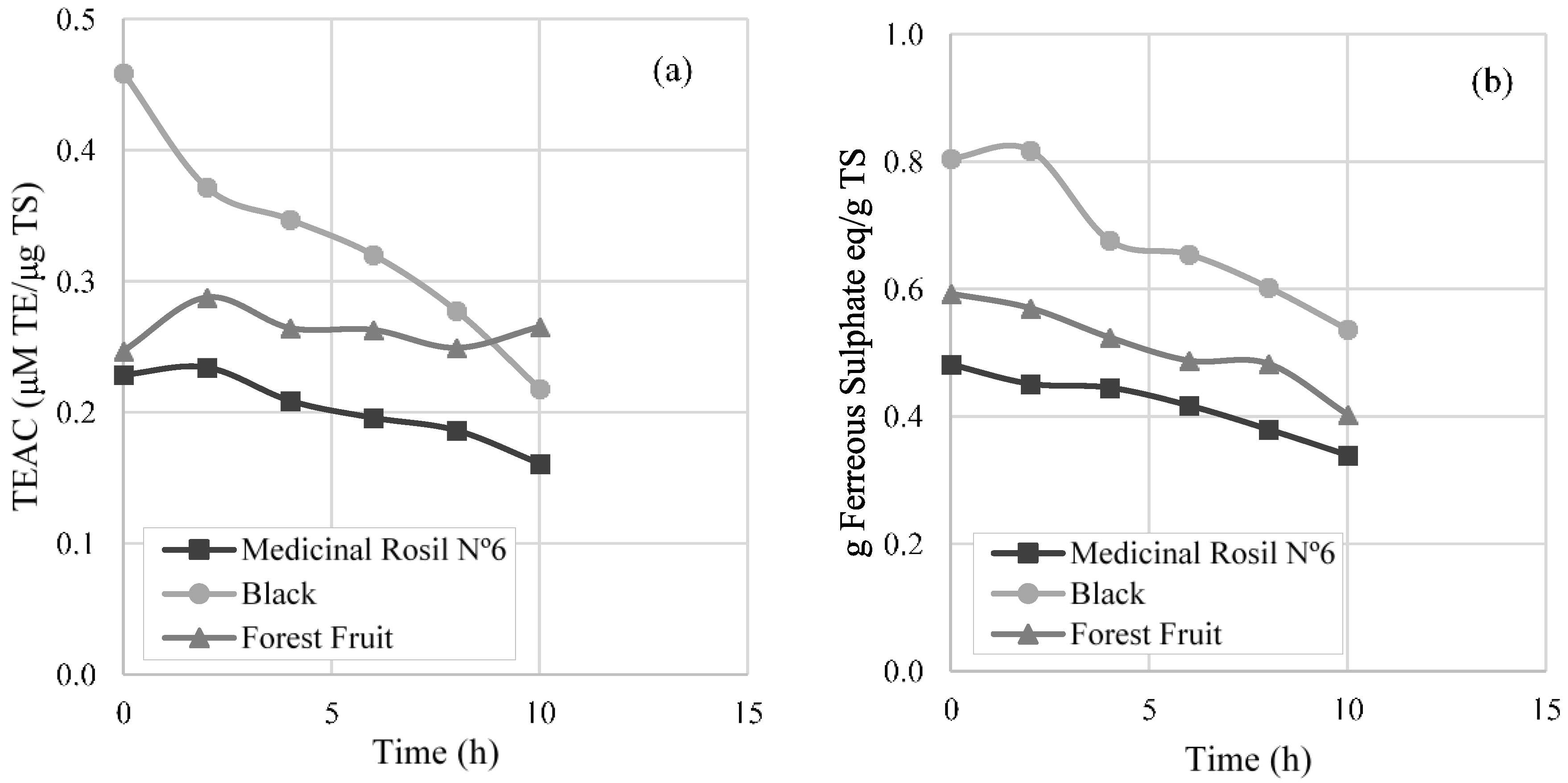
3.3.3. Antioxidant Activity
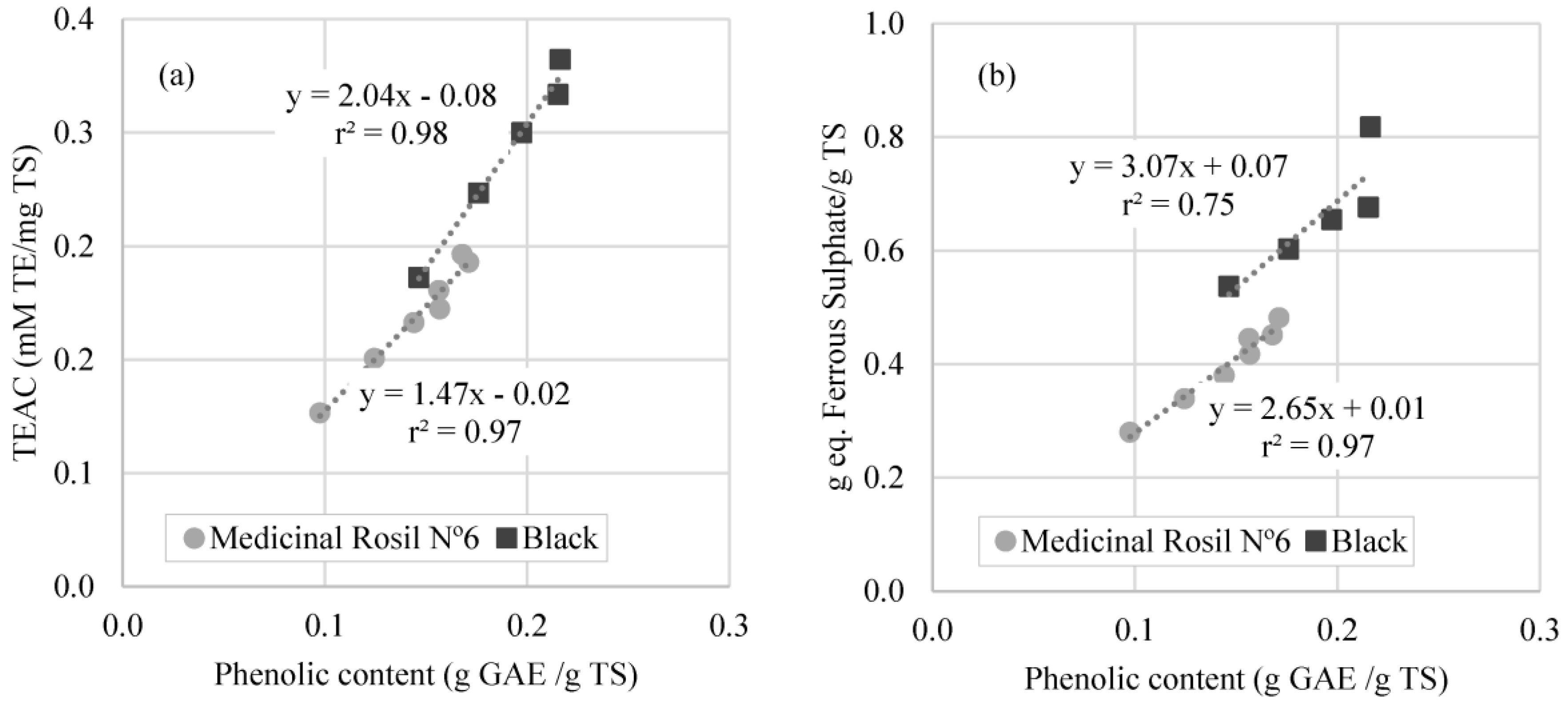
3.3.4. Correlation between Antioxidant Activity and Total Phenolic Content
3.3.5. Concentration Process Scale-Up
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saberi, H. Tea: A Global History; Reaktion Books: London, UK, 2010. [Google Scholar]
- Torun, M.; Rácz, G.; Fogarassy, E.; Vatai, G.; Dinçer, C.; Topuz, A.; Özdemir, F. Concentration of sage (Salvia fruticosa Miller) extract by using integrated membrane process. Sep. Purif. Technol. 2014, 132, 244–251. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, S.; Yuan, H.; Tang, P.; Yin, J. Analysis of cream formation in green tea concentrates with different solid concentrations. J. Food Sci. Technol. 2012, 49, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, S.; Riera, F.A.; Álvarez, R.; Coca, J.; Cuperus, F.P.; Bouwer, S.T.; Boswinkel, G.; Gemert, R.W.; Veldsink, J.W.; Giorno, L.; et al. A new integrated membrane process for producing clarified apple juice and apple juice aroma concentrate. J. Food Eng. 2000, 46, 109–125. [Google Scholar] [CrossRef]
- Cassano, A.; Drioli, E.; Galaverna, G.; Marchelli, R.; Silvestro, G.D.; Cagnasso, P. Clarification and concentration of citrus and carrot juices by integrated membrane processes. J. Food Eng. 2003, 57, 153–163. [Google Scholar] [CrossRef]
- Galaverna, G.; Silvestro, G.D.; Cassano, A.; Sforza, S.; Dossena, A.; Drioli, E.; Marchelli, R. A new integrated membrane process for the production of concentrated blood orange juice: Effect on bioactive compounds and antioxidant activity. Food Chem. 2008, 106, 1021–1030. [Google Scholar] [CrossRef]
- Alves, V.D.; Coelhoso, I.M. Orange juice concentration by osmotic evaporation and membrane distillation: A comparative study. J. Food Eng. 2006, 74, 125–133. [Google Scholar] [CrossRef]
- Petrotos, K.B.; Lazarides, H.N. Osmotic concentration of liquid foods. J. Food Eng. 2001, 49, 201–206. [Google Scholar] [CrossRef]
- Barbe, A.; Bartley, J.; Jacobs, A.; Johnson, R. Retention of volatile organic flavour/fragrance components in the concentration of liquid foods by osmotic distillation. J. Membr. Sci. 1998, 145, 67–75. [Google Scholar] [CrossRef]
- Nii, S.; Jebson, R.; Cussler, E. Membrane evaporators. J. Membr. Sci. 2002, 201, 149–159. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Drioli, E. Physico-chemical parameters of cactus pear (Opuntia ficus-indica) juice clarified by microfiltration and ultrafiltration processes. Desalination 2010, 250, 1101–1104. [Google Scholar] [CrossRef]
- Onsekizoglu, P.; Bahceci, K.S.; Acar, M.J. Clarification and the concentration of apple juice using membrane processes: A comparative quality assessment. J. Membr. Sci. 2010, 352, 160–165. [Google Scholar] [CrossRef]
- Rodrigues, R.B.; Menezes, H.C.; Cabral, L.C.; Dornier, M.; Rios, G.M.; Reynes, M. Evaluation of reverse osmosis and osmotic evaporation to concentrate camu-camu juice (Myrciaria dubia). J. Food Eng. 2004, 63, 97–102. [Google Scholar] [CrossRef]
- Cissé, M.; Vaillant, F.; Bouquet, S.; Pallet, D.; Lutin, F.; Reynes, M.; Dornier, M. Athermal concentration by osmotic evaporation of roselle extract, apple and grape juices and impact on quality. Innov. Food Sci. Emerg. Technol. 2011, 12, 352–360. [Google Scholar] [CrossRef]
- Hasanoglu, A.; Rebolledo, F.; Plaza, A.; Torres, A.; Romero, J. Effect of the operating variables on the extraction and recovery of aroma compounds in an osmotic distillation process coupled to a vacuum membrane distillation system. J. Food Eng. 2012, 111, 632–641. [Google Scholar] [CrossRef]
- Souza, A.L.R.; Pagani, M.M.; Dornier, M.; Gomes, F.S.; Tonon, R.V.; Cabral, L.M.C. Concentration of camu–camu juice by the coupling of reverse osmosis and osmotic evaporation processes. J. Food Eng. 2013, 119, 7–12. [Google Scholar] [CrossRef]
- Zambra, C.; Romero, J.; Pino, L.; Saavedra, A.; Sanchez, J. Concentration of cranberry juice by osmotic distillation process. J. Food Eng. 2015, 144, 58–65. [Google Scholar] [CrossRef]
- Alves, V.D.; Coelhoso, I.M. Study of mass and heat transfer in the osmotic evaporation process using hollow fibre membrane contactors. J. Membr. Sci. 2007, 289, 249–257. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, P.O. The Properties of Gases and Liquids, 5th ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Alves, V.D.; Coelhoso, I.M. Mass transfer in osmotic evaporation: Effect of process parameters. J. Membr. Sci. 2002, 208, 171–179. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Asssay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical method to Evaluate Antioxidant Activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Rampp, M.; Buttersack, C.; Lüdemann, H. c,T-Dependence of viscosity and the self-diffusion coefficients in some aqueous carbohydrate solutions. Carbohydr. Res. 2000, 328, 561–572. [Google Scholar] [CrossRef]
- Tamas, J.; Ujszaszy, K. Diffusion of H218O molecules in certain aqueous salt solutions. Magy. Kem. Foly. 1966, 72, 447. [Google Scholar]
- Viegas, R.C.; Rodríguez, M.; Luque, S.; Alvarez, J.R.; Coelhoso, I.M.; Crespo, J.G. Mass transfer correlations in membrane extraction: Analysis of wilson-plot methodology. J. Membr. Sci. 1998, 145, 129–142. [Google Scholar] [CrossRef]
- Pekal, A.; Drózdz, P.; Biesaga, M.; Pyrzynska, K. Evaluation of the antioxidant properties of fruit and flavoured black teas. Eur. J. Nutr. 2011, 50, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, M.; Reese, R.N.; Tulio, A., Jr.; Scheerens, J.C.; Miller, A.R. Modified 2,2-Azino-bis-3-ethylbenzothiazoline-6-sulfonic Acid (ABTS) Method to Measure Antioxidant Capacity of Selected Small Fruits and Comparison to Ferric Reducing Antioxidant Power (FRAP) and 2,2′-Diphenyl-1-picrylhydrazyl (DPPH) Methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Boxin, O.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E. Analysis of Antioxidant Activities of Common Vegetables Employing Oxygen Radical Absorbance Capacity (ORAC) and Ferric Reducing Antioxidant Power (FRAP) Assays: A Comparative Study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chem. 2006, 99, 835–841. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]

| Tea | Ingredients |
|---|---|
| Medicinal Rosil No. 6 tea | Avocado (Persea americana) |
| Boldo (Peumus boldus) | |
| Gorse flower (Genista tridentata) | |
| Horsetail (Equisetum giganteum) | |
| Herb Robert (Geranium robertianum) | |
| St. John’s weed (Hypericum perforatum) | |
| Dandelion (Taraxacum officinale) | |
| Black tea | Camellia Sinensis (Camellia sinensis) |
| Forest Fruit tea | Camellia Sinensis (Camellia sinensis) |
| Raspberry (Rubus idaeus) | |
| Cherry (Prunus) | |
| Blackberry (Morus) | |
| Redcurrant (Ribes rubrum) |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, M.P.; Alves, V.D.; Coelhoso, I.M. Concentration of Tea Extracts by Osmotic Evaporation: Optimisation of Process Parameters and Effect on Antioxidant Activity. Membranes 2017, 7, 1. https://doi.org/10.3390/membranes7010001
Marques MP, Alves VD, Coelhoso IM. Concentration of Tea Extracts by Osmotic Evaporation: Optimisation of Process Parameters and Effect on Antioxidant Activity. Membranes. 2017; 7(1):1. https://doi.org/10.3390/membranes7010001
Chicago/Turabian StyleMarques, Marisa P., Vítor D. Alves, and Isabel M. Coelhoso. 2017. "Concentration of Tea Extracts by Osmotic Evaporation: Optimisation of Process Parameters and Effect on Antioxidant Activity" Membranes 7, no. 1: 1. https://doi.org/10.3390/membranes7010001








